Structure and Properties of Protective Coatings Deposited by Pulsed Cathodic Arc Evaporation in Ar, N2, and C2H4 Environments using the TiC–NiCr–Eu2O3 Cathode
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sahoo, C.K.; Masanta, M. Microstructure and mechanical properties of TiC–Ni coating on AISI304 steel produced by TIG cladding process. J. Mater. Process. Technol. 2017, 240, 126–137. [Google Scholar] [CrossRef]
- Wei, Q.; Pang, X.; Zhou, J.; Chen, C. High temperature spectral selective TiC–Ni/Mo cermet-based coatings for solar thermal systems by laser cladding. Solar Energy 2018, 171, 247–257. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Z.; Pu, F.; Xia, Z.; Sun, G.; Zhang, L.; Shi, C.; Zhang, Z. Microstructure and dry-sliding wear properties of TiC/CaF2/γ–Ni self-lubricating wear-resistant composite coating produced by co-axial powder feeding plasma transferred arc (PTA) cladding process. Surf. Coat. Technol. 2018, 345, 61–69. [Google Scholar] [CrossRef]
- Huang, S.; Sun, D.; Wang, W.; Xu, H. Microstructures and properties of in-situ TiC particles reinforced Ni-based composite coatings prepared by plasma spray welding. Ceram. Int. 2015, 41, 12202–12210. [Google Scholar] [CrossRef]
- Qi, X.; Eigen, N.; Aust, E.; Gärtner, F.; Klassen, T.; Bormann, R. Two-body abrasive wear of nano- and microcrystalline TiC–Ni-based thermal spray coatings. Surf. Coat. Technol. 2006, 200, 5037–5047. [Google Scholar] [CrossRef]
- Kartal, M.; Buyukbayram, I.; Alp, A.; Akbulut, H. Production of pulse electrodeposited Ni–TiC nanocomposite coatings. Mater. Today Proc. 2017, 4, 6982–6989. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.; Sheveyko, A.; Shvindina, N.; Levashov, E.; Shtansky, D. Comparative study of Ti–C–Ni–Al, Ti–C–Ni–Fe, and Ti–C–Ni–Al/Ti–C–Ni–Fe coatings produced by magnetron sputtering, electro-spark deposition, and a combined two-step process. Ceram. Int. 2018, 44, 7637–7646. [Google Scholar] [CrossRef]
- Grandin, M.; Nedfors, N.; Sundberg, J.; Jansson, U.; Wiklund, U. Ti–Ni–C nanocomposite coatings evaluated in a sliding electrical contact application. Surf. Coat. Technol. 2015, 276, 210–218. [Google Scholar] [CrossRef]
