Abstract
Carbonated hydroxyapatite derivatives (CHAp) and its metallic derivatives (Ag, Sr, Ba, K, Zn) have been prepared and characterized in this paper and their coating capacity on some model stone samples have been evaluated and discussed. These compounds were characterized by using several analytical tools, including X-ray diffraction analysis (XRD), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), to determine the purity of the CHAp sample. The XRD and FTIR results confirmed the presence of AB-carbonated type CHAp. The thermal analysis (TGA) established two stages of weight loss that occured during the heating process: The first weight loss between 30–225 °C corresponding to the partial carbonate release from OH-channel and the second one between 226–700 °C, corresponding to some thermal reactions, possibly to the generation of calcium phosphate. The efficiency and suitability of these products on model stone samples were evaluated by monitoring the resistance to artificial weather (freeze–thaw), and pore structure changes (surface area, pore volume, pore diameter). Meanwhile, optical microscopy (OM) and Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM–EDS) techniques showed the particles size and surface morphology of the samples, as well as information on its chemical composition. Also, the compressive strength of these new compounds as coatings revealed a homogeneity and strengthen of these model stone samples.
1. Introduction
Nowadays, one of the main difficulties in preserving objects and heritage monuments is to select the most adequate treatment methods for stone protection, able to preserve their original appearance, without any detrimental effect on their aesthetic properties [1]. The protective products must preserve the stones, to bring it to a state like the original one and prevent further damage. In general, to apply a coating to the surface of the stone.
The requirements for the consolidation of products consist of: Good stability, compatibility with the substrate [2] and adequate penetration depth. A good consolidant should first reduce the rate of degradation of the stone surface and then improve its mechanical properties.
The main challenges in stone protection are related to the creation of a barrier against water penetration and protection of the stone surface against pollutants and the deposition of organic/inorganic particles, while ensuring aesthetic compatibility with the substrate (i.e., color changes at acceptable intervals), a minimal change in water vapor permeability and reversibility of the treatment [3]. In this perspective, hydrophobic coatings [4], antifouling treatments [5] and nanoparticle self-cleaning bands [6] are currently at the center of research on stone conservation [7,8].
However, at present, there are no standard procedures and/or unique assessment methods supporting the effectiveness of enhancers and protection products [9].
A good consolidant should have a good penetration depth and adhesion to the substrate. The bonding capacity depends to a large extent, both the chemical composition of the product (including solvent and concentration) and the stone surface. In particular, the products with a composition-like stone are preferred. Among the new products synthesized for preservation of culture heritage materials, some hybrid formulations have been recently proposed for inorganic [10] and organic materials [11,12].
Another aspect concerning the consolidant is the method of application [11,13], which plays a key role, significantly influencing the transfer of laboratory procedures to on-site practice. There are some application methods: Immersion, which can be easily controlled in the laboratory, but is hardly reproducible on site where it maximizes the penetration depth very hard; poulticing, to control even in laboratory conditions [13]; spraying, which delivers high amounts of consolidant at one time, causing a large consolidant runoff and consumption (besides a higher solvent evaporation due to nebulisation) [11], but a limited penetration depth [10]; and brushing, which often lacks an adequate control of all the parameters that finally determine the treatment efficacy [3,12]. Usually, the brushing method is preferred because it assures an efficient application and a relatively controlled balance between penetration and solvent evaporation [13,14]. Nanomaterials exhibit good chemical–physical features for application in the conservation science of artwork, being able to slow down the degradation processes of artifacts, by comparison with traditional methods, widely described in literature [15]. Up to now, the most used consolidants are nano hydroxyapatite, Ca10(PO4)6(OH)2, already used for travertine, marble and limestone chalk stone consolidation, and used as models for architectural monuments [16].
The aim of this paper is to synthesize and to test some consolidants based on carbonated hydroxyapatite (CHAp), which is quite similar with hydroxyapatite [17,18,19]. By comparison with Hap, which has a crystal structure very similar to that of calcite, enabling epitaxial growth over calcareous matrices [20], in carbonated hydroxyapatite (CHAp) the CO32− ions can replace both OH− and PO43− ions and thus the last form has a high special surface area than the previous one [21]. In the case of carbonated hydroxyapatite, a decrease in the a-axis and increase in the c-axis in the apatite lattice is registered, due to the incorporation of B-type CO32− substitution into the HAp structure [22], a decrease of the crystalinity which suggests a decrease in the crystallite size and crystallinity of the apatite phase [23,24], given by the highest amount of CO32− in the apatite structure [25].
Carbonated hydroxyapatite derivatives (CHAp) and its metallic derivatives (Silver (Ag); Strontium (Sr); Barium (Ba); Potassium (K); Zinc (Zn)) have been prepared and characterized by X-ray diffraction analysis (XRD), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR). The efficiency and suitability of these products on model stone samples, applied by brushing (B) and spraying (S), were evaluated by monitoring the resistance to artificial weather (freeze–thaw) and pore structure changes (surface area, pore volume, pore diameter). Also, the mechanical strengths of these new compounds as coatings for model stone samples revealed good adherence, homogeneity and strength of these layers.
2. Materials and Methods
2.1. Specimens Samples Preparation
Different samples were prepared from sand and a mixture of gypsum with lime (95%–5%) (1:4) (40 mm × 40 mm × 40 mm) in order to distinguish the difference between non-treated/treated samples and to observe the influence of consolidant used during the treatment. Cubic specimens (diameter: 4 cm, height: 4 cm) have been prepared in a silicon resin matrice in order to avoid the dispersion of the solid matter or evaporation of the solvent from the lateral sides. All specimens were stored under controlled conditions (T = 20 °C, 65% RH) for 1 month. After the drying process, the substrates were left to cool for two hours in a desiccator and adequately treated with the new prepared compounds, and their appearance was examined.
