Condensation of Model Lipid Films by Cholesterol: Specific Ion Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Langmuir Film Balance
2.2.2. Brewster Angle Microscope
2.2.3. Surface Potential
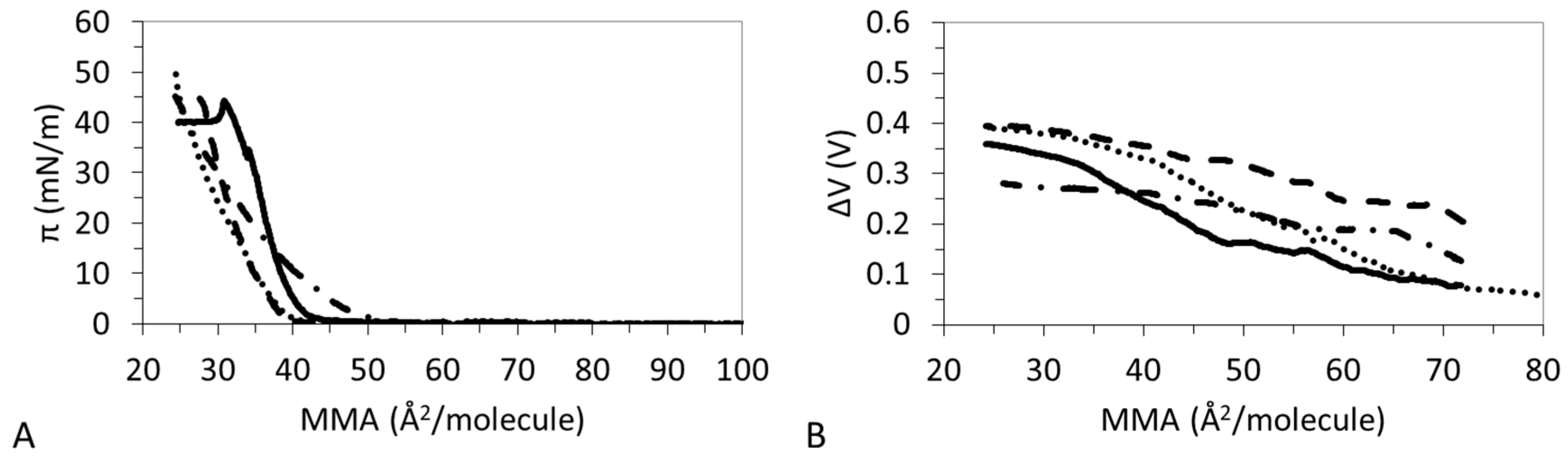
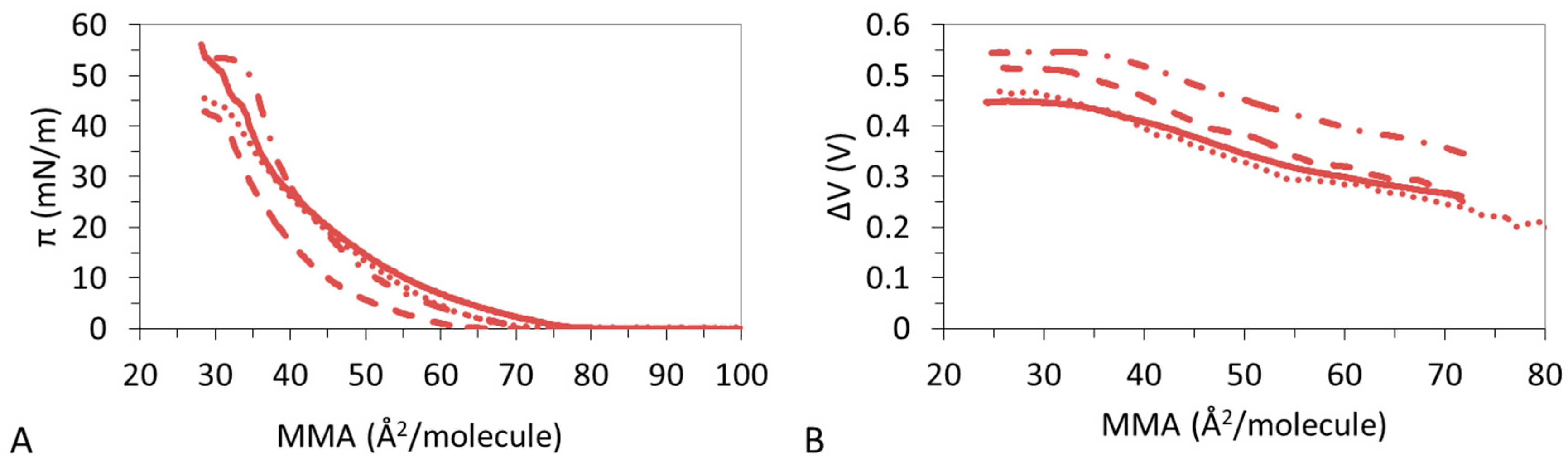
3. Results
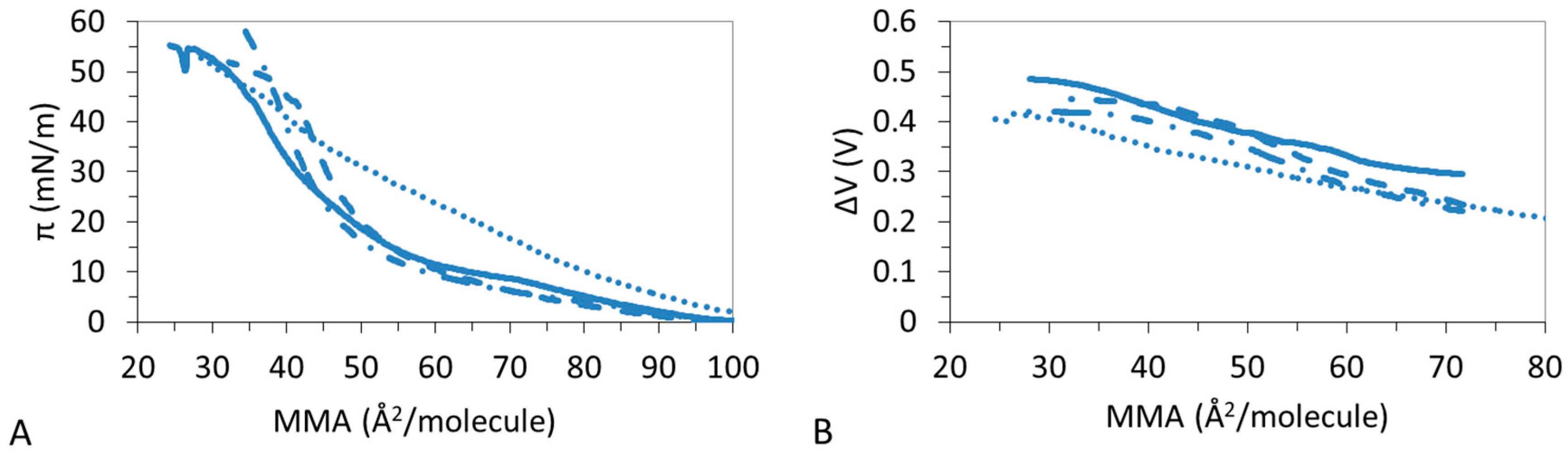
3.1. π-A Isotherms
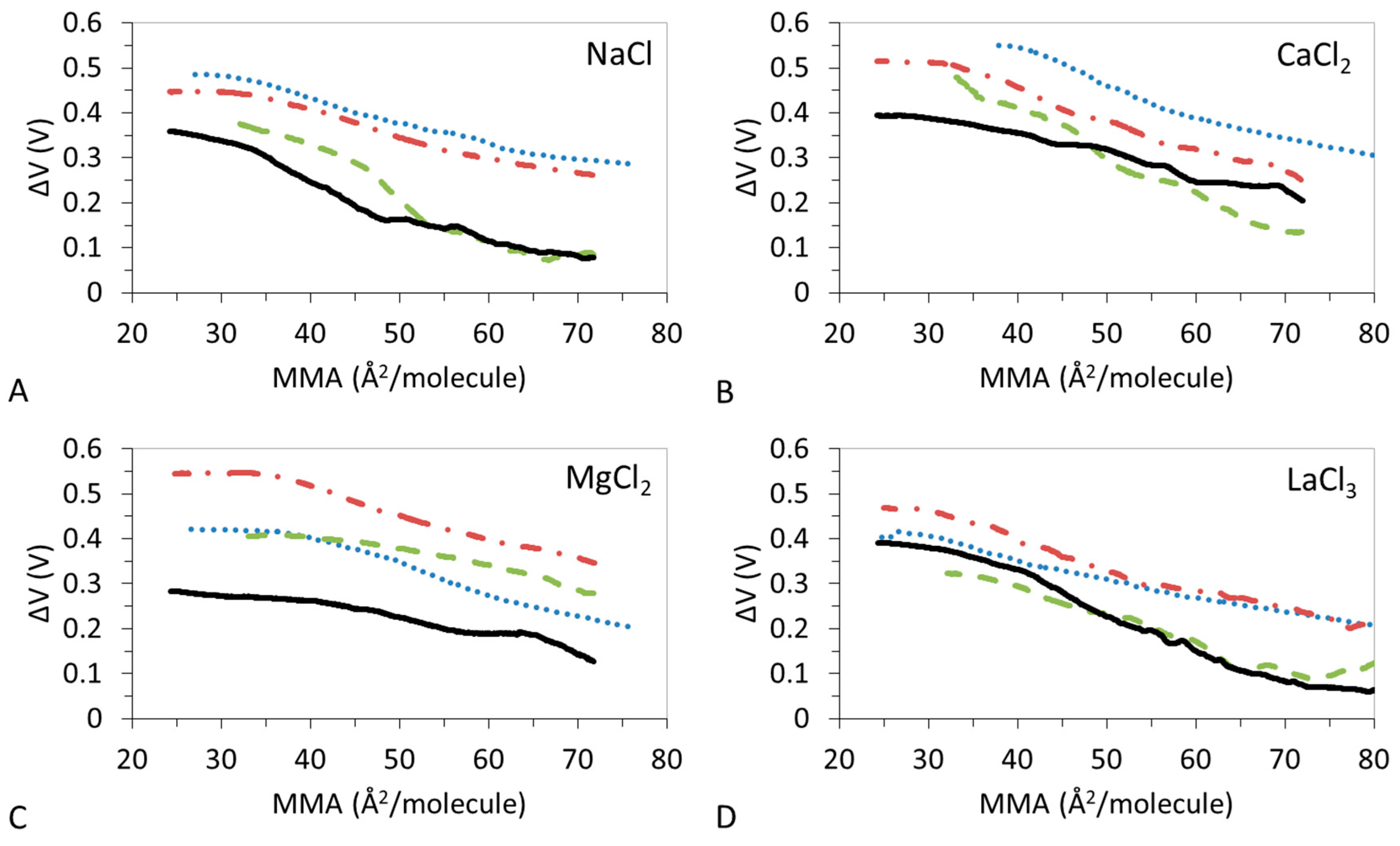
3.2. ΔV–A Isotherms
