Strategies of Anode Materials Design towards Improved Photoelectrochemical Water Splitting Efficiency
Abstract
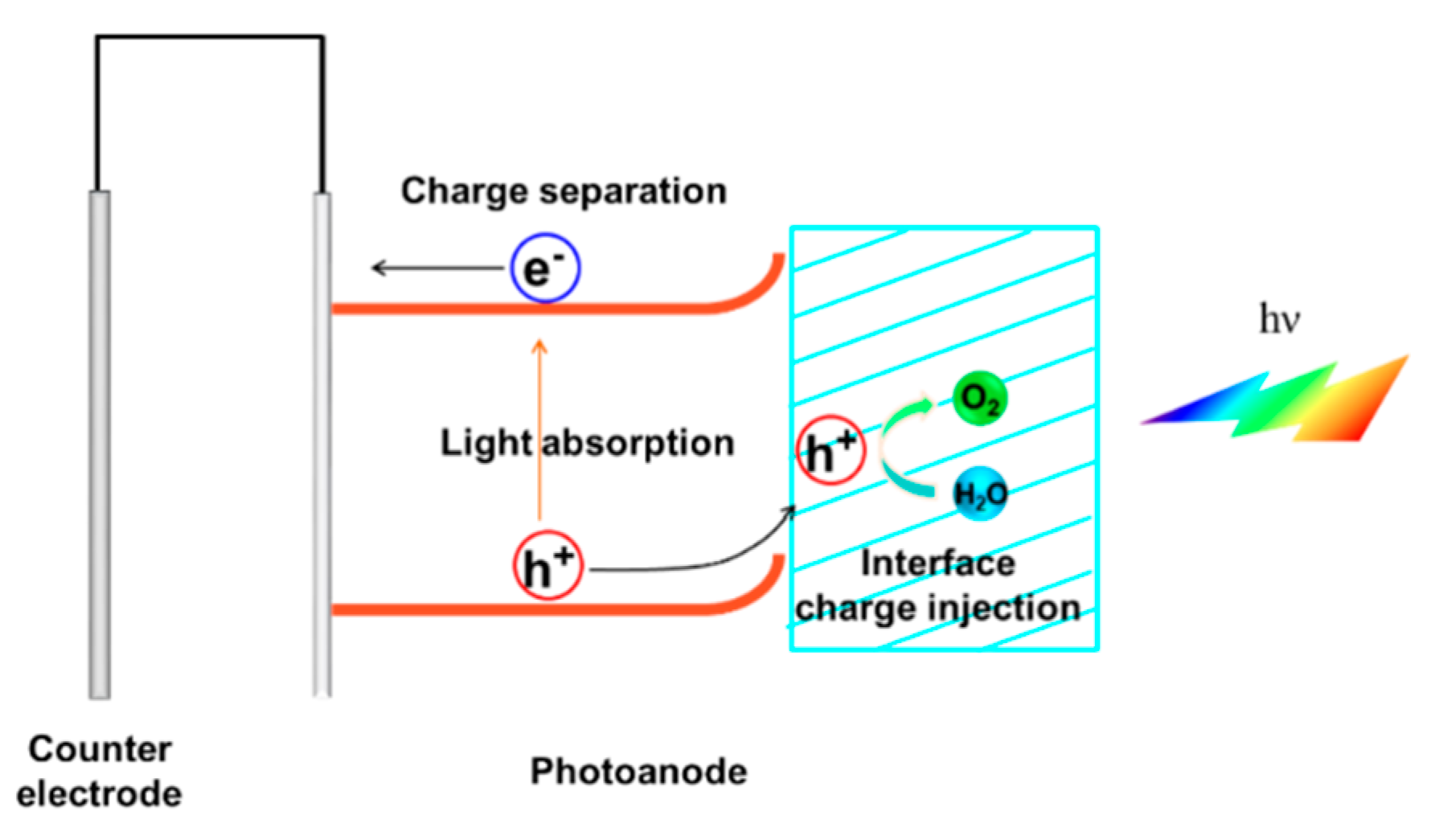
:1. Introduction
2. Strategies to Enhance the Light Absorption
2.1. Nanostructure Formation
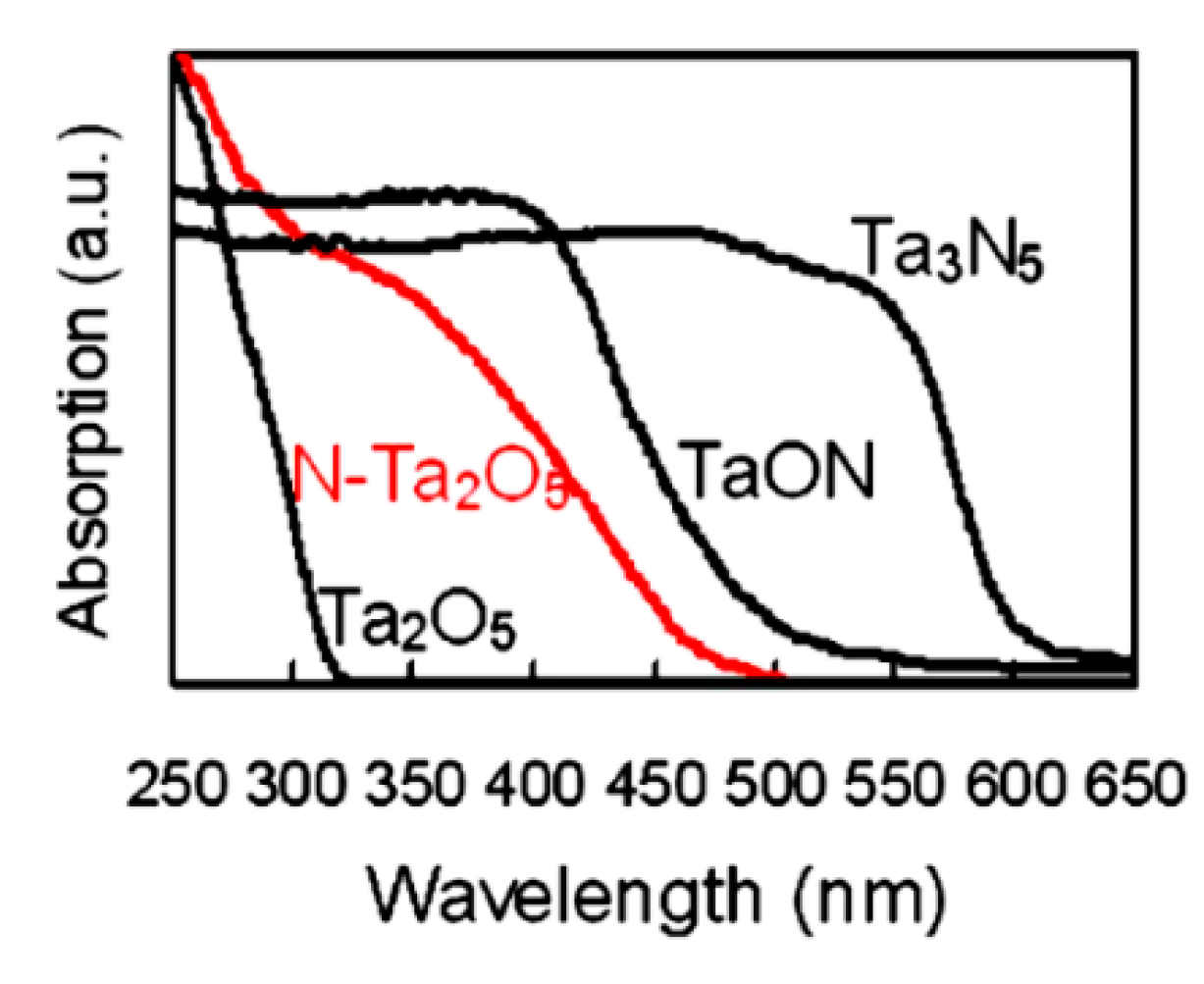
2.2. Band Engineering
2.3. Dual Absorber
3. Strategies to Improve the Charge Separation
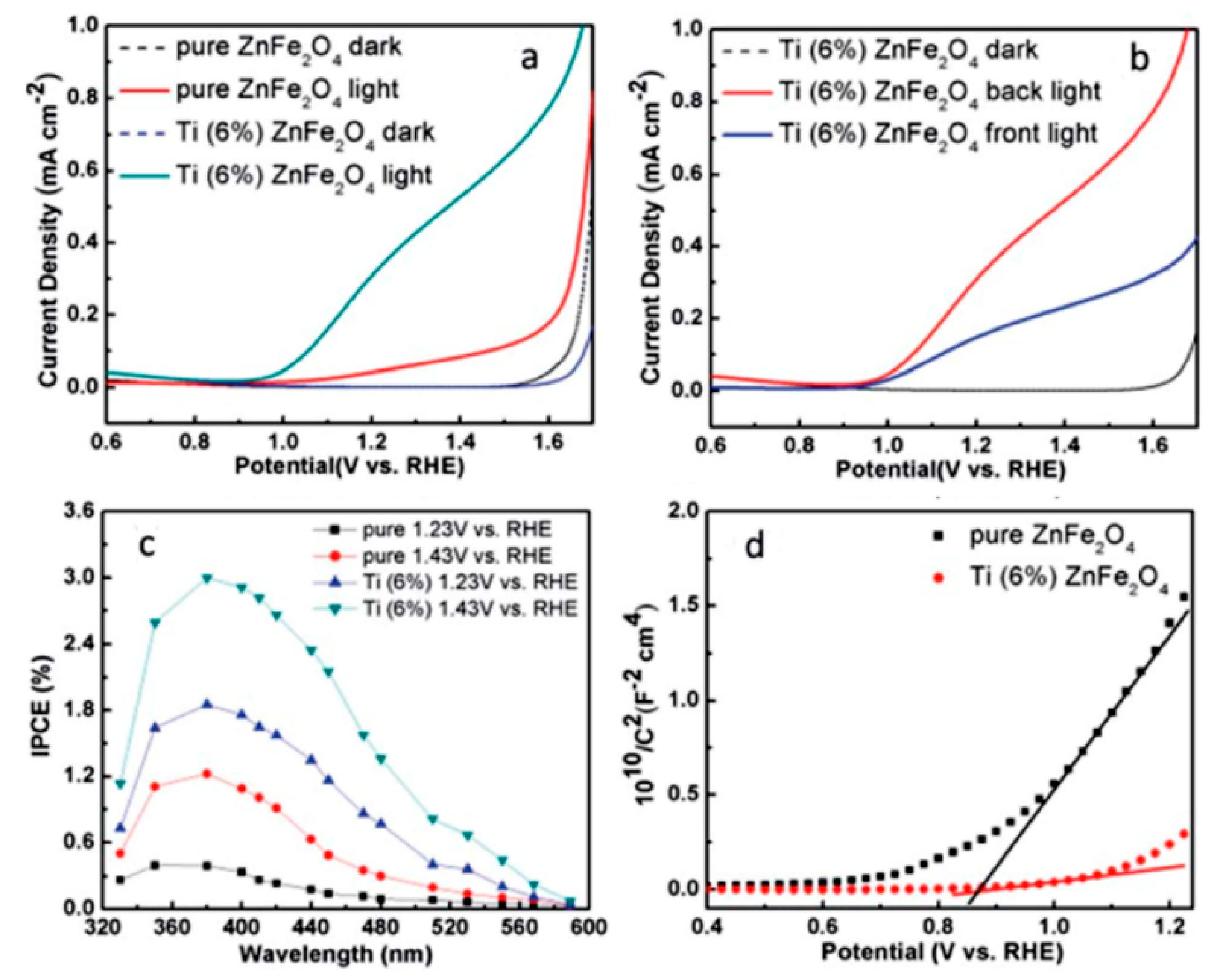
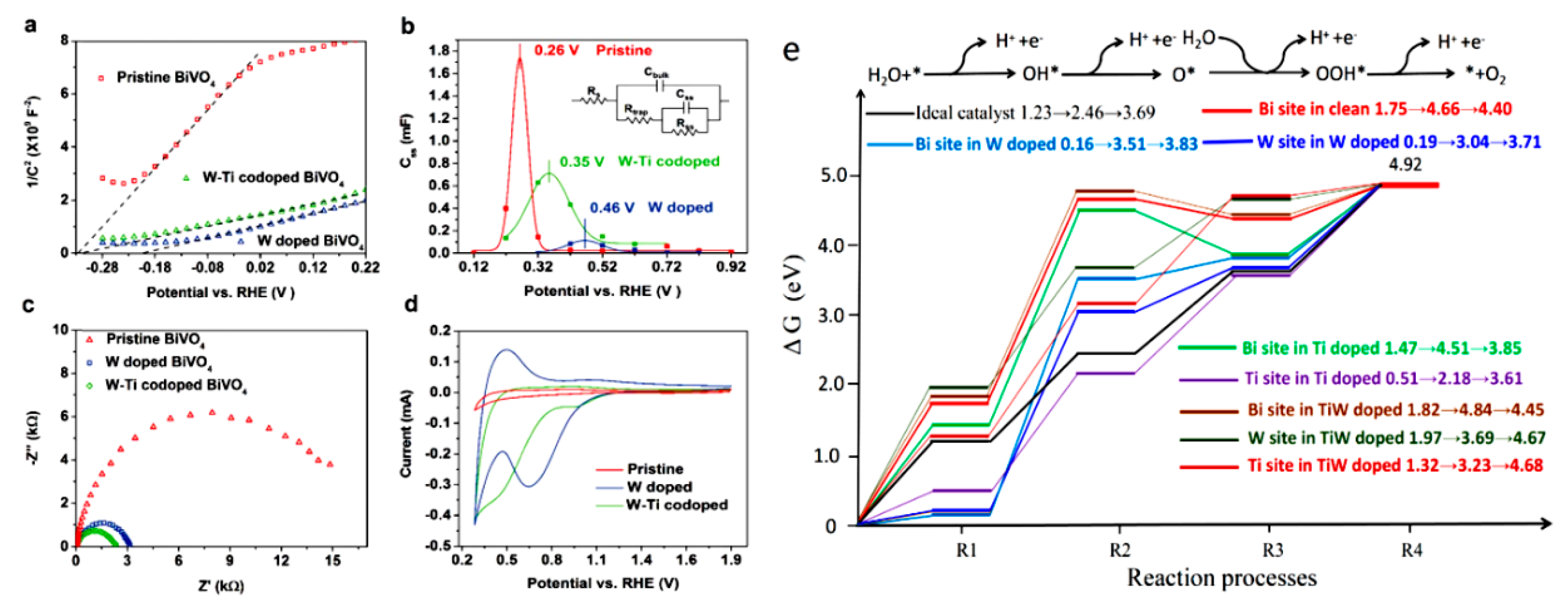
3.1. Doping
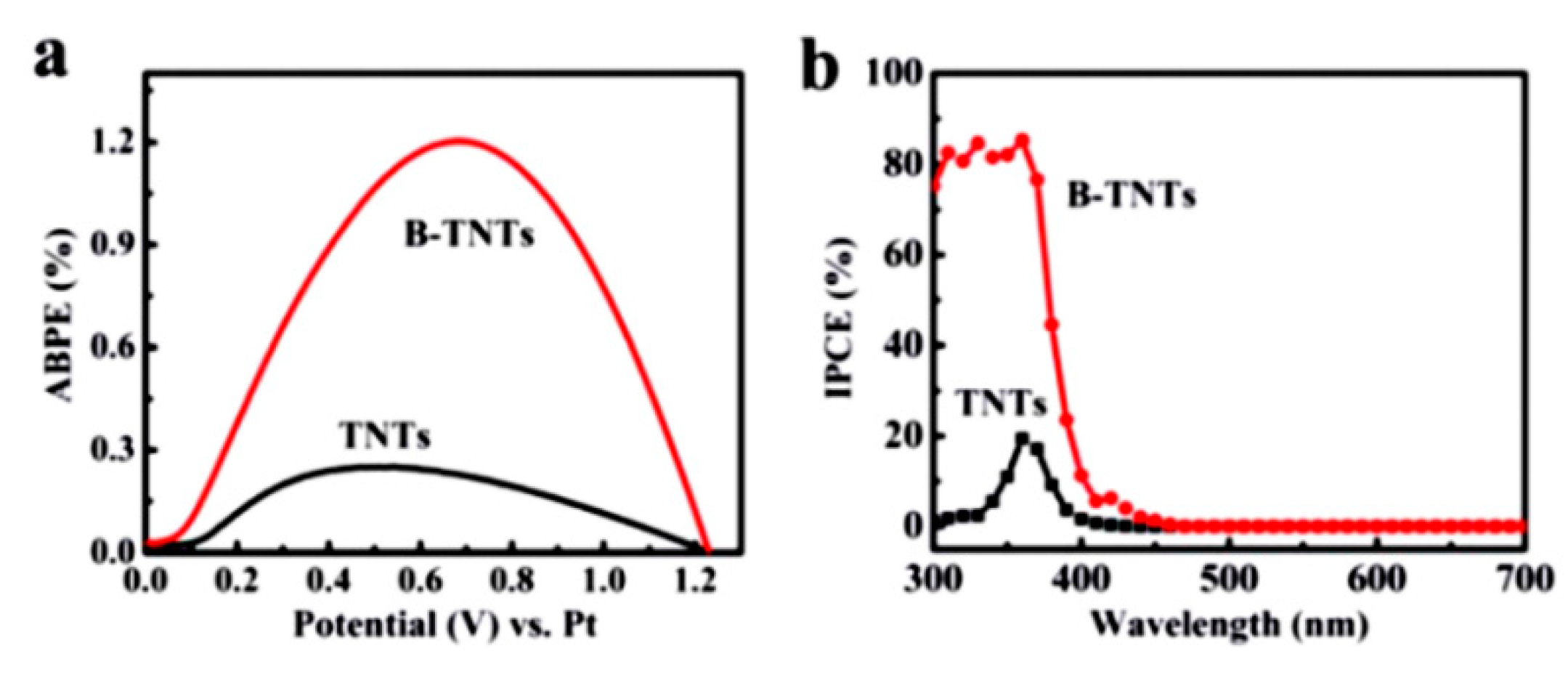
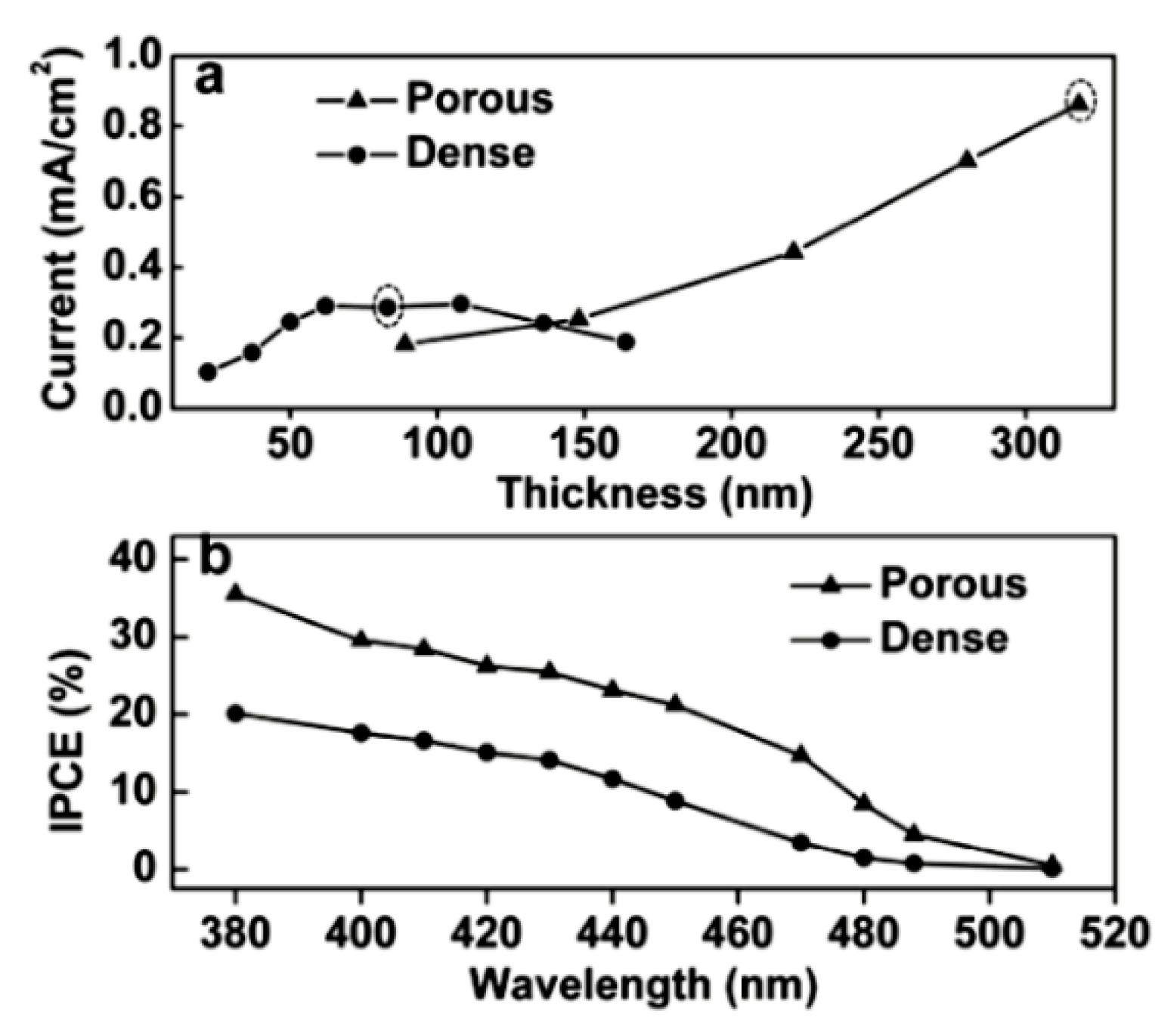
3.2. Nanostructure to Shorten the Diffusion Length
3.3. Heterojunction
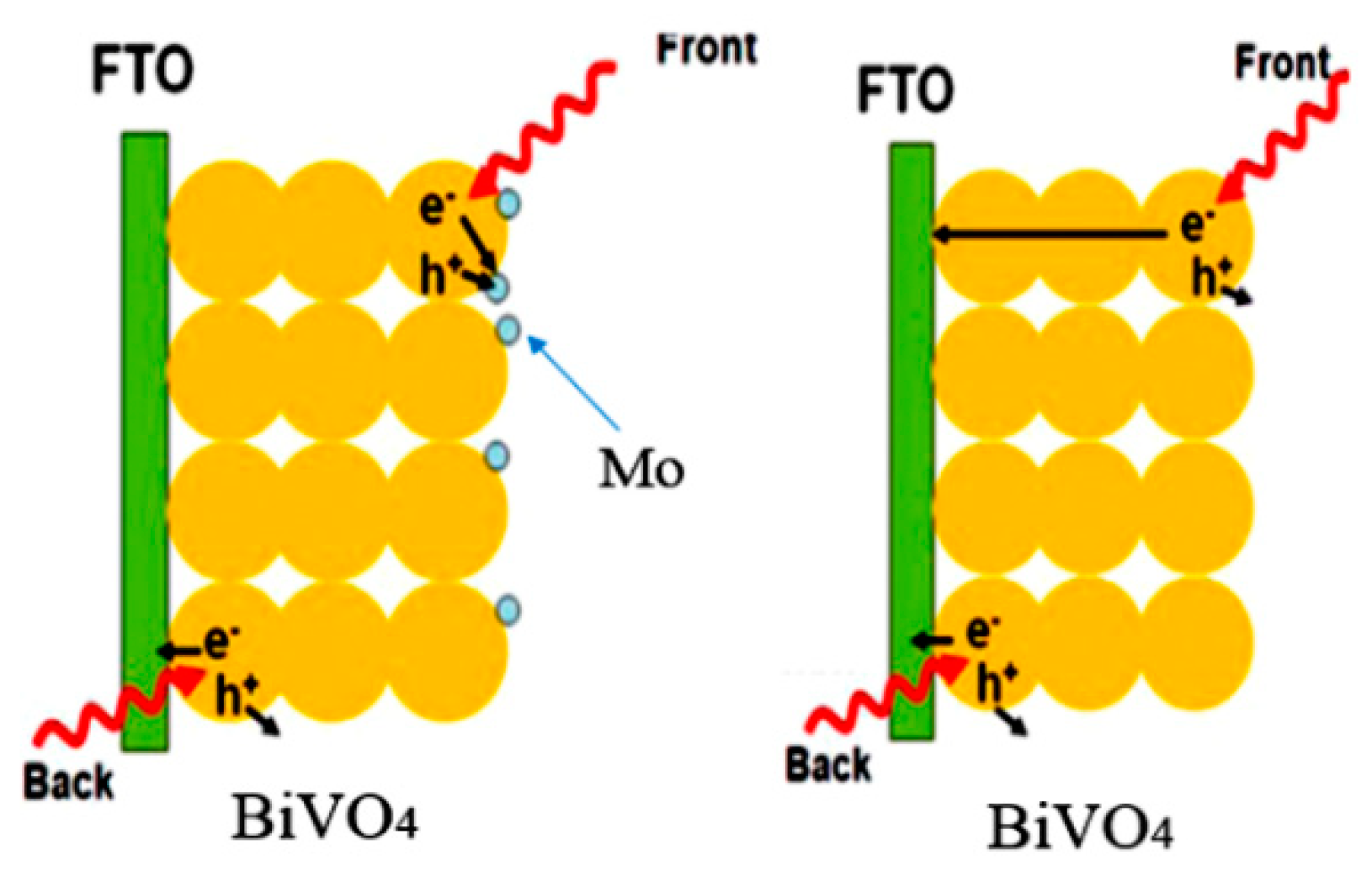
3.4. Internal Electric Field to Improve the Charge Separation
4. Strategies to Enhance the Surface Charge Injection
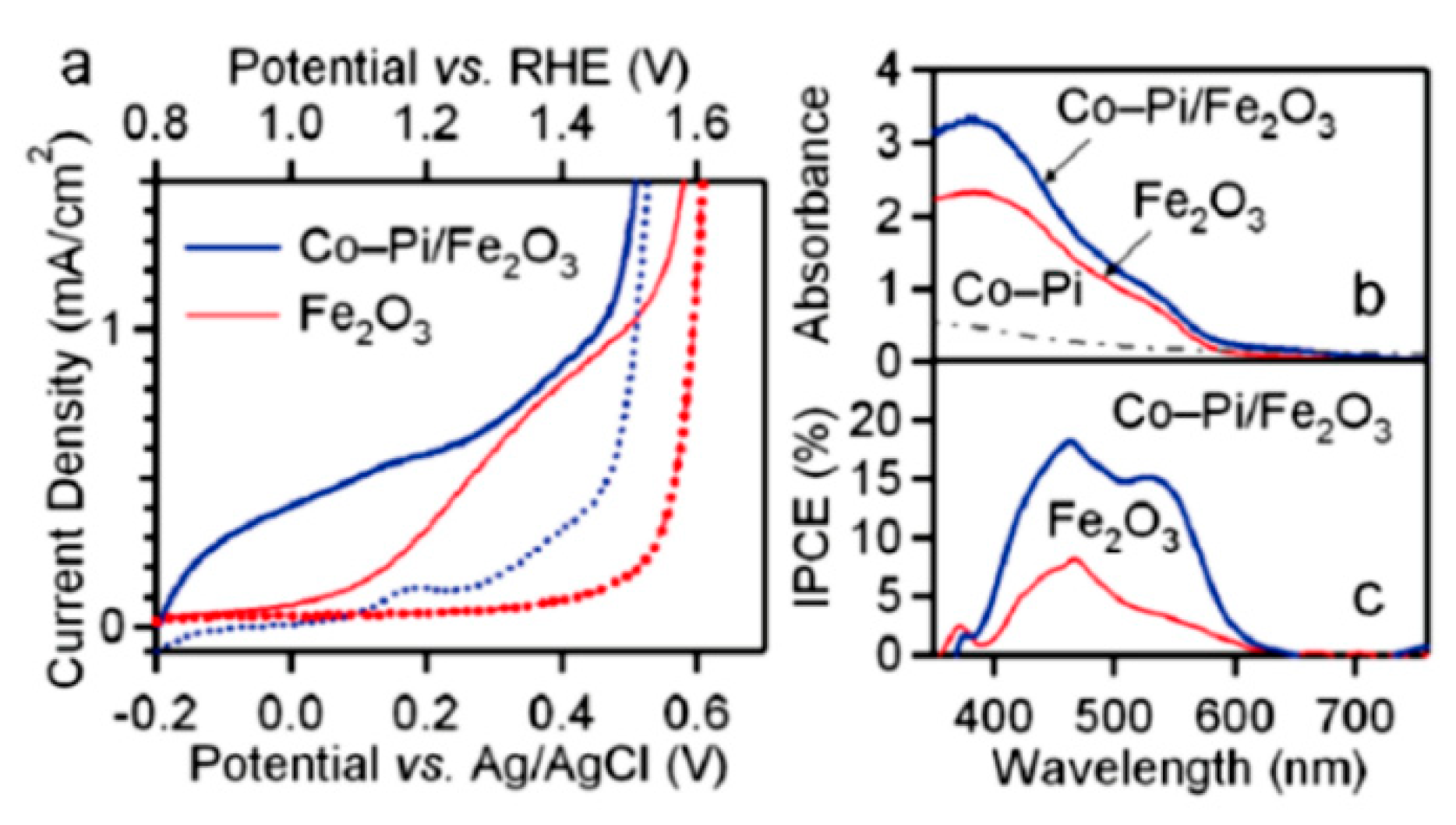
4.1. Catalyst Loading
4.2. Surface Treatment and Surface Passivation
4.3. Active Sites
5. Conclusions and Outlook
Funding
Conflicts of Interest
References
- van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: Berlin, Germany, 2012; pp. 13–69. [Google Scholar]
- Inoue, H.; Shimada, T.; Kou, Y.; Nabetani, Y.; Masui, D.; Takagi, S.; Tachibana, H. The water oxidation bottleneck in artificial photosynthesis: How can we get through it? an alternative route involving a two-electron process. ChemSusChem 2011, 4, 173–179. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Lin, L.; Wygant, B.R.; Liu, Y.; Zhu, Y.; Zheng, Y.; Mullins, C.B.; Zhao, Y.; Zhang, X. Highly efficient photoelectrochemical water splitting from hierarchical WO3/BiVO4 nanoporous sphere arrays. Nano Lett. 2017, 17, 8012–8017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, A.; Green, M.A.; Ferrazza, F. 19.8% efficient “honeycomb” textured multicrystalline and 24.4% monocrystalline silicon solar cells. Appl. Phys. Lett. 1998, 73, 1991–1993. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, Z. Enhanced photoelectrochemical water splitting performance using morphology-controlled BiVO4 with W doping. Beilstein J. Nanotechnol. 2017, 8, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Liu, W.; Chen, W.; Zhou, G.; Hsu, P.-C.; Zhang, R.; Liang, Z.; Fan, S.; Zhang, Y.; Cui, Y. Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells. Sci. Adv. 2016, 2, e1501764. [Google Scholar] [CrossRef]