2.2. Application of the Carbonated Hydroxyapatite Derivatives
The nanoparticles were suspended in distilled water with conductivity <2 s/cm, due to its higher boiling point and higher surface tension, responsible for a low kinetic stability. They were applied by spraying, an application method commonly adopted in the practice of conservation, for a good penetration and deposition in depth. The procedure was repeated with up to 3 consecutive applications, in order to improve the consolidation effect. The interval between consecutive applications was defined at 6 h, for achieving a complete evaporation of the solvent. The applications were performed under controlled conditions (50% RH, T = 20 °C, air speed <0.1 m/s). The treated specimens were then stored at 65% RH, T = 20 °C, air speed <0.1 m/s for over 1 months, in order to enhance the nanoparticles deposition.
2.3. CHAp Synthesis
Carbonated hydroxyapatite (CHAp), as very fine and uniform sized powder, was obtained by a nanoemulsion technique adapted from Zhou et al. [26]. CHAp nanoparticles were synthesized by mixing aqueous solutions of (NH4)2HPO4 and NH4HCO3, under magnetic stirring. The pH of the aqueous solution was adjusted to 11 using sodium hydroxide 1 M. Then an acetone solution of Ca(NO3)2·4H2O at a molar ratio of Ca2+:PO43−:CO32− = 1.67:1:0.5 was introduced into the flask. The reaction was carried out at room temperature. The precipitate was vacuum filtered using a Buchner funnel and washed with distilled water. The reaction product was freeze-dried overnight and calcined the next day at 900 °C for 4 h. Doped CHAp with different metal ions (Ag+, Sr2+, Ba2+, Zn2+, K+) was synthetized by a similar microemulsion method, the metal nitrate solution (AgNO3, Sr(NO3)2, Ba(NO3)2, Zn(NO3)2, KNO3) being added in the Ca(NO3)2·4H2O acetone solution, in concentration of 5 mole% reported to Ca2+. These compounds have been applied by brushing and spraying using an aqueous solution of 0.25g/L on the non-treated samples.
2.4. Chemical Characterization Methods
X-ray diffraction measurements of powder samples were carried out with a Rigaku Ultima IV diffractometer (Rigaku, Tokyo, Japan) using a Cu Kα radiation (λ = 1.54 Å). In this experiment the accelerating voltage of the generator radiation was set at 40 kV and the emission current at 200 mA. The diffractograms were recorded in parallel beam geometry over 2θ = 5° to 100° continuously at a scan rate of 4°/min.
Fourier transformed infrared spectroscopy (ATR–FTIR) was recorded with a Vertex 80 spectrometer (Bruker Optik GMBH, Billerica, MA, USA) equipped with DRIFT accessory, in the range of 2000–400 cm−1, because the range 4000–2000 cm−1 did not show major changes for these samples.
Thermogravimetric analyses of CHAp were performed using a Pyris 1 TGA analyzer (Perkin–Elmer TGA-7, Waltham, MA, USA) with a scan range from 50–700 °C and a constant heating rate of 10 °C/min under continuous nitrogen.
For porosity determination, the nitrogen adsorption/desorption isotherms were recorded at 77 K in the relative pressure range p/po = 0.005–1.0 using NOVA2200e Gas Sorption Analyzer (Quantachrome, Boynton Beach, FL, USA). Data processing was performed using NovaWin version 11.03 software. Prior to adsorption measurements, the samples were degassed for 4 h at 180 °C under vacuum. The specific surface area was determined by the standard Brunauer–Emmett–Teller (BET) equation. The total pore volume was estimated from the volume adsorbed at a relative pressure p/po close to unity. Pore size distribution and mesopores volume were obtained from desorption branch of the isotherm by applying the Barrett–Joyner–Halenda (BJH) model. The t-plot method was used to estimate the micropore surface area and external surface area.
Also, the OM was recorded by a Primo Star ZEISS optical microscope (Carl Zeiss, Oberkochen, Germany) that offers the possibility of investigating the samples in transmitted light at a magnification between 4–100×. The equipment had a digital video camera attached (Axiocam 105, Carl Zeiss, Oberkochen, Germany) which, by the microscope software (Zen Pro), allowed real-time data acquisition. The obtained images could easily be converted from 2D in 3D format through its software for a better view.
The Scanning Electron Microscopy with Energy Dispersive Spectroscopy (SEM–EDS) was obtained with a SU-70 (Hitachi, Japan) microscope, with a magnification range of 30×–800,000×.
Freeze–thaw aging test: For the freeze-thaw test (20 cycles) STAS 6200/15 83, the sample shall be dried in oven at 105 ± 5 °C for 1 h up to the constant mass (m1). The samples were immersed in distilled water for 15 min at room temperature. The samples were kept in the freezer for 3 h at −18 ± 5 °C and then thawed in water. The freeze–thaw operation is repeated until 20 cycles are performed, after which the mass losses during the freeze–thaw process is expressed as gelivity coefficient, Equation (1).
where:
μg = (m2 − m3/m1) × 100%
m1–the initial mass of the sample, determined after drying at 105 °C to the constant mass and before saturation with distilled water, in grams;
m2–mass of sample saturated with water, determined before the first freeze–thaw cycle, in grams;
m3–defrost mass of the sample, determined after drying at 105 °C to the constant mass and before saturation with distilled water, in grams.
2.5. Mechanical Testing Method
The mechanical strength test was performed with a Silver Schmidt Hammer Proceq test hammer, type L-0.735 Nm impact energy, according to ASTM C805. The strength range of the Silver Schmidt test hammer is from 10–100 N/mm2. Ten replicates within the test location with a minimum spacing of 25 mm between each two testing points and a minimum edge distance of 25 mm, have been recorded. The hammer was positioned at 90° downward, and the rebound number value is calculated as the average of the readings within this test location, in order to find a relationship between surface hardness and compressive strength with an acceptable error. The compressive strength was calculated using the Equation (2) (for 10th percentile curve range), after apparatus manual indications.
compressive strength = 2.77 × e^(0.048 × Q)
The results have been obtained for the untreated and treated specimens with CHAp, Ag-CHAp, Sr-CHAp, Ba-CHAp, Zn-CHAp, K-CHAp solutions of 0.25 g/L solution applied by brushing and spraying on the stone surface.
