Hygroexpansion, Surface Roughness and Porosity Affect the Electrical Resistance of EVOH-Aluminum- Coated Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Used Papers
2.2. Preparation of Aqueous EVOH Solutions
2.3. Laboratory-Scale EVOH Coating
2.4. Pilot-Scale EVOH Coating
2.5. EVOH Coating Weight Determination
2.6. Physical Vapor Deposited (PVD) Aluminum Coating
- PC/Al: Aluminum was deposited on the paper side, that has been pigment coated;
- PC/EVOH/Al: The paper was first coated with EVOH on the paper side, that has been pigment coated, and then aluminum was deposited on top of the EVOH;
- noPC/Al: Aluminum was deposited on the paper side, which has not been pigment coated;
- noPC/EVOH/Al: The paper was first coated with EVOH on the paper side, that has not been clay coated, then aluminum deposited was on top of the EVOH.
2.7. Aluminum Coating Weight Determination
2.8. Scanning Electron Microscopy (SEM)
2.9. Determination of Sheet Resistance via Eddy Currents at Different Relative Humdities
2.10. Sorption Isotherm
2.11. Hygroexpansion
2.12. Surface Roughness
2.13. Statistical Methods
3. Results and Discussion
3.1. Effect of Polymer Coatings on Paper Hygroexpansion
 ) but the absorption was the same regardless of the side of the paper to which the EVOH was applied (PC
) but the absorption was the same regardless of the side of the paper to which the EVOH was applied (PC  or noPC
or noPC  ). This reflected the relatively low coating weight (~1–3 g/m2) compared to the paper grammage (65–80 g/m2). In contrast, hygroexpansion (Figure 4, right column) was strongly affected by the side to which the EVOH was applied. The effect was not visible in the case of Adicar WSHGM, as the differences in roughness and coating weight between noPC and PC was low. The hygroexpansion was higher for the EVOH-coated papers than for the pure paper, but highest when EVOH was applied to the noPC side (
). This reflected the relatively low coating weight (~1–3 g/m2) compared to the paper grammage (65–80 g/m2). In contrast, hygroexpansion (Figure 4, right column) was strongly affected by the side to which the EVOH was applied. The effect was not visible in the case of Adicar WSHGM, as the differences in roughness and coating weight between noPC and PC was low. The hygroexpansion was higher for the EVOH-coated papers than for the pure paper, but highest when EVOH was applied to the noPC side (  ). This indicated that the EVOH penetrated further into the noPC side of the paper because the surface contained more pores and channels (see images presented in [1]). EVOH thus fills the voids in the noPC paper and occupies space that the fibers would otherwise fill during expansion. This interpretation is supported by reference [28]. Because this space between the fibers is now occupied by EVOH, the paper expands further, particularly on the noPC side where more EVOH has penetrated between the fibers. This is because EVOH does not prevent water molecules from permeating towards the fibers, but it allows water molecules to permeate towards the fibers, which leads to fiber hygroexpansion. Moreover, the EVOH itself absorbs water and swells, which further intensifies the effect (lacquer hygroexpansion of ~5% and moisture content of 30% at 100% RH at 23 °C).