- André, B.; Lewin, E.; Jansson, U.; Wiklund, U. Friction and contact resistance of nanocomposite Ti–Ni–C coatings. Wear 2011, 270, 555–566. [Google Scholar] [CrossRef]
- Daniel, J.; Souček, P.; Bernátová, K.; Zábranský, L.; Stupavská, M.; Buršíková, V.; Vašina, P. Investigation of the influence of Ni doping on the structure and hardness of Ti–Ni–C coatings. J. Nanomater. 2017, 2017, 6368927. [Google Scholar] [CrossRef]
- Levashov, E.; Kudryashov, A.; Vakaev, P.; Shtansky, D.; Malochkin, O.; Gammel, F.; Suchentrunk, R.; Moore, J.; Shtansky, D. The prospects of nanodispersive powders application in surface engineering technologies. Surf. Coat. Technol. 2004, 180, 347–351. [Google Scholar] [CrossRef]
- Rajabi, A.; Ghazali, M.; Syarif, J.; Daud, A. Development and application of tool wear: A review of the characterization of TiC-based cermets with different binders. Chem. Eng. J. 2014, 255, 445–452. [Google Scholar] [CrossRef]
- Tan, Y.; He, L.; Wang, X.; Hong, X.; Wang, W. Tribological properties and wear prediction model of TiC particles reinforced Ni-base alloy composite coatings. Trans. Nonferr. Met. Soc. China 2014, 24, 2566–2573. [Google Scholar] [CrossRef]
- Zohari, S.; Sadeghian, Z.; Lotfi, B.; Broeckmann, C. Application of spark plasma sintering (SPS) for the fabrication of in situ Ni–TiC nanocomposite clad layer. J. Alloy. Compd. 2015, 633, 479–483. [Google Scholar] [CrossRef]
- Bin, C.; Tan, Y.; Long, H.; Hua, T.; Li, G. Tribological properties of TiC particles reinforced Ni-based alloy composite coatings. Trans. Nonferr. Met. Soc. China 2013, 23, 1681–1688. [Google Scholar]
- Chen, S.; Xiong, W.; Yao, Z.; Zhang, G.; Chen, X.; Huang, B.; Yang, Q. Corrosion behavior of Ti(C,N)–Ni/Cr cermets in H2SO4 solution. Int. J. Refract. Met. Hard Mater. 2014, 47, 139–144. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Chen, X.; Chi, C.; Wang, M.; Han, X.; Xie, Y. Influence of Cr addition on the interface purification of vacuum brazed NiCr–Cr3C2 coatings on single crystal superalloy. Surf. Coat. Technol. 2017, 325, 200–209. [Google Scholar] [CrossRef]
- Solecka, M.; Radziszewska, A.; Rutkowski, B. New insight on study of Ni-base alloy clad layer after oxidation at 650 °C. Corros. Sci. 2019, 149, 244–248. [Google Scholar] [CrossRef]