3. Results
3.1. Crystal Structure
Table 1 summarizes the results of the phase purity and the average crystallite sizes calculated for the most intense peak of CHAp (0 0 2) for all the investigated samples by XRD and Scherrer Equation (3):
where:
L = (K × λ)/(β × cosθ)

Table 1.
Summary of the XRD characterization of pure and substituted CHAp.
L = crystallite size;
K = Scherrer constant (0.9);
λ = X-ray wavelength (1.54 Å);
β = peak width at half maximum intensity (in radians), from the apparatus programme;
θ = Bragg angle, from the apparatus programme;
By analyzing this table, it could be observed that all the samples matched with the standard JCPDS reference (09-432) file for synthetic carbonated hydroxyapatite. XRD confirmed the phase purity of the synthesized carbonate hydroxyapatite [27] and no additional phases (brushite) were identified, this being an indication for these compounds’ purity.
Figure 1 shows the characteristic XRD spectra for all samples. When all the prepared samples were analyzed by X-ray diffraction, their patterns were characteristic of the hexagonal apatite phase [28,29]. Few XRD peaks are characteristic of carbonated hydroxyapatite (with 2θ from 26–40) (Figure 1).

Figure 1.
XRD diagrams for CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
3.2. Molecular Structure
The major functional groups in this case are the carbonate, hydroxyl, phosphate groups, identified by FTIR. The observed vibrational peaks, summarized in Figure 2, are characteristic of carbonated hydroxyapatite compounds, similar to other literature data [27,30]. For a proper identification of the specific bands of these compounds, the IR region was chosen between 2000–400 cm−1.
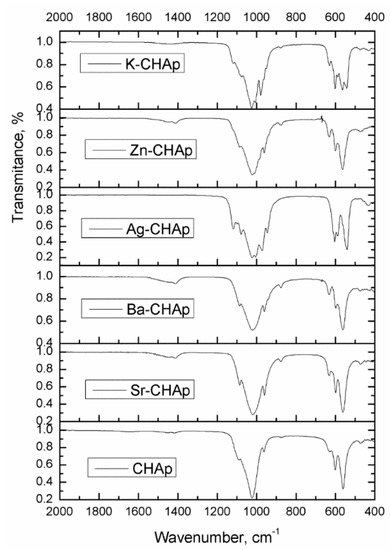
Figure 2.
FTIR spectra for CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
3.3. Thermal Properties
The thermogravimetric analysis of the samples carried out from 30–1000 °C in air atmosphere using a heating rate of 10 °C/min is shown in Figure 3. The TGA result shows that there are two stages of weight loss that occurs during the heating process: The first weight loss between 30–225 °C and the second one between 226–700 °C, corresponding to some thermal reactions [31].

Figure 3.
The TGA diagram for CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
3.4. The Efficiency and Suitability of These Products on Model Stone Samples
The efficiency and suitability of these products on model stone samples were evaluated by compressive strength, to artificial weather (freeze–thaw), water absorption test in relationship with porosimetry measurements. Except the pore structure changes (surface area, pore volume, pore diameter), the variation of the surface area and pore diameter (by brushing and spraying), is represented in Figure 4.
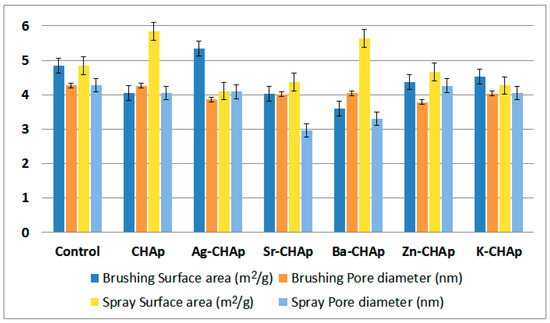
Figure 4.
Surface area and pore diameter parameters for CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
With the Silver Schmidt test hammer measures the speed of the impact as well as of the rebound immediately before and after the impact, calculating the amount of energy that can be recovered. It is one of the traditionally used methods for evaluating the mechanical properties of the rocks (compressive strength). The rebound number measurements for the untreated and treated with carbonated hydroxyapatite (by brushing and spraying the specimens) are shown in Figure 5. The measurements of rebound number have been taken and then the compressive strength determined as per ASTM C805.

Figure 5.
Mechanical strength for control, CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
For the water absorption test, the samples were dried in the oven at 40 °C for 8 h, cooled at room temperature and then weighed (W1). After that, the samples were immersed in distilled water for 24 h. At the end, they were removed from distilled water and weighed (W2). The water absorption content was calculated with the Equation (4), and graphical represented in Figure 6.
water absorption = [(W2 − W1)/W1] × 100%
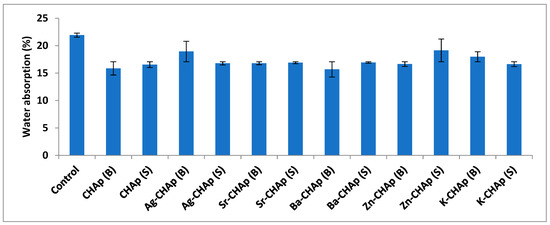
Figure 6.
Water absorption for control, CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
The freeze–thaw operation is repeated until 20 cycles are performed, after which the mass losses during the freeze–thaw process is expressed as gelivity coefficient (Figure 7).
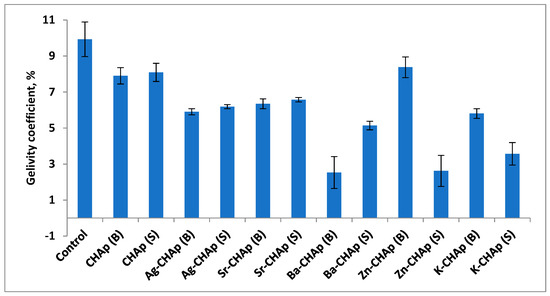
Figure 7.