). This indicated that the EVOH penetrated further into the noPC side of the paper because the surface contained more pores and channels (see images presented in [1]). EVOH thus fills the voids in the noPC paper and occupies space that the fibers would otherwise fill during expansion. This interpretation is supported by reference [28]. Because this space between the fibers is now occupied by EVOH, the paper expands further, particularly on the noPC side where more EVOH has penetrated between the fibers. This is because EVOH does not prevent water molecules from permeating towards the fibers, but it allows water molecules to permeate towards the fibers, which leads to fiber hygroexpansion. Moreover, the EVOH itself absorbs water and swells, which further intensifies the effect (lacquer hygroexpansion of ~5% and moisture content of 30% at 100% RH at 23 °C). 3.2. Effect of EVOH Pre-coating on Effective Resistivity of the Aluminum Coating
- The resistivity of aluminum applied to EVOH-coated surfaces was lower than on surfaces without EVOH. This supports the observation described previously [1];
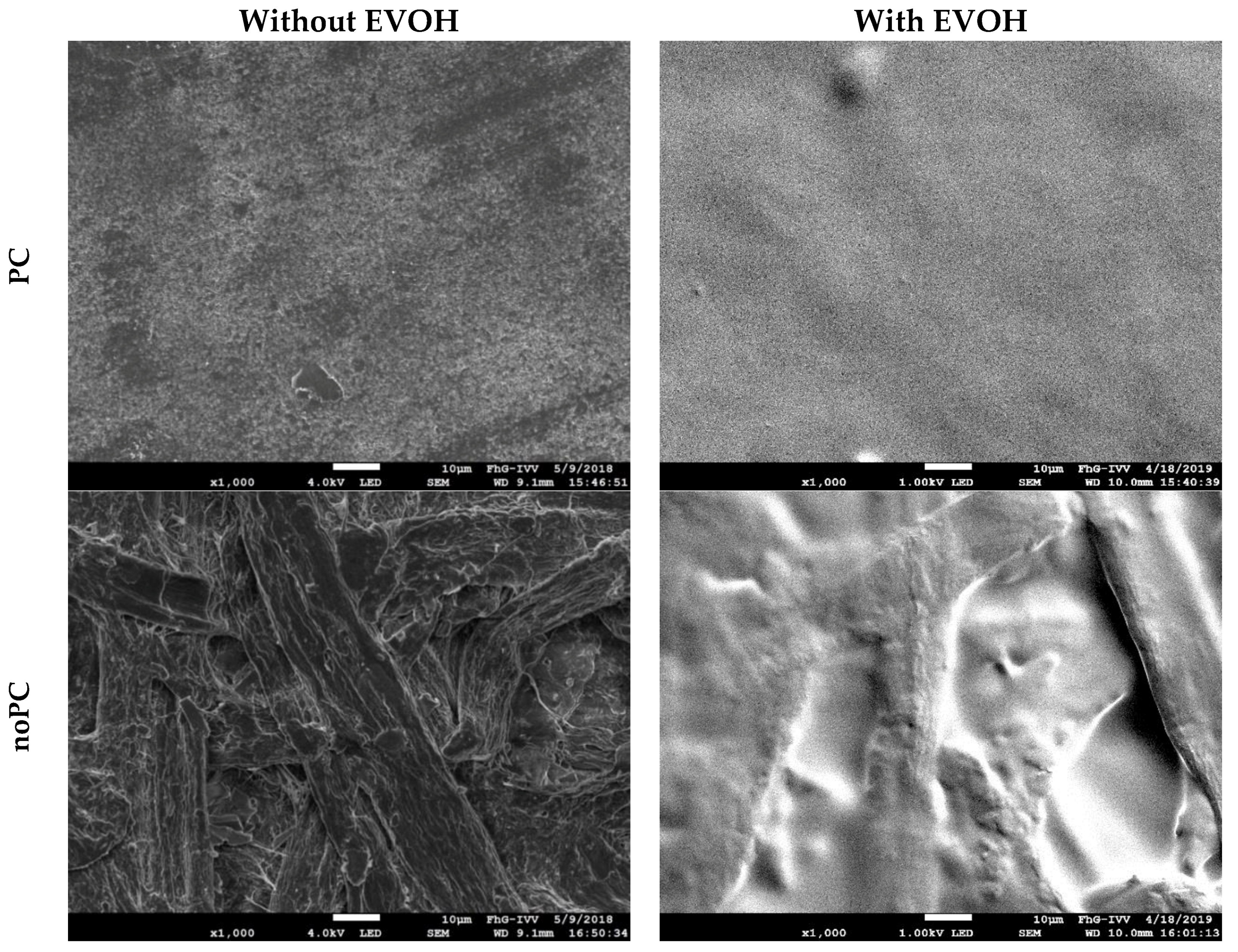
- However, the roughness of noPC with EVOH (
, RZ = 9.5 µm) was much higher than PC (
RZ = 3.8 µm). Nevertheless, noPC with EVOH (
) had a lower resistivity than PC (
);
- This indicates that, not only the roughness, but also the precise morphology—including pores and microchannels—were decisive factors. EVOH coatings may fill up pores and microchannels, but they did not affect the micrometer-scale roughness significantly (compare Figure 3);
- This filling up of voids facilitated the formation of a closed aluminum layer because it reduced the severity of defects in the aluminum coating. As shown in Section 3.1, the filling up of voids promoted hygroexpansion and EVOH should, therefore, be applied on the PC side of the paper.