- Levashov, E.A.; Malochkin, O.V.; Kudryashov, A.E.; Gammel, F.; Suchentrunk, R. Effects of nanocrystalline powders additions on the characteristics of combustion process, phase- and structure-formation, and properties of SHS alloys on titanium carbide base. J. Mater. Synth. Process. 2002, 10, 231–236. [Google Scholar] [CrossRef]
- Quazi, M.; Fazal, M.A.; Haseeb, A.; Yusof, F.; Masjuki, H.; Arslan, A. Effect of rare earth elements and their oxides on tribo-mechanical performance of laser claddings: A review. J. Rare Earths 2016, 34, 549–564. [Google Scholar] [CrossRef]
- Sun, S.; Fu, H.; Ping, X.; Guo, X.; Lin, J.; Lei, Y.; Wu, W.; Zhou, J. Effect of CeO2 addition on microstructure and mechanical properties of in-situ (Ti, Nb)C/Ni coating. Surf. Coat. Technol. 2019, 359, 300–313. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Sytchenko, A.D.; Kudryashov, A.E.; Levashov, E.A.; Shtansky, D.V. The effect of Eu2O3 additive to the TiCNiCr electrode on the formation of electrospark coatings. Tech. Phys. Lett. 2018, 44, 753–755. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Sytchenko, A.D.; Kudryashov, A.E.; Levashov, E.A. Protective coatings produced by electro-spark deposition with TiCNiCr–(Eu2O3) electrodes. CIS Iron Rev. 2018, 16, 57–62. [Google Scholar] [CrossRef]
- Sanchette, F.; Ducros, C.; Schmitt, T.; Steyer, P.; Billard, A. Nanostructured hard coatings deposited by cathodic arc deposition: From concepts to applications. Surf. Coat. Technol. 2011, 205, 5444–5453. [Google Scholar] [CrossRef]
- Mattox, D.M. The Foundations of Vacuum Coating Technology, 2nd ed.; Elsevier/William Andrew: Norwich, NY, USA, 2018; p. 378. [Google Scholar]
- Martin, P. Review of the filtered vacuum arc process and materials deposition. Thin Films 2001, 394, 1–14. [Google Scholar] [CrossRef]
- Karlsson, L.; Hultman, L.; Johansson, M.; Sundgren, J.-E.; Ljungcrantz, H. Growth, microstructure, and mechanical properties of arc evaporated TiCxN1−x (0 ≤ x ≤ 1) films. Surf. Coat. Technol. 2000, 126, 1–14. [Google Scholar] [CrossRef]
- Grimm, W.; Weihnacht, V. Properties of super-hard carbon films deposited by pulsed arc process. Vacuum 2010, 85, 506–509. [Google Scholar] [CrossRef]