Gelivity coefficient for control, CHAp and Me-CHAp (Me = Sr, Ag, Ba, Zn, K).
4. Discussion
The XRD patterns shown in Figure 1, indicated that Me-CHAp samples are very similar to that of pure CHAPp and were in accordance with ASTM data (Card 9-432). The diffraction peaks (2θ = 25.879° for (002), 31.775° for (211), 31.196° for (112), 32.902° for (300), 33.684° for (202), 35.597° for (301) and 39.853° for (130) respectively), are sharp and well resolved, indicating the obtained phase pure, well crystallized hydroxyapatites [32]. The unit cell parameters of all samples are presented in Table 2.

Table 2.
The lattice parameters a and c for synthesized carbonated hydroxyapatites.
The lattice parameters a and c increased with increasing ionic radium of metallic ions. The substitution of Ca (0.99 Å) with Zn (0.74 Å), Sr (1.12 Å), Ag (1.26 Å), Ba (1.35 Å), and K (1.38 Å) in CHAp lattice could be a cause of increasing of a and c lattice parameters. Changes in cell parameters may imply that all the metals substituted for calcium in the CHAp lattice corresponded with an increase in lattice parameters (except for Zn, which has a smaller ionic radius than Ca). It is also possible that lattice parameter changes occurred due to silver ion and other site substitutions in the crystal lattice. These results are in accordance with previous reports by [30]. Moreover, the inclusion of the metals with a larger ionic radius compared to Ca2+, caused the expansion of the lattice parameters (mainly along the c axis) and the increase of cell volume [33,34,35]. According to results given in Table 2, all Me-CHAp samples have lower crystallinity than pure hydroxyapatite.
On the other hand, the substitution of strontium and silver can cause phase shifting to lower 2θ indicating an increase in the lattice parameters, which can be attributed to the higher ionic radius of Sr (1.12 Å) and Ag (1.26 Å), as compared to Ca2+ [35].
Broadening of the peaks due to the reduction in the crystallite size and increase in the lattice disorder, are attributed to the divalent metal+ substitution in the HAp lattice [36]. Also, the average crystallite sizes, calculated after Scherrer equation suggests that the hydroxyapatite samples could be considered as nano-CHAp (Table 1). The results show that insertion of the metals into CHAp lattice led to a higher SBET that is correlated with smaller crystallite sizes of these samples [37].
The bands from 1455 and 1419 cm−1 have been ascribed to carbonate and phosphate IR bands. When the carbonate IR bands are at 1455 and 1419 cm−1 (ascribed to CO32− and PO34−) a B type substitution is identified, whereas the bands are at 1550 and 1530 cm−1 (ascribed to OH and PO34−) a A type substitution is present (Figure 2).
The bands from 879, 873 and 866 cm−1 are similar and characteristic of all the samples. This means that the carbonate ions are present in the CHAp structure either carbonate ions substituting OH− groups (type A–879 cm−1) or carbonate ions substituting the phosphate ions (type B-873 cm−1). Phosphate bands could be identified at 605, 575 and 563 cm−1. The FTIR bands obtained confirm that the synthesized samples could be AB type: CO32− is substituting PO43−. Similar results have been reported by Padila [30].
The TGA result shows that there are two stages of weight loss that occur during the heating process. The first weight loss of 8.3 wt.% is observed from 30 to 225 °C due to the removal of trapped water in the sample. The second weight loss of approximately 31.6 wt.% occurs from 226 to 700 °C and was attributed to the decomposition of the other components (CO32− according to the weight loss observed in TGA curves between 550–950 °C [31,38].
The results obtained for the non-treated and treated specimens with carbonated hydroxyapatite solutions 0.25 g/L concentration, revealed that by brushing, acicular crystals of approximately 500 nm in length and 10 nm in width were obtained (Figure 8); and the second one, when the solution was sprayed on the stone surface, agglomerates of spheroidal crystals of approximately 10 ± 20 nm in diameter were obtained (marked by arrows).
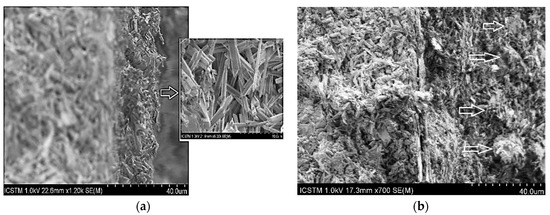
Figure 8.
SEM images for BaCHAp applied by brushing and spraying: (a) Ba-CHAp applied by brushing, (b) Ba-CHAp applied by spraying.
By optical microscopy (Figure 9) it was possible to observe that the untreated specimen presents a whitish, highly porous surface (Figure 9a–c), while the treated specimen shows a more homogeneous surface with no chromatic alteration macroscopically visible. Looking more in detail to the microstructure, it can be observed that the nanoparticles partially filled the superficial pores of the original matrix. The thickness of the deposited layer depends on the consolidant type, higher for Ag-CHAp and mostly similar for Ba-CHAp, Sr-CHAp and CHAp. The layer thickness of the other consolidants is very thin, between 8–10 μm, this being a possible reason for the low efficacy of them.
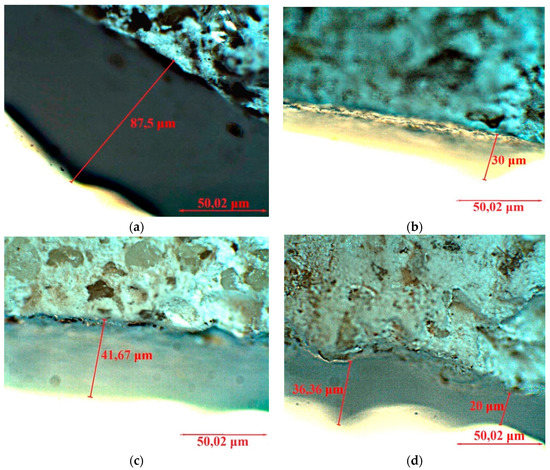
Figure 9.