- Feng, X.; Shankar, K.; Varghese, O.K.; Paulose, M.; Latempa, T.J.; Grimes, C.A. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis details and applications. Nano Lett. 2008, 8, 3781–3786. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.N.; Amal, R.; Scott, J.; Ng, Y.H. Concentration-mediated band gap reduction of Bi2MoO6 photoanodes prepared by Bi3+ cation insertions into anodized MoO3 thin films: Structural, optical, and photoelectrochemical properties. ACS Appl. Energy Mater. 2018, 1, 3955–3964. [Google Scholar] [CrossRef]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–353. [Google Scholar] [CrossRef]
- Lou, S.N.; Ng, Y.H.; Ng, C.; Scott, J.; Amal, R. Harvesting, storing and utilising solar energy using MoO3: Modulating structural distortion through pH adjustment. ChemSusChem 2014, 7, 1934–1941. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Liu, G.; Yin, L.C.; Wang, J.; Niu, P.; Zhen, C.; Xie, Y.; Cheng, H.M. A red anatase TiO2 photocatalyst for solar energy conversion. Energy Environ. Sci. 2012, 5, 9603–9610. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Li, S.; Grote, F.; Zhang, X.; Zhang, H.; Wang, R.; Lei, Y.; Pan, B.; Xie, Y. Controllable disorder engineering in oxygen-incorporated MoS2 ultrathin nanosheets for efficient hydrogen evolution. J. Am. Chem. Soc. 2013, 135, 17881–17888. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Saeki, S.; Suzuki, T.; Kajino, T.; Motohiro, T. Dual functional modification by N doping of Ta2O5: P-type conduction in visible-light-activated N-doped Ta2O5. Appl. Phys. Lett. 2010, 96, 142111. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN: ZnO solid solution as a photocatalyst for visible-light-driven overall water splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free gydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhao, W.; Yang, C.; Yin, H.; Lin, T.; Shan, Y.; Xie, Y.; Gu, H.; Huang, F. Black TiO2 nanotube arrays for high-efficiency photoelectrochemical water-splitting. J. Mater. Chem. A 2014, 2, 8612–8616. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Bai, Y.; Yun, J.H.; Liu, G.; Wan, L. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv. Mater. 2018, 30, 1800486. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jang, J.W.; Jo, Y.H.; Abdi, F.F.; Lee, Y.H.; Van De Krol, R.; Lee, J.S. Hetero-type dual photoanodes for unbiased solar water splitting with extended light harvesting. Nat. Commun. 2016, 7, 13380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, M.T.; Du, C.; Wang, D. Hematite/Si nanowire dual-absorber system for photoelectrochemical water splitting at low applied potentials. J. Am. Chem. Soc. 2012, 134, 12406–12409. [Google Scholar] [CrossRef] [PubMed]