- Warcholinski, B.; Gilewicz, A.; Kuprin, A.; Tolmachova, G.; Ovcharenko, V.; Kuznetsova, T.; Zubar, T.; Khudoley, A.; Chizhik, S. Mechanical properties of Cr-O-N coatings deposited by cathodic arc evaporation. Vacuum 2018, 156, 97–107. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, F.V.; Shirmanov, N.A.; Sheveiko, A.N.; Levashov, E.A.; Petrzhik, M.I.; Shtanskii, D.V. Nanostructural wear-resistant coatings produced on metal-cutting tools by electric-arc evaporation and magnetronic sputtering. Russ. Eng. Res. 2010, 30, 910–920. [Google Scholar] [CrossRef]
- Chang, C.-L.; Chen, Y.-W. Effect of the carbon content on the structure and mechanical properties of Ti–Si–C coatings by cathodic arc evaporation. Surf. Coat. Technol. 2010, 205, S1–S4. [Google Scholar] [CrossRef]
- Johnson, L.; Rogström, L.; Johansson, M.; Odén, M.; Hultman, L. Microstructure evolution and age hardening in (Ti,Si)(C,N) thin films deposited by cathodic arc evaporation. Thin Films 2010, 519, 1397–1403. [Google Scholar] [CrossRef]
- Lugscheider, E.; Knotek, O.; Zimmermann, H.; Hellmann, S. Investigation of the mechanical and structural properties of Ti–Hf–C–N arc PVD coatings. Surf. Coat. Technol. 1999, 116, 239–243. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Kuptsov, K.A.; Novikov, A.V.; Shtansky, D. Ti–Cr–B–N coatings prepared by pulsed cathodic-arc evaporation of ceramic TiCrB target produced by SHS. Prot. Met. Phys. Chem. Surfaces 2013, 49, 677–681. [Google Scholar] [CrossRef]
- Siemroth, P.; Schulke, T.; Witke, T. High-current arc—A new source for high-rate deposition. Surf. Coat. Technol. 1994, 68, 314–319. [Google Scholar] [CrossRef]
- Engers, B.; Fuchs, H.; Schultz, J.; Hettkamp, E.; Mecke, H. Comparison of substrate temperature and deposition rate between modified pulsed arc process and d.c. arc process. Surf. Coat. Technol. 2000, 133, 121–125. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Pierson, J.-F.; Billard, A.; Belmonte, T.; Michel, H.; Frantz, C. Influence of oxygen flow rate on the structural and mechanical properties of reactively magnetron sputter-deposited Zr–B–O coatings. Thin Films 1999, 347, 78–84. [Google Scholar] [CrossRef]