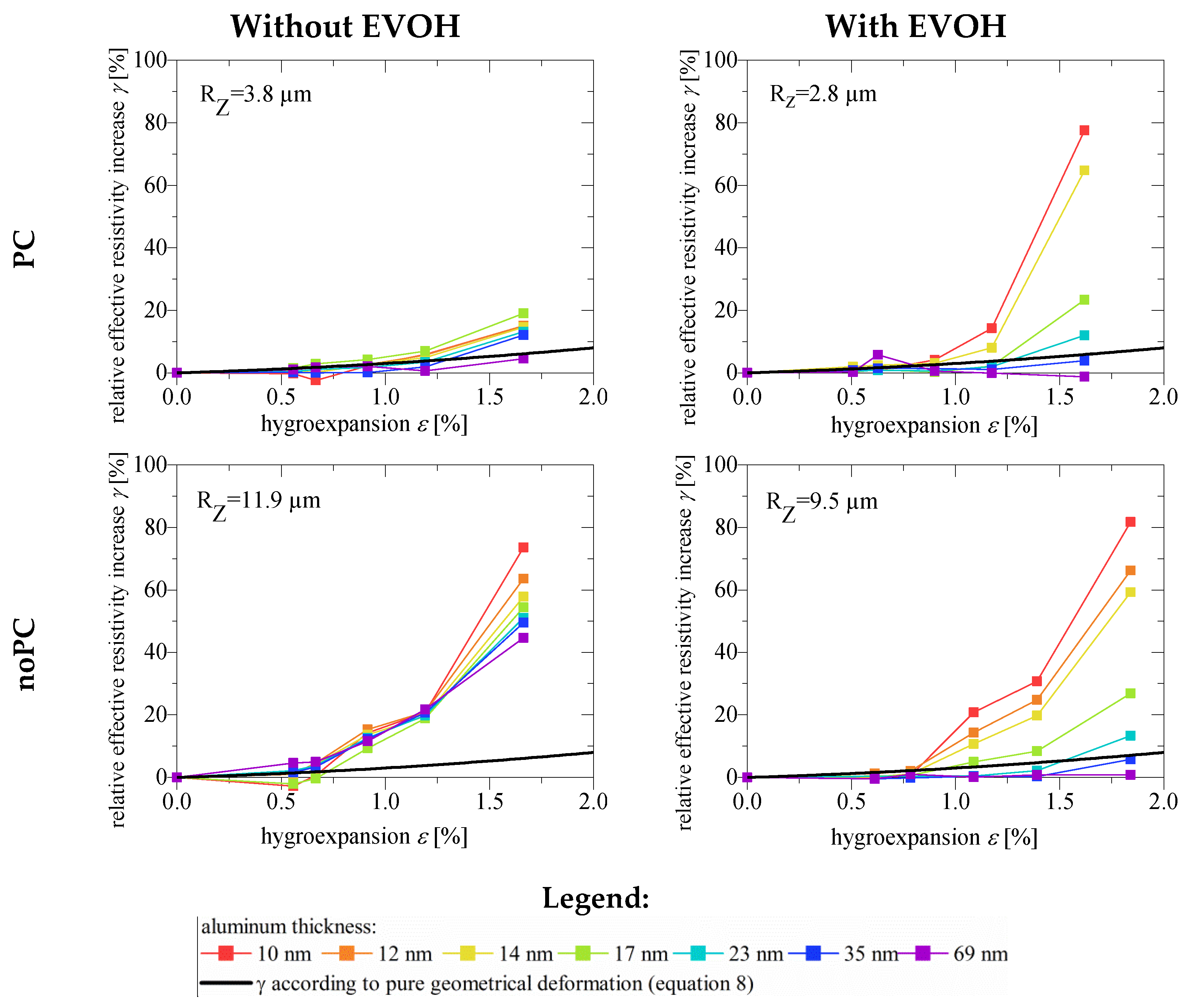
3.3. Effect of EVOH Pre-Coating on the Increase in Resistivity during Hygroexpansion
- The relative effective resistivity increase γ correlated with hygroexpansion ε;
- The maximum relative effective resistivity increase γmax at 95% RH was partially higher on EVOH-coated surfaces (Figure 6). This was because the initial effective resistivity values ρEFF (Figure 5) were much lower; probably due to fewer initial defects. Therefore, the addition of only a few more defects increased the resistivity by a much greater degree. This means that γmax also depended on the initial ρEFF before hygroexpansion, which was higher on rough and porous surfaces;
- In the ideal case (smooth substrate surface, low hygroexpansion, and thick coatings) γ was equal to the value expected, according to the geometrical deformation model in Equation (8). This indicated that no additional defects occurred during hygroexpansion and that no additional defects should be expected in the case of smooth substrates and thick coatings (>35 nm);
- On EVOH-coated surfaces, the effect of the aluminum thickness (d) was more explicit. Thinner coatings (<35 nm) led to a higher relative effective resistivity increase γ. Coatings >35 nm are, therefore, recommended;