- Brillet, J.; Yum, J.-H.; Cornuz, M.; Hisatomi, T.; Solarska, R.; Augustynski, J.; Graetzel, M.; Sivula, K. Highly efficient water splitting by a dual-absorber tandem cell. Nat. Photonics 2012, 6, 824. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Zhang, P.; Zhang, J.; Li, A.; Gong, J. Enhanced surface reaction kinetics and charge separation of p–n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 2015, 137, 8356–8359. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wang, Z.; Gu, J.; Xiao, S.; Yuan, Y.; Zhu, Y.; Zhang, Y.; Mai, W.; Yang, S. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Kim, J.K.; Shi, X.; Jeong, M.J.; Park, J.; Han, H.S.; Kim, S.H.; Guo, Y.; Heinz, T.F.; Fan, S.; Lee, C.L.; et al. Enhancing Mo:BiVO4 Solar Water Splitting with Patterned Au Nanospheres by Plasmon-Induced Energy Transfer. Adv. Energy Mater. 2018, 8, 1701765. [Google Scholar] [CrossRef]
- Thimsen, E.; Formal, F.L.; Grätzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef]
- Mascaretti, L.; Dutta, A.; Kment, Š.; Shalaev, V.M.; Boltasseva, A.; Zbořil, R.; Naldoni, A. Plasmon-enhanced photoelectrochemical water splitting for efficient renewable energy storage. Adv. Mater. 2019, 1805513. [Google Scholar] [CrossRef]
- Zhao, X.; Feng, J.; Chen, S.; Huang, Y.; Sum, T.C.; Chen, Z. New insight into the roles of oxygen vacancies in hematite for solar water splitting. Phys. Chem. Chem. Phys. 2017, 19, 1074–1082. [Google Scholar] [CrossRef]
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Berglund, S.P.; Rettie, A.J.E.; Hoang, S.; Mullins, C.B. Incorporation of Mo and W into nanostructured BiVO4 films for efficient photoelectrochemical water oxidation. Phys. Chem. Chem. Phys. 2012, 14, 7065–7075. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Wang, X.; Qian, Q.; Zhang, S.; Li, Z.; Zou, Z. A facile spray pyrolysis method to prepare Ti-doped ZnFe2O4 for boosting photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 7571–7577. [Google Scholar] [CrossRef]
- Lumley, M.A.; Choi, K.S. Investigation of pristine and (Mo, W)-doped Cu11V6O26 for use as photoanodes for solar water splitting. Chem. Mater. 2017, 29, 9472–9479. [Google Scholar] [CrossRef]
- Jo, W.J.; Jang, J.W.; Kong, K.; Kang, H.J.; Kim, J.Y.; Jun, H.; Parmar, K.P.S.; Lee, J.S. Phosphate doping into monoclinic BiVO4 for enhanced photoelectrochemical water oxidation activity. Angew. Chem. Int. Ed. 2012, 51, 3147–3151. [Google Scholar] [CrossRef]