- Lengauer, W. Transition Metal Carbides, Nitrides, and Carbonitrides; Wiley: Hoboken, NJ, USA, 2008; pp. 202–252. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U. Control of crystallinity in sputtered Cr–Ti–C films. Acta Mater. 2013, 61, 6352–6361. [Google Scholar] [CrossRef]
- Wdowik, U.; Twardowska, A.; Mȩdala-Wa̧sik, M. Lattice dynamics of binary and ternary phases in Ti–Si–C system: A combined Raman spectroscopy and density functional theory study. Mater. Chem. Phys. 2015, 168, 58–65. [Google Scholar] [CrossRef]
- Escobar-Alarcón, L.; Medina, V.; Camps, E.; Romero, S.; Fernández, M.; Solís-Casados, D. Microstructural characterization of Ti–C–N thin films prepared by reactive crossed beam pulsed laser deposition. Appl. Surf. Sci. 2011, 257, 9033–9037. [Google Scholar] [CrossRef]
- Bolelli, G.; Colella, A.; Lusvarghi, L.; Puddu, P.; Rigon, R.; Sassatelli, P.; Testa, V. Properties of HVOF-sprayed TiC–FeCrAl coatings. Wear 2019, 36–51. [Google Scholar] [CrossRef]
- Dilawar, N.; Mehrotra, S.; Varandani, D.; Kumaraswamy, B.; Haldar, S.; Bandyopadhyay, A. A Raman spectroscopic study of C-type rare earth sesquioxides. Mater. Charact. 2008, 59, 462–467. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, G.; Wang, X.; Lv, G.; Zhou, L.; Chen, H.; Pang, H.; Yang, S. Influence of pulsed substrate bias on the structure and properties of Ti–Al–N films deposited by cathodic vacuum arc. Appl. Surf. Sci. 2012, 258, 7274–7279. [Google Scholar] [CrossRef]
- Fager, H.; Andersson, J.; Lu, J.; Jöesaar, M.J.; Odén, M.; Hultman, L. Growth of hard amorphous TiAlSiN thin films by cathodic arc evaporation. Surf. Coat. Technol. 2013, 235, 376–382. [Google Scholar] [CrossRef]
- Glatz, S.; Koller, C.; Bolvardi, H.; Kolozsvári, S.; Riedl, H.; Mayrhofer, P. Influence of Mo on the structure and the tribomechanical properties of arc evaporated Ti–Al–N. Surf. Coat. Technol. 2017, 311, 330–336. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yang, S.-J.; Wang, D.-Y. Characterization of TiCr(C,N)/amorphous carbon coatings synthesized by a cathodic arc deposition process. Thin Films 2007, 515, 4722–4726. [Google Scholar] [CrossRef]