Optical microscopy of the untreated and treated by spraying the specimens: (a) Ag-CHAp, (b) Ba-CHAp, (c) SrCHAp, (d) CHAp.
The layer varies between 30–90 μm, which is in good agreement with other literature report [39]. By brushing, the layer is thickened, as SEM images shown (Figure 10a–d).
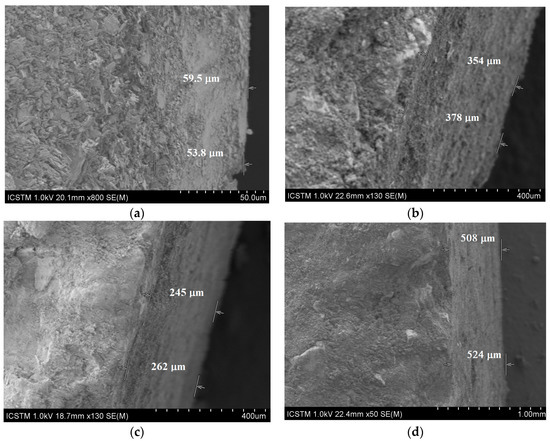
Figure 10.
SEM images for brushed specimens: (a) CHAp, (b) Ba-CHAp, (c) Sr-CHAp, (d) Ag-CHAp.
As humidity plays a key role in stone deterioration, a detailed understanding of the processes involved and imposed is required. The amount of water presented in a building stone, its actual material moisture, is a function of several parameters. The specific inner surface represents the interface for any process between the atmosphere and the solid material. There is a close interrelation between material moisture and mechanical properties [40].
From the above images it can be observed that the layer obtained by brushing the carbonated hydroxyapatite derivatives on model samples has different thicknesses: 53–59 μm for CHAp (Figure 10a) and thicker from 245 to 524 μm for its metallic derivatives (Figure 10b–d). For CHAp derivatives with divalent metals (Sr, Ba), the layer obtained by application is between 262 and 378 μm, while for the monovalent derivative of CHAp (Ag) the thickest layer of 540 μm is obtained. The reason lies in the tendency to agglomerate of these metallic derivatives, due to the large surface-to-volume ratio (Figure 4), more accentuated at Ag-CHAp than Sr-CHAp and Ba-CHAp.
If the pore volume, determined by porosity, is quite similar (0.008–0.01 cc/g), the surface area and pore diameter are different and depend on the consolidant type: It is highest for Ag-CHAp applied by brushing and for CHAp and Ba-CHAp applied by spraying. Meanwhile, the pore diameter is the smallest for Sr-CHAp and Ba-CHAp applied by spraying. From these data, it could be concluded that the application type is decisive and contribute to the layer homogenity applied on the stone surface. For the control specimens, these values are not different at all. They are similar both for brushing and for spraying (Figure 4).
Measurement of mechanical properties of the samples in all stages (i.e., by brushing and by spraying) was achieved by the test of compression strength. It could be observed that by comparison with the control samples, all the treated samples presented higher compressive strength values for the samples treated by brushing than those treated by spraying (Figure 11a–d) correlated with an increased rebound number (Figure 5). The addition of the nanoparticles on the specimen surface enhanced the durability of stone compared to the samples not treated, due to the role of nanoparticles in reinforcing the stone, improving their interaction with the stone grains.
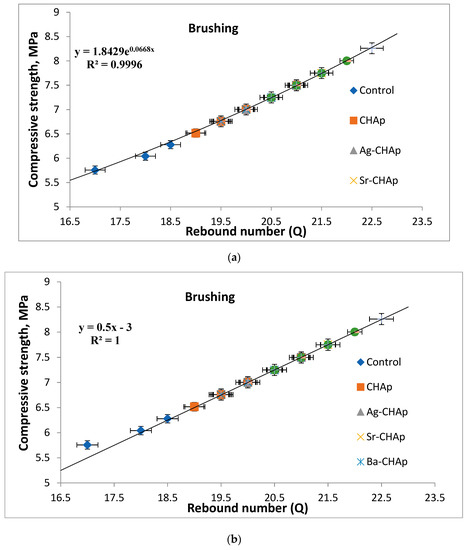

Figure 11.
The compressive strength vs. rebound index relationship for model stones coated with carbonated hydroxyapatite derivatives. (a) samples treated by brushing, exponential graphical representation; (b) samples treated by brushing, linear graphical representation; (c) samples treated by spraying, exponential graphical representation; (d) samples treated by spraying, linear graphical representation.
It was observed that the results of Zn-CHAp nanoparticles gave the highest values of compressive strength. This can be attributed to the effect of the high reactivity, crystallites size, nanoparticles size and high compatibility with the substrates, similar with other cases reported in the literature [41]. The higher the crystallites size, the higher the compressive strength. The chemical composition of nanosized consolidant can exhibit improvements in thermal, physical, and mechanical properties, because of the strong synergistic effects between the stone surface and CHAp on both the molecular and nanometric scales [42].
This paper presents the compressive strength vs. rebound index relationship for model stones. Linear and exponential relationship between average rebound number and compressive strength was established using the least square method. The linear model was found to be the better one with a regression coefficient of 1.0 than the exponential model with a regression coefficient of 0.9996, which indicates the acceptability of the linear model for predicting compressive strength of model stones, Figure 11a–d.
As humidity plays a key role in stone deterioration, a detailed understanding of the processes involved and imposed is required. The amount of water presented in a building stone, its actual material moisture, is a function of several parameters. The specific inner surface represents the interface for any process between the atmosphere and the solid material. There is a close interrelation between material moisture and mechanical properties [40].