- Yang, Z.; Chang, B.; Zou, J.; Qiao, J.; Gao, P.; Zeng, Y.; Li, H. Comparison between gradient-doping GaAs photocathode and uniform-doping GaAs photocathode. Appl. Opt. 2007, 46, 7035–7039. [Google Scholar] [CrossRef]
- Abdi, F.F.; Han, L.; Smets, A.H.; Zeman, M.; Dam, B.; Van De Krol, R. A bismuth vanadate–cuprous oxide tandem cell for overall solar water splitting. Nat. Commun. 2013, 4, 2195. [Google Scholar] [CrossRef]
- Krol, R.; Liang, Y.; Schoonman, J. Solar hydrogen production with nanostructured metal oxides. J. Mater. Chem. 2008, 18, 2311–2320. [Google Scholar] [CrossRef]
- Kim, T.W.; Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, W.; Feng, J.; Li, M.; Li, Z.; Yu, T.; Zou, Z. Quantitative analysis and visualized evidence for high charge separation efficiency in a solid-liquid bulk heterojunction. Adv. Energy Mater. 2014, 4, 1301785. [Google Scholar] [CrossRef]
- Zhu, X.; Guijarro, N.; Liu, Y.; Schouwink, P.; Wells, R.A.; Formal, F.L.; Sun, S.; Gao, C.; Sivula, K. Spinel structural disorder influences solar-water-splitting performance of ZnFe2O4 nanorod photoanodes. Adv. Mater. 2018, 30, 1801612. [Google Scholar] [CrossRef]
- Iwase, A.; Yoshino, S.; Takayama, T.; Ng, Y.H.; Amal, R.; Kudo, A. Water splitting and CO2 reduction under visible light irradiation using Z-Scheme systems consisting of metal sulfides, CoOx-loaded BiVO4, and a reduced graphene oxide electron mediator. J. Am. Chem. Soc. 2016, 138, 10260–10264. [Google Scholar] [CrossRef]
- Booshehri, A.Y.; Goh, S.C.; Hong, J.D.; Jiang, R.; Xu, R. Effect of depositing silver nanoparticles on BiVO4 in enhancing visible light photocatalytic inactivation of bacteria in water. J. Mater. Chem. A 2014, 2, 6209–6217. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P. Optimization of photoelectrochemical water splitting performance on hierarchicalTiO2 nanotube arrays. Energy Environ. Sci. 2012, 5, 6506–6512. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical properties of the semiconducting electrode WO2. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.-X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting–a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Su, J.; Guo, L.; Bao, N.; Grimes, C.A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 2011, 11, 1928–1933. [Google Scholar] [CrossRef]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously efficient light absorption and charge separation in WO3/BiVO4 core/shell nanowire photoanode for photoelectrochemical water oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, J.; Yang, S.; Xu, Y.; Cai, X.; Li, X.; Zhang, X.; Peng, F.; Fang, Y. Electrodeposition of Cu2O/g-C3N4 heterojunction film on an FTO substrate for enhancing visible light photoelectrochemical water splitting. Chin. J. Catal. 