- On surfaces without EVOH, the effect of the aluminum thickness was less explicit. On non-EVOH coated surfaces, the effect of aluminum thickness on the increase in γ was lower because the aluminum already contained many defects before hygroexpansion, due to its roughness and porosity (Figure 3). Hence the additional defects due to hygroexpansion did not significantly affect the resistivity value. The EVOH decreased the roughness (RZ) and the areal density of pores and microchannels. For practical applications, this means that even by applying thicker aluminum coatings, the negative effect of roughness and pores during hygroexpansion cannot be reduced. Thus, a polymer pre-coating such as EVOH is indispensable;
- The effect of aluminum thickness on the maximum relative effective resistivity increase γmax (at 95% RH) is shown in Figure 7. When the paper was coated with EVOH, γ is affected to a greater degree by aluminum thickness. When the aluminum thickness was approximately 30–40 nm on EVOH-coated surfaces, γmax did not decrease any further. For practical applications, this means that the maximum resistance against hygroexpansion was reached at this thickness;
- Accordingly, the crack onset strain (COS) increased with aluminum thickness and decreasing substrate roughness;
- Although the hygroexpansion was higher in the presence of EVOH than in its absence, γmax on EVOH coated paper was only a little higher. The increase in hygroexpansion due to the EVOH coating was, therefore, not a major hindrance to the production of flexible and closed aluminum coatings.