Transport and distribution of H2O, fluid water and vapor in porous materials are governed by different processes. In the assumed case of a dry material, as a first step, its surface starts to adsorb single H2O molecules from the atmosphere to form a layer. The only transport mechanism which occurs is vapor transport. With rising air humidity, the number of layers of water molecules on the surface increases. Within those multimolecular layers of the water, surface flow occurs as a secondary and more efficient transport mechanism. Additionally, at a certain content, the water layers merge into the small pores due to capillary condensation, which is effective up to some 10−7 m pore radius. With an increase of water content in the system, capillary forces, which are active in the pore radius range between some 10−7–10−3 m, start to govern the internal flow. Finally, when the material is completely filled with water, saturation flow occurs. Similar, but inverse are the processes when a water saturated material is subjected to drying. The removal of H2O stops when desorption out of the material and adsorption of air humidity reach equilibrium.
When water from the stone surface evaporates faster, a larger active evaporation surface cools occur. Partial saturation enhances this effect. This shows that the surface roughness expressed in the specific inner surface is a determining parameter. The amount of water sorption uptake correlates mainly with the inner surface area, determined by the N2-BET method.
Samples with medium specific surface areas have a lower uptake and the lowest values can be observed in samples with the lowest specific surface areas. So, the samples with high specific inner surfaces, exhibit the highest moisture uptake values.
The specimens that supported the freeze–thaw cycles were first dried, weighed, and later immersed in H2O for saturation throughout 48 h, consistent with the standardized procedure represented in EN12371. The freeze–thaw aging was performed on 15 specimens and lasted 20 cycles. At the end of freeze–thaw aging procedure the dry mass of the specimens was assessed (Figure 12).

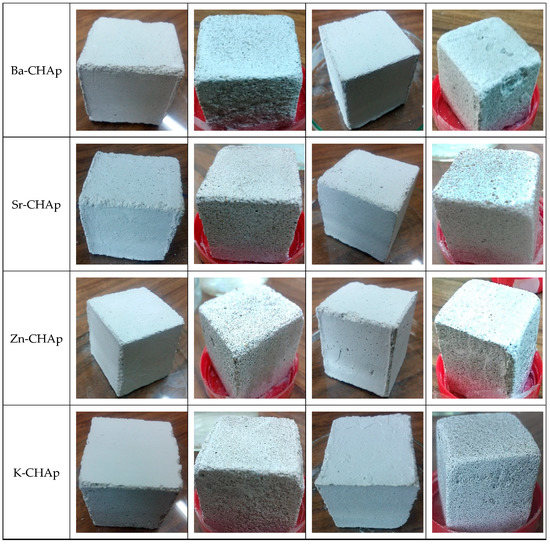
Figure 12.
Stone samples with metallic hydroxyapatite applied by brushing and spraying.
The deterioration of the stones involves mechanical ruptures along the edges, fractures due to the weathering, grooves in depth, shrinkage cracks and brown patina. Salt incrustation may cover the stone surface and fill the fissures of blocks. The deterioration/weathering condition could be poorly weathered stone surfaces and extremely weathered stone surfaces.
By correlation between the visual appearances of samples treated with gelivity values for the samples treated with various carbonated hydroxyapatite a similar degradation tendency could be observed. The control samples undergo similar degradations after the 20 freeze–thaw cycles, as shown in Figure 7, and more pronounced in the samples treated by spraying with two exceptions of the samples treated with Zn-CHAp and K-CHAp.
The most pronounced degradations are observed in the case of Ba-CHAp, applied by spraying, most probably due to Ba ionic radium 1.35 Å, too high to cover the homogeneous samples.
The higher the gelivity value, the higher the degradation rate and smaller the protection capacity of the carbonated hydroxyapatite derivatives coatings.
The spraying procedure is a fast application, requires one application, leads to a smooth finish free of brush/roller marks, can get into hard to reach areas, but has some disadvantages: Long prep and clean up time, uneven coverage (sometimes too thick), uses 2–3 times as much paint as brushing, poor adhesion, cannot paint on windy days. By comparison, brushing has an excellent control, very good adhesion, even uniform coverage, gets paint into nooks and crannies better than spraying. But it is a slow and laborious application, can require 2 or more coats, can leave brush marks and obstructions which make for a difficult application. It is slow, but an even, uniform coat of paint could be obtained and much better adhesion than with spraying alone [20,43,44].
After analyzing these results, it could be concluded that the brushing procedure could offer a more efficient coating of the model stone specimens, while the spraying procedure, evenly induces good compressive strength, produces mechanical ruptures along the edges (Zn-CHAp, Ba-CHAp, Ag-CHAp), fractures (Zn-CHAp, Ba-CHAp, Ag-CHAp), grooves in depth (Zn-CHAp, K-CHAp, Ba-CHAp, Sr-CHAp), shrinkage cracks (CHAp), brown patina (Zn-CHAp).
5. Conclusions
Carbonated hydroxyapatite derivatives (CHAp) and its metallic derivatives (Ag, Sr, Ba, K, Zn) have been prepared and characterized in this paper and their coating capacity has been evaluated and discussed. These compounds were characterized using several analytical tools, XRD, TGA, FTIR, OM and SEM–EDS techniques.
The efficiency and suitability of these products on model stone samples were evaluated by monitoring the resistance to artificial weather (freeze–thaw), and pore structure changes (surface area, pore volume, pore diameter). Also, the mechanical strengths of these new compounds as coatings for model stone samples revealed homogeneous and strong layers deposited on the stone surface. By correlation, between the visual appearance of samples treated with gelivity values and the samples treated with various carbonated hydroxyapatite, a similar degradation tendency could be observed that is more pronounced in the samples treated by spraying with two exceptions of the samples treated with Zn-CHAp and K-CHAp. The higher the gelivity value, the higher the degradation rate and smaller protection capacity of the carbonated hydroxyapatite derivatives coatings.