2017, 38, 365–371. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Cheng, H.; Jiao, S.; Takeda, O.; Zhu, H. High-performance p-Cu2O/n-TaON heterojunction nanorod photoanodes passivated with an ultrathin carbon sheath for photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 3758–3768. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Zhao, X.; Su, H.; Chen, Z. Anisotropic electronic characteristics, adsorption, and stability of low-index BiVO4 surfaces for photoelectrochemical applications. ACS Appl. Mater. Interfaces 2018, 10, 5475–5484. [Google Scholar] [CrossRef]
- Wang, D.; Kanhere, P.; Li, M.; Tay, Q.; Tang, Y.; Huang, Y.; Sum, T.C.; Mathews, N.; Sritharan, T.; Chen, Z. Improving Photocatalytic H2 Evolution of TiO2 via Formation of {001}−{010} Quasi-Heterojunctions. J. Phys. Chem. C 2013, 117, 22894–22902. [Google Scholar] [CrossRef]
- Kment, S.; Riboni, F.; Pausova, S.; Wang, L.; Wang, L.; Han, H.; Hubicka, Z.; Krysa, J.; Schmuki, P.; Zboril, R. Photoanodes based on TiO2 and α-Fe2O3 for solar water splitting—Superior role of 1D nanoarchitectures and of combined heterostructures. Chem. Soc. Rev. 2017, 46, 3716–3769. [Google Scholar] [CrossRef]
- Huang, X.; Wang, K.; Wang, Y.; Wang, B.; Zhang, L.; Gao, F.; Zhao, Y.; Feng, W.; Zhang, S.; Liu, P. Enhanced charge carrier separation to improve hydrogen production efficiency by ferroelectric spontaneous polarization electric field. Appl. Catal. B-Environ. 2018, 227, 322–329. [Google Scholar] [CrossRef]
- Li, L.; Salvador, P.A.; Rohrer, G.S. Photocatalysts with internal electric fields. Nanoscale 2014, 6, 24–42. [Google Scholar] [CrossRef] [Green Version]
- Giocondi, J.L.; Rohrer, G.S. The influence of the dipolar field effect on the photochemical reactivity of Sr2Nb2O7 and BaTiO3 microcrystals. Top. Catal. 2008, 49, 18–23. [Google Scholar] [CrossRef]
- Schultz, A.M.; Zhang, Y.; Salvador, P.A.; Rohrer, G.S. Effect of crystal and domain orientation on the visible-light photochemical reduction of Ag on BiFeO3. ACS Appl. Mater. Interfaces 2011, 3, 1562–1567. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Burbure, N.V.; Gamalski, A.; Rohrer, G.S. Composition dependence of the photochemical reduction of Ag by Ba1−xSrxTiO3. Chem. Mater. 2010, 22, 3527–3534. [Google Scholar] [CrossRef]