3.4. Effect of Drying Contraction on Electrical Resistivity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindner, M.; Heider, J.; Reinelt, M.; Langowski, H.-C. Hygroexpansion and surface roughness cause defects and increase the electrical resistivity of physical vapor deposited aluminum coatings on paper. Coatings 2019, 9, 33. [Google Scholar] [CrossRef]
- Lindner, M.; Schmid, M. Thickness measurement methods for physical vapor deposited Aluminum Coatings in Packaging Applications: A Review. Coatings 2017, 7, 9. [Google Scholar] [CrossRef]
- Kijima, T.; Yamakawa, I. Effect of beating condition on shrinkage during drying. Jpn. Tappi J. 1978, 32, 722–727. [Google Scholar] [CrossRef]
- Salmén, L.; Boman, R.; Fellers, C.; Htun, M. The implications of fiber and sheet structure for the hygroexpansivity of paper [curl, shrinkage, beating, fines]. Nord. Pulp Pap. Res. J. (Sweden) 1987, 4, 127. [Google Scholar] [CrossRef]
- Antonsson, S.; Mäkelä, P.; Fellers, C.; Lindström, M.E. Comparison of the physical properties between hardwood and softwood pulps. Nord. Pulp Pap. Res. J. 2009, 24, 409–414. [Google Scholar] [CrossRef]
- Mendes, A.H.T.; Kim, H.Y.; Ferreira, P.J.T.; Park, S.W. The importance of the measurement of paper differential CD shrinkage. O PAPEL 2012, 73, 45–50. [Google Scholar]
- Fahey, D.J.; Chilson, W. Mechanical treatments for improving dimensional stability of paper. Rev. Process. Non-Refereed (Other) 1963, 46, 393–399. [Google Scholar]
- Sampson, W.W.; Yamamoto, J. The drying shrinkage of cellulosic fibres and isotropic paper sheets. J. Mater. Sci. 2011, 46, 541–547. [Google Scholar] [CrossRef]
- Lif, J.O. Hygro-viscoelastic stress analysis in paper web offset printing. Finite Elem. Anal. Des. 2006, 42, 341–366. [Google Scholar] [CrossRef]
- Alfthan, J. The Effect of Humidity Cycle Amplitude on Accelerated Tensile Creep of Paper. Mech. Time-Depend. Mater. 2004, 8, 289–302. [Google Scholar] [CrossRef]
- Dickerman, G.K.; Savage, R.L. Method of Making Printable Coated Paper. U.S. Patent 2949382, 28 February 1960. [Google Scholar]
- DeMatte, M.L.; Kelly, S.T. Coated Paper for Inkjet Printing. U.S. Patent 5985424A, 16 November 1999. [Google Scholar]
- Kuroyama, Y.; Ohmura, T.; Yamazaki, Y.; Nanri, Y. Cast-Coated Paper for Ink Jet Recording and Production Method Thereof. U.S. Patent 5755929, 26 May 1998. [Google Scholar]
- Paunonen, S. Influence of Moisture on the Performance of Polyethylene Coated Solid Fiberboard and Boxes. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Noway, October 2010. [Google Scholar]
- DIN EN ISO 3274:1998-04. Geometrical Product Specifications (GPS)—Surface Texture: Profile method—Nominal Characteristics of Contact (stylus) Instruments (ISO 3274:1996), German version EN ISO 3274:1997; Beuth: Berlin, Germany, 1998.
- DIN EN ISO 4288:1998-04. Geometrical Product Specifications (GPS)—Surface Texture: Profile method—Rules and Procedures for the Assessment of Surface Texture (ISO 4288:1996), German version EN ISO 4288:1997; Beuth: Berlin, Germany, 1998.