The brushing procedure could offer a more efficient coating of the model stone specimens, while the spraying procedure, evenly induces good compressive strengths, produces mechanical ruptures along the edges (Zn-CHAp, Ba-CHAp, Ag-CHAp), fractures (Zn-CHAp, Ba-CHAp, Ag-CHAp), grooves in depth (Zn-CHAp, K-CHAp, Ba-CHAp, Sr-CHAp), shrinkage cracks (CHAp) and brown patina (Zn-CHAp).
Author Contributions
Conceptualization, R.-M.I. and M.-L.I.; Formal analysis, R.-M.I. and M.-L.I.; Funding acquisition, R.-M.I.; Investigation, R.-M.I., L.I., G.V., M.E.G., R.E.A., G.-I.R., R.M.G., S.T., I.A.B., M.-L.I., I.D.D., A.I.G. and C.R.; Project administration, R.-M.I.; Software, G.-I.R.; Supervision, R.-M.I. and M.-L.I.; Visualization, R.-M.I. and L.I.; Writing—original draft, R.-M.I.; Writing—review & editing, R.-M.I. and L.I.
Funding
This paper received the financial support of the projects: PN-III-P1-1.2-PCCDI-2017-0476 from UEFISCDI-MECI, Romania.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sassoni, E.; Graziani, G.; Franzoni, E. An innovative phosphate-based consolidant for limestone. Part 1: Effectiveness and compatibility in comparison with ethyl silicate. Constr. Build. Mater. 2016, 102, 918–930. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P. Hydrophobic and superhydrophobic coatings for limestone and marble conservation. In Smart Composite Coatings and Membranes; Montemor, M.F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 421–452. [Google Scholar]
- Ruffolo, S.A.; Macchia, A.; La Russa, M.F.; Mazza, L.; Urzì, C.; De Leo, F.; Barberio, M.; Crisci, G.M. Marine antifouling for underwater archaeological sites: TiO2 and Ag-Doped TiO2. Int. J. Photoenergy 2013, 2013, 251647. [Google Scholar] [CrossRef] [PubMed]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Sierra-Fernandez, A.; Gomez-Villalba, L.S.; Rabanal, M.E.; Fort, R. New nanomaterials for applications in conservation and restoration of stony materials: A review. Materiales de Construcción 2017, 67, 325. [Google Scholar] [CrossRef]
- Zendri, E.; Balliana, E.; Izzo, F.C.; Di Crescenzo, M.M.; Falchi, L.; Sgobbi, M.; Biscontin, G. The choise of parameters for the monitoring and the maintenance of architectural stone surfaces. Int. J. Herit. Digital Era 2012, 1 (Suppl. 1), 331–335. [Google Scholar] [CrossRef]
- Delgado Rodrigues, J. Stone consolidation: Research and practice. In Int. Symp. on Works of Art and Conservation Science Today; Aristotle University of Thessaloniki: Thessaloniki, Greece, 2010. [Google Scholar]
- Salazar-Hernández, C.; Cervantes, J.; Puy-Alquiza, M.J.; Miranda, R. Conservation of building materials of historic monuments using a hybrid formulation. J. Cult. Herit. 2015, 16, 185–191. [Google Scholar] [CrossRef]
- Pinto, A.F.; Rodrigues, J.D. Stone consolidation: The role of treatment procedures. J. Cult. Herit. 2008, 9, 38–53. [Google Scholar] [CrossRef]
- Pinna, D.; Salvadori, B.; Porcinai, S. Evaluation of the application conditions of artificial protection treatments on salt-laden limestones and marble. Constr. Build. Mater. 2011, 25, 2723–2732. [Google Scholar] [CrossRef]
- Pinto, A.P.F.; Rodrigues, J.D. Consolidation of carbonate stones: Influence of treatment procedures on the strengthening action of consolidants. J. Cult. Herit. 2012, 13, 154–166. [Google Scholar] [CrossRef]
- Tabasso, M.L.; Simon, S. Testing methods and criteria for the selection/evaluation of products for the conservation of porous building materials. Stud. Conserv. 2006, 51 (Suppl. 1), 67–82. [Google Scholar] [CrossRef]
- Ion, R.M.; Turcanu-Caruţiu, D.; Fierăscu, R.C.; Fierăscu, I.; Bunghez, I.R.; Ion, M.L.; Teodorescu, S.; Vasilievici, G.; Rădiţoiu, V. Caoxite-hydroxyapatite composition as consolidating material for the chalk stone from Basarabi–Murfatlar churches ensemble. Appl. Surf. Sci. 2015, 358, 612–618. [Google Scholar] [CrossRef]
- Ion, R.M.; Fierascu, R.C.; Fierascu, I.; Senin, R.M.; Ion, M.L.; Leahu, M.; Turcanu-Carutiu, D. Influence of Fântâniṭa lake (chalk lake) water on the degradation of basarabi–murfatlar churches. In Engineering Geology for Society and Territory-Volume 8; Springer: Cham, Switzerland, 2015; pp. 543–546. [Google Scholar]
- Gibson, I.R.; Bonfield, W. Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J. Biomed. Mater. Res. 2002, 59, 697–708. [Google Scholar] [CrossRef]
- Ellies, L.G.; Nelson, D.G.A.; Featherstone, J.D.B. Crystallographic structure and surface morphology of sintered carbonated apatites. J. Biomed. Mater. Res. 1988, 22, 541–553. [Google Scholar] [CrossRef]
- Kovaleva, E.S.; Shabanov, M.P.; Putlyaev, V.I.; Tretyakov, Y.D.; Ivanov, V.K.; Silkin, N.I. Bioresorbable carbonated hydroxyapatite Ca10−xNax(PO4)6−x(CO3)x(OH)2 powders for bioactive materials preparation. Cent. Eur. J. Chem. 2009, 7, 168–174. [Google Scholar] [CrossRef]
- Sassoni, E.; Naidu, S.; Scherer, G.W. The use of hydroxyapatite as a new inorganic consolidant for damaged carbonate stones. J. Cult. Herit. 2011, 12, 346–355. [Google Scholar] [CrossRef]