- Li, J.; Cai, L.; Shang, J.; Yu, Y.; Zhang, L. Giant enhancement of internal electric field boosting bulk charge separation for photocatalysis. Adv. Mater. 2016, 28, 4059–4064. [Google Scholar] [CrossRef]
- Cui, X.; Li, Y.; Sun, N.; Dua, J.; Lia, X.; Yang, H.; Hao, X. Double perovskite Bi2FeMoxNi1−xO6 thin films: Novel ferroelectric photovoltaic materials with narrow bandgap and enhanced photovoltaic performance. Sol. Energy Mater. Sol. C 2018, 187, 9–14. [Google Scholar] [CrossRef]
- Han, H.; Riboni, F.; Karlicky, F.; Kment, S.; Goswami, A.; Sudhagar, P.; Yoo, J.; Wang, L.; Tomanec, O.; Petr, M.; et al. α-Fe2O3/TiO2 3D hierarchical nanostructures for enhanced photoelectrochemical water splitting. Nanoscale 2017, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.H.; Choi, W.; Park, H. Photoelectrochemical and photocatalytic behaviors of hematite-decorated Titania nanotube arrays: Energy level mismatch versus surface specific reactivity. J. Phys. Chem. C 2011, 115, 7134–7142. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic water splitting: Recent progress and future challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Zhong, D.K.; Sun, J.; Inumaru, H.; Gamelin, D.R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Ping, Y.; Galli, G.A.; Choi, K.S. Simultaneous enhancements in photon absorption and charge transport of bismuth vanadate photoanodes for solar water splitting. Nat. Commun. 2015, 6, 8769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, F.; Cheng, W.; Su, H.; Zhao, X.; Liu, Q. Smoothing surface trapping states in 3D coral-like CoOOH-wrapped-BiVO4 for efficient photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2018, 10, 6228–6234. [Google Scholar] [CrossRef]
- Zhang, W.; Li, R.; Zhao, X.; Chen, Z.; Law, A.W.K.; Zhou, K. A cobalt-based metal–organic framework as cocatalyst on BiVO4 photoanode for enhanced photoelectrochemical water oxidation. ChemSusChem 2018, 11, 2710–2716. [Google Scholar] [CrossRef]
- Shi, X.; Choi, I.Y.; Kan, Z.; Kwon, J.; Dong, Y.K.; Lee, J.K.; Sang, H.O.; Kim, J.K.; Park, J.H. Efficient photoelectrochemical hydrogen production from bismuth vanadate-decorated tungsten trioxide helix nanostructures. Nat. Commun. 2014, 5, 4775. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X. Enhanced photoelectrochemical water-splitting performance of semiconductors by surface passivation layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Li, Z.; Yu, T.; Zou, Z. Effects of surface electrochemical pretreatment on the photoelectrochemical performance of Mo-Doped BiVO4. J. Phys. Chem. C 2012, 116, 5076–5081. [Google Scholar] [CrossRef]