- Witke, T.; Schuelke, T.; Schultrich, B.; Siemroth, P.; Vetter, J. Comparison of filtered high-current pulsed arc deposition (φ-HCA) with conventional vacuum arc methods. Surf. Coat. Technol. 2000, 126, 81–88. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Chen, W.-J.; Chen, J.-H.; Chang, C.-L. Deposition and characterization of TiBCN films by cathodic arc plasma evaporation. Thin Films 2009, 517, 5044–5049. [Google Scholar] [CrossRef]
- Eriksson, A.; Ghafoor, N.; Jensen, J.; Näslund, L.-Å.; Johansson, M.; Sjölén, J.; Odén, M.; Hultman, L.; Rosén, J. Arc deposition of Ti–Si–C–N thin films from binary and ternary cathodes—Comparing sources of C. Surf. Coat. Technol. 2012, 213, 145–154. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Levashov, E.; Petrzhik, M.; Shtansky, D.; Kiryukhantsev-Korneev, P.; Sheveyko, A.; Valiev, R.; Gunderov, D.; Prokoshkin, S.; Korotitskiy, A.; Smolin, A.; et al. Nanostructured titanium alloys and multicomponent bioactive films: Mechanical behavior at indentation. Mater. Sci. Eng. A 2013, 570, 51–62. [Google Scholar] [CrossRef]
- Martinez, D.M.; Cartes, C.L.; Justo, A.; Fernández, A.; Sanchez-Lopez, J.C. Self-lubricating Ti–C–N nanocomposite coatings prepared by double magnetron sputtering. Solid State Sci. 2009, 11, 660–670. [Google Scholar] [CrossRef]
- Kuptsov, K.; Kiryukhantsev-Korneev, P.; Sheveyko, A.; Shtansky, D.; Shtansky, D. Comparative study of electrochemical and impact wear behavior of TiCN, TiSiCN, TiCrSiCN, and TiAlSiCN coatings. Surf. Coat. Technol. 2013, 216, 273–281. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, G.; Ma, H.; Lin, G. Effect of pulsed bias voltage on the structure and mechanical properties of Ti–C–N composite films by pulsed bias arc ion plating. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 333, 1–5. [Google Scholar] [CrossRef]
- Chen, R.; Tu, J.; Liu, D.; Mai, Y.; Gu, C. Microstructure, mechanical and tribological properties of TiCN nanocomposite films deposited by DC magnetron sputtering. Surf. Coat. Technol. 2011, 205, 5228–5234. [Google Scholar] [CrossRef]