- Yasukawa, A.; Nakajima, M.; Kandori, K.; Ishikawa, T. Preparation and characterization of carbonated barium hydroxyapatites. J. Colloid Interface Sci. 1999, 212, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.G.; Featherstone, J.D. Preparation, analysis, and characterization of carbonated apatites. Calcif. Tissue Int. 1982, 34 (Suppl. 2), S69–S81. [Google Scholar] [PubMed]
- Jäger, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef]
- Miquel, J.L.; Facchini, L.; Legrand, A.P.; Marchandise, X.; Lecouffe, P.; Chanavaz, M.; Donazzan, M.; Rey, C.; Lernaitre, J. Characterisation and conversion study into natural living bone of calcium phosphate bioceramics by solid state NMR spectroscopy. Clin. Mater. 1990, 5, 115–125. [Google Scholar] [CrossRef]
- Calderin, L.; Stott, M.J.; Rubio, A. Electronic and crystallographic structure of apatites. Phys. Rev. B 2003, 67, 134106. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, M.; Cheung, W.L.; Guo, B.C.; Jia, D.M. Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–110. [Google Scholar] [CrossRef]
- Barralet, J.; Knowles, J.; Best, S.; Bonfield, W. Thermal decomposition of synthesised carbonate hydroxyapatite. J. Mater. Sci. Mater. Med. 2002, 13, 529–533. [Google Scholar] [CrossRef]
- Mayer, I.; Apfelbaum, F.; Featherstone, J.D.B. Zinc ions in synthetic carbonated hydroxyapatites. Arch. Oral Biol. 1994, 39, 87–90. [Google Scholar] [CrossRef]
- Kostov-Kytin, V.V.; Dyulgerova, E.; Ilieva, R.; Petkova, V. Powder X-ray diffraction studies of hydroxyapatite and β-TCP mixtures processed by high energy dry milling. Ceram. Int. 2018, 44, 8664–8671. [Google Scholar] [CrossRef]
- Padilla, S.; Izquierdo-Barba, I.; Vallet-Regí, M. High specific surface area in nanometric carbonated hydroxyapatite. Chem. Mater. 2008, 20, 5942–5944. [Google Scholar] [CrossRef]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The effect of temperature on the properties of hydroxyapatite precipitated from calcium hydroxide and phosphoric acid. Thermochim. Acta 2001, 374, 13–22. [Google Scholar] [CrossRef]
- Stanić, V.; Janaćković, D.; Dimitrijević, S.; Tanasković, S.B.; Mitrić, M.; Pavlović, M.S.; Krstic, A.; Jovanovic, D.; Raicevic, S. Synthesis of antimicrobial monophase silver-doped hydroxyapatite nanopowder for bone tissue engineering. Appl. Surf. Sci. 2011, 257, 4510–4518. [Google Scholar] [CrossRef]
- Terra, J.; Dourado, E.R.; Eon, J.G.; Ellis, D.E.; Gonzalez, G.; Rossi, A.M. The structure of strontium-doped hydroxyapatite: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2009, 11, 568–577. [Google Scholar] [CrossRef]
- Panda, R.N.; Hsieh, M.F.; Chung, R.J.; Chin, T.S. FTIR, XRD, SEM and solid state NMR investigations of carbonate-containing hydroxyapatite nano-particles synthesized by hydroxide-gel technique. J. Phys. Chem. Solids 2003, 64, 193–199. [Google Scholar] [CrossRef]
- Bianchi, M.; Degli Esposti, L.; Ballardini, A.; Liscio, F.; Berni, M.; Gambardella, A.; Leeuwenburgh, S.C.G.; Sprio, S.; Tampieri, A.; Iafisco, M. Strontium doped calcium phosphate coatings on poly (etheretherketone)(PEEK) by pulsed electron deposition. Surf. Coat. Technol. 2017, 319, 191–199. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and characterization of hydroxyapatite crystals: A review study on the analytical methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Zyman, Z.; Rokhmistrov, D.; Ivanov, I.; Epple, M. The influence of foreign ions on the crystal lattice of hydroxyapatite upon heating. Mater. Werkst. Entwickl. Fert. Prüfung Eig. Anwend. Tech. Werkst. 2006, 37, 530–532. [Google Scholar] [CrossRef]
- Skwarek, E. Thermal analysis of hydroxyapatite with adsorbed oxalic acid. J. Therm. Anal. Calorim. 2015, 122, 33–45. [Google Scholar] [CrossRef]
- Novoselov, A.; Konstantinov, A.; Leonova, L.; Soktoev, B.; Morgalev, S. Carbonate neoformations on modern buildings and engineering structures in tyumen city, Russia: structural features and development factors. Geosciences 2019, 9, 128. [Google Scholar] [CrossRef]
- Franzen, C.; Mirwald, P. Moisture content of natural stone: static and dynamic equilibrium with atmospheric humidity. Environ. Geol. 2004, 46, 391–401. [Google Scholar] [CrossRef]
- Giorgi, R.; Ambrosi, M.; Toccafondi, N.; Baglioni, P. Nanoparticles for cultural heritage conservation: Calcium and barium hydroxide nanoparticles for wall painting consolidation. Chem. A Eur. J. 2010, 16, 9374–9382. [Google Scholar] [CrossRef]
- Dei, L.; Salvadori, B. Nanotechnology in cultural heritage conservation: Nanometric slaked lime saves architectonic and artistic surfaces from decay. J. Cult. Herit. 2006, 7, 110–115. [Google Scholar] [CrossRef]
- Sandrolini, F.; Franzoni, E.; Cuppini, G. Predictive diagnostics for decayed ashlars substitution in architectural restoration in Malta. Mater. Eng. Modena 2000, 11, 323–338. [Google Scholar]
- Karatasios, I.; Theoulakis, P.; Kalagri, A.; Sapalidis, A.; Kilikoglou, V. Evaluation of consolidation treatments of marly limestones used in archaeological monuments. Constr. Build. Mater. 2009, 23, 2803–2812. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).