- Wang, S.; Chen, P.; Yun, J.H.; Hu, Y.; Wang, L. An electrochemically treated BiVO4 photoanode for efficient photoelectrochemical water splitting. Angew. Chem. Int. Ed. 2017, 56, 8500–8504. [Google Scholar] [CrossRef]
- Riha, S.C.; Klahr, B.M.; Tyo, E.C.; Seifert, S.; Vajda, S.; Pellin, M.J.; Hamann, T.W.; Martinson, A.B.F. Atomic layer deposition of a submonolayer catalyst for the enhanced photoelectrochemical performance of water oxidation with hematite. ACS Nano 2013, 7, 2396–2405. [Google Scholar] [CrossRef]
- Hisatomi, T.; Le Formal, F.; Cornuz, M.; Brillet, J.; Tétreault, N.; Sivula, K.; Grätzel, M. Cathodic shift in onset potential of solar oxygen evolution on hematite by 13-group oxide overlayers. Energy Environ. Sci. 2011, 4, 2512–2515. [Google Scholar] [CrossRef]
- Le Formal, F.; Tetreault, N.; Cornuz, M.; Moehl, T.; Gr€atzel, M.; Sivula, K. Passivating surface states on water splitting hematite photoanodes with alumina overlayers. Chem. Sci. 2011, 2, 737–743. [Google Scholar] [CrossRef]
- Abe, R.; Higashi, M.; Domen, K. Facile fabrication of an efficient oxynitride TaON photoanode for overall water splitting into H2 and O2 under visible light irradiation. J. Am. Chem. Soc. 2010, 132, 11828–11829. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Highly stable water splitting on oxynitride TaON photoanode system under visible light irradiation. J. Am. Chem. Soc. 2012, 134, 6968–6971. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Zhou, X.; Li, C. A theoretical study on the mechanism of photocatalytic oxygen evolution on BiVO4 in aqueous solution. Chem. Eur. J. 2013, 19, 1320–1326. [Google Scholar] [CrossRef]
- Zhao, Z.Y. Single water molecule adsorption and decomposition on the low-index stoichiometric rutile TiO2 surfaces. J. Phys. Chem. C 2014, 118, 4287–4295. [Google Scholar] [CrossRef]
- Valdés, Á.; Brillet, J.; Grätzel, M.; Gudmundsdóttir, H.; Hansen, H.A.; Jónsson, H.; Klüpfel, P.; Kroes, G.J.; Formal, F.L.; Man, I.C.; et al. Solar hydrogen production with semiconductor metal oxides: New directions in experiment and theory. Phys. Chem. Chem. Phys. 2012, 14, 49–70. [Google Scholar] [CrossRef]