- Zhang, F.; He, J.; Chen, K.; Qin, Y.; Li, C.; Yin, F. Microstructure evolution and mechanical properties of TiCN–Cr nano/micro composite coatings prepared by reactive plasma spraying. Appl. Surf. Sci. 2018, 427, 905–914. [Google Scholar] [CrossRef]
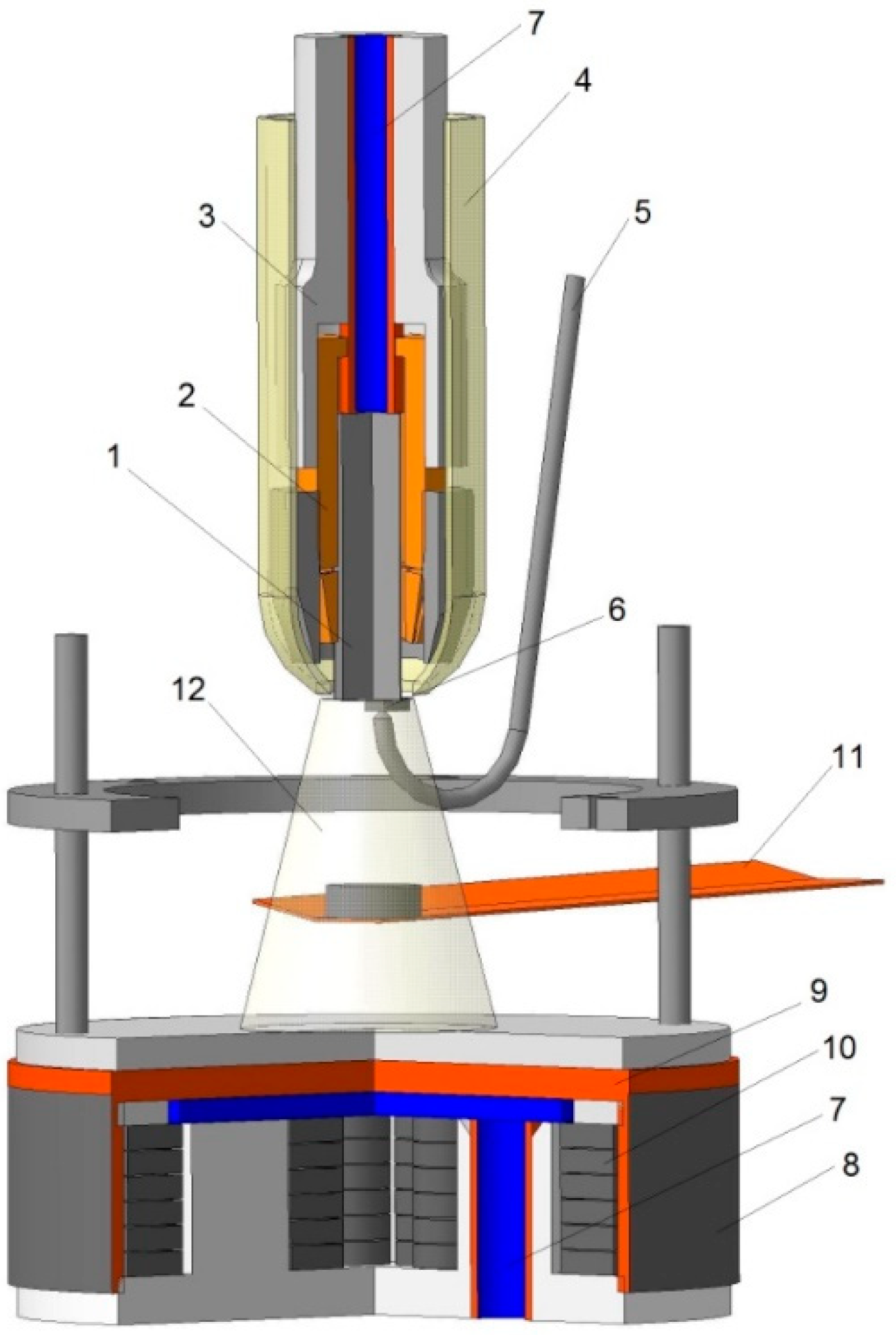

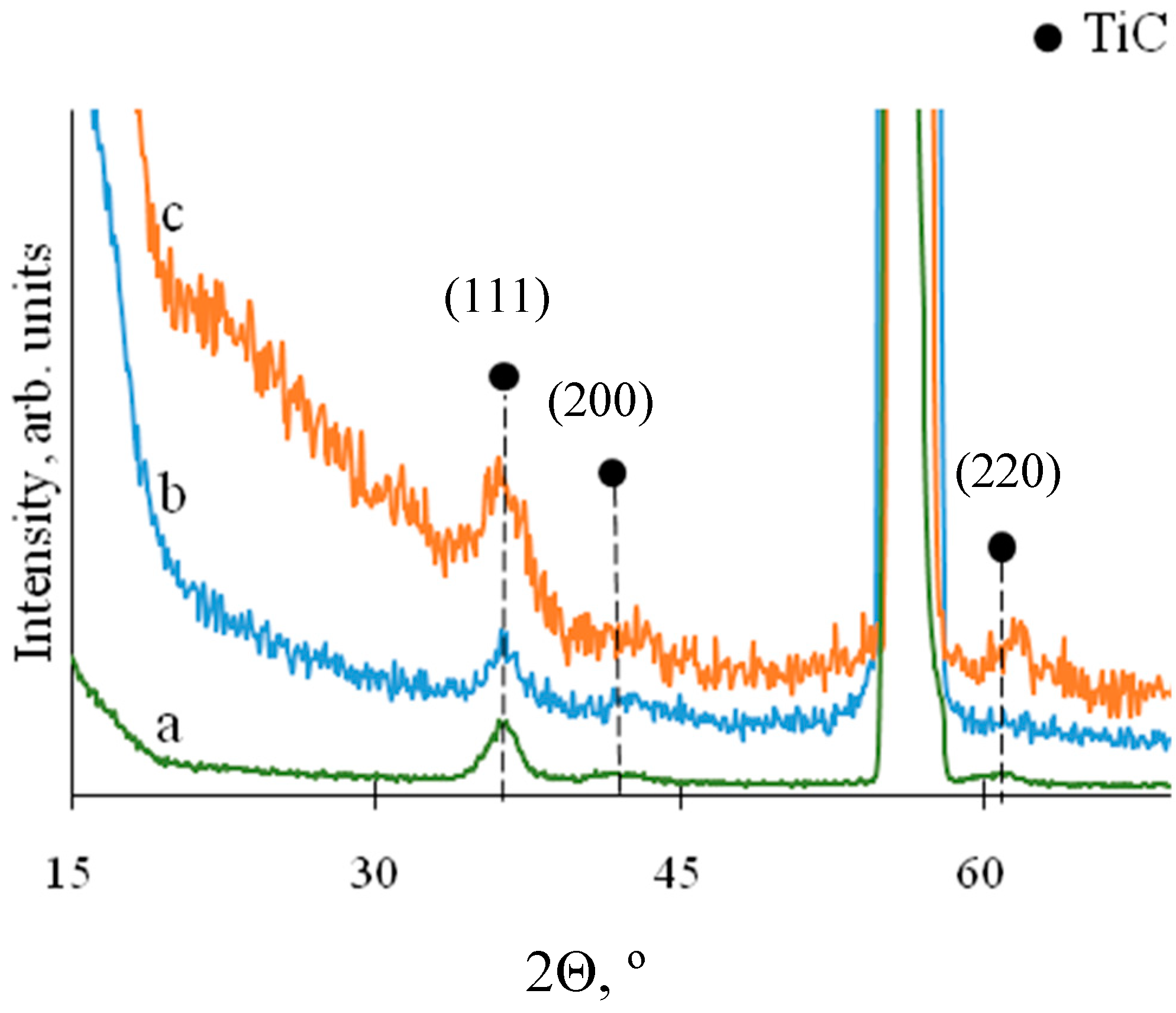




| Coatings | Environment | Content, at.% | |||||
|---|---|---|---|---|---|---|---|
| C | Ti | Cr | Ni | Eu | N | ||
| 1 | Ar | 52.9 | 39.7 | 4.0 | 2.9 | 0.5 | 0 |
| 2 | C2H4 | 86.8 | 10.9 | 1.6 | 0.8 | 0 | 0 |
| 3 | N2 | 23.2 | 18.4 | 3.2 | 1.5 | 0.4 | 53.2 |
| Cathode | – | 45.6 | 45.7 | 5.3 | 2.9 | 0.4 | 0 |
| Coating | Environment | H, GPa | E, GPa | W, % | H/E | H3/E2, GPa |
|---|---|---|---|---|---|---|
| 1 | Ar | 20.2 | 166 | 92 | 0.12 | 0.30 |
| 2 | C2H4 | 17.0 | 191 | 84 | 0.09 | 0.13 |
| 3 | N2 | 16.5 | 152 | 90 | 0.11 | 0.19 |
| Coating | Environment | φ, V | icor, μA/cm2 |
|---|---|---|---|
| 1 | Ar | 0.30 | 0.45 |
| 2 | C2H4 | 0.38 | 0.23 |
| 3 | N2 | 0.28 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiryukhantsev-Korneev, P.; Sytchenko, A.; Sheveyko, A.; Vorotilo, S. Structure and Properties of Protective Coatings Deposited by Pulsed Cathodic Arc Evaporation in Ar, N2, and C2H4 Environments using the TiC–NiCr–Eu2O3 Cathode. Coatings 2019, 9, 230. https://doi.org/10.3390/coatings9040230
Kiryukhantsev-Korneev P, Sytchenko A, Sheveyko A, Vorotilo S. Structure and Properties of Protective Coatings Deposited by Pulsed Cathodic Arc Evaporation in Ar, N2, and C2H4 Environments using the TiC–NiCr–Eu2O3 Cathode. Coatings. 2019; 9(4):230. https://doi.org/10.3390/coatings9040230
Chicago/Turabian StyleKiryukhantsev-Korneev, Philipp, Alina Sytchenko, Alexander Sheveyko, and Stepan Vorotilo. 2019. "Structure and Properties of Protective Coatings Deposited by Pulsed Cathodic Arc Evaporation in Ar, N2, and C2H4 Environments using the TiC–NiCr–Eu2O3 Cathode" Coatings 9, no. 4: 230. https://doi.org/10.3390/coatings9040230
APA StyleKiryukhantsev-Korneev, P., Sytchenko, A., Sheveyko, A., & Vorotilo, S. (2019). Structure and Properties of Protective Coatings Deposited by Pulsed Cathodic Arc Evaporation in Ar, N2, and C2H4 Environments using the TiC–NiCr–Eu2O3 Cathode. Coatings, 9(4), 230. https://doi.org/10.3390/coatings9040230