- Zhang, Z.; Britt, I.J.; Tung, M.A. Permeation of oxygen and water vapor through EVOH films as influenced by relative humidity. J. Appl. Polym. Sci. 2001, 82, 1866–1872. [Google Scholar] [CrossRef]
- Johansson, C.; Clegg, F. Hydrophobically modified poly (vinyl alcohol) and bentonite nanocomposites thereof: Barrier, mechanical, and aesthetic properties. J. Appl. Polym. Sci. 2015, 132, 41737. [Google Scholar] [CrossRef]
- Lindner, M.; Höflsauer, F.; Heider, J.; Reinelt, M.; Langowski, H.-C. Comparison of thickness determination methods for physical-vapor-deposited aluminum coatings in packaging applications. Thin Solid Film. 2018, 666, 6–14. [Google Scholar] [CrossRef]
- Kaden, H. Wirbelströme und Schirmung in der Nachrichtentechnik; Springer: Heidelberg, Germany, 2007. [Google Scholar]
- Küpfmüller, K.; Mathis, W.; Reibiger, A. Theoretische Elektrotechnik: Eine Einführung; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Lu, N.; Wang, X.; Suo, Z.; Vlassak, J. Metal films on polymer substrates stretched beyond 50%. Appl. Phys. Lett. 2007, 91, 221909. [Google Scholar] [CrossRef]
- Sorg, H. Praxis der Rauheitsmessung und Oberflächenbeurteilung; Hanser: Munich, Germany, 1995. [Google Scholar]
- Ridgway, C.J.; Gane, P.A. Bulk density measurement and coating porosity calculation for coated paper samples. Nord. Pulp Pap. Res. J. 2003, 18, 24–31. [Google Scholar] [CrossRef]
- Oliver, J.; Agbezuge, L.; Woodcock, K. A diffusion approach for modelling penetration of aqueous liquids into paper. Colloids Surf. A Physicochem. Eng. Asp. 1994, 89, 213–226. [Google Scholar] [CrossRef]
- Roberts, R.J. Liquid Penetration into Paper. Ph.D. Thesis, Australian National University September, Canberra, Australia, September 2010. [Google Scholar]
- Hyväluoma, J.; Raiskinmäki, P.; Jäsberg, A.; Koponen, A.; Kataja, M.; Timonen, J. Simulation of liquid penetration in paper. Phys. Rev. E 2006, 73, 036705. [Google Scholar] [CrossRef] [PubMed]
- Viguie, J.; Dumont, P.J.J.; Mauret, E.; du Roscoat, S.R.; Vacher, P.; Desloges, I.; Bloch, J.F. Analysis of the hygroexpansion of a lignocellulosic fibrous material by digital correlation of images obtained by X-ray synchrotron microtomography: application to a folding box board. J. Mater. Sci. 2011, 46, 4756–4769. [Google Scholar] [CrossRef]
- Polywka, A.; Stegers, L.; Krauledat, O.; Riedl, T.; Jakob, T.; Görrn, P. Controlled Mechanical Cracking of Metal Films Deposited on Polydimethylsiloxane (PDMS). Nanomaterials 2016, 6, 168. [Google Scholar] [CrossRef] [PubMed]






| Product Name | Grammage [g/m2] | Supplier |
|---|---|---|
| Metalkote | 65 | Ahlstrom-Munksjö |
| Algro Finesse T | 70 | Sappi Europe SA |
| Adicar WS HGM | 80 | Cham Paper Group |
| Adicar 2 | 80 | Cham Paper Group |
| Labelcar MTS | 65 | Cham Paper Group |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindner, M.; Reinelt, M.; Gilch, T.; Langowski, H.-C. Hygroexpansion, Surface Roughness and Porosity Affect the Electrical Resistance of EVOH-Aluminum- Coated Paper. Coatings 2019, 9, 295. https://doi.org/10.3390/coatings9050295
Lindner M, Reinelt M, Gilch T, Langowski H-C. Hygroexpansion, Surface Roughness and Porosity Affect the Electrical Resistance of EVOH-Aluminum- Coated Paper. Coatings. 2019; 9(5):295. https://doi.org/10.3390/coatings9050295
Chicago/Turabian StyleLindner, Martina, Matthias Reinelt, Tobias Gilch, and Horst-Christian Langowski. 2019. "Hygroexpansion, Surface Roughness and Porosity Affect the Electrical Resistance of EVOH-Aluminum- Coated Paper" Coatings 9, no. 5: 295. https://doi.org/10.3390/coatings9050295
APA StyleLindner, M., Reinelt, M., Gilch, T., & Langowski, H.-C. (2019). Hygroexpansion, Surface Roughness and Porosity Affect the Electrical Resistance of EVOH-Aluminum- Coated Paper. Coatings, 9(5), 295. https://doi.org/10.3390/coatings9050295