- Sun, Y.F.; Liu, Q.H.; Gao, S.; Cheng, H.; Lei, F.C.; Sun, Z.H.; Jiang, Y.; Su, H.B.; Wei, S.Q.; Xie, Y. Pits confined in ultrathin cerium (IV) oxide for studying catalytic centers in carbon monoxide oxidation. Nat. Commun. 2013, 4, 2899. [Google Scholar] [CrossRef]
- Sun, Y.F.; Lei, F.C.; Gao, S.; Pan, B.C.; Zhou, J.F.; Xie, Y. Atomically thin tin dioxide sheets for efficient catalytic oxidation of carbon monoxide. Angew. Chem. Int. Ed. 2013, 52, 10569. [Google Scholar] [CrossRef]
- Sun, Y.F.; Gao, S.; Lei, F.C.; Liu, J.W.; Liang, L.; Xie, Y. Atomically-thin non-layered cobalt oxide porous sheets for highly efficient oxygen-evolving electrocatalysts. Chem. Sci. 2014, 5, 3976–3982. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Chen, W.; Su, H.; Chen, Z. Theoretical insight into the mechanism of photoelectrochemical oxygen evolution reaction on BiVO4 anode with oxygen vacancy. J. Phys. Chem. C 2017, 121, 18702–18709. [Google Scholar] [CrossRef]
- Wendt, S.; Sprunger, P.T.; Lira, E.; Madsen, G.K.H.; Li, Z.; Hansen, J.O.; Matthiesen, J.; Blekinge-Rasmussen, A.; Lægsgaard, E.; Hammer, B.; Besenbacher, F. The role of interstitial sites in the Ti3D defect state in the band gap of Titania. Science 2008, 320, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, J.; Chen, S.; Chen, Z. An investigation on the role of W doping in BiVO4 photoanodes used for solar water splitting. Phys. Chem. Chem. Phys. 2018, 20, 13637–13645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, J.; Wu, B.; Banerjee, A.; Chakraborty, S.; Feng, J.; Zhao, Z.; Chen, S.; Ahuja, R.; Sum, T.C.; Chen, Z. Simultaneous enhancement in charge separation and onset potential for water oxidation in a BiVO4 photoanode by W–Ti codoping. J. Mater. Chem. A 2018, 6, 16965–16974. [Google Scholar] [CrossRef]















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Zhao, S.; Zhao, X.; Chen, Z. Strategies of Anode Materials Design towards Improved Photoelectrochemical Water Splitting Efficiency. Coatings 2019, 9, 309. https://doi.org/10.3390/coatings9050309
Hu J, Zhao S, Zhao X, Chen Z. Strategies of Anode Materials Design towards Improved Photoelectrochemical Water Splitting Efficiency. Coatings. 2019; 9(5):309. https://doi.org/10.3390/coatings9050309
Chicago/Turabian StyleHu, Jun, Shuo Zhao, Xin Zhao, and Zhong Chen. 2019. "Strategies of Anode Materials Design towards Improved Photoelectrochemical Water Splitting Efficiency" Coatings 9, no. 5: 309. https://doi.org/10.3390/coatings9050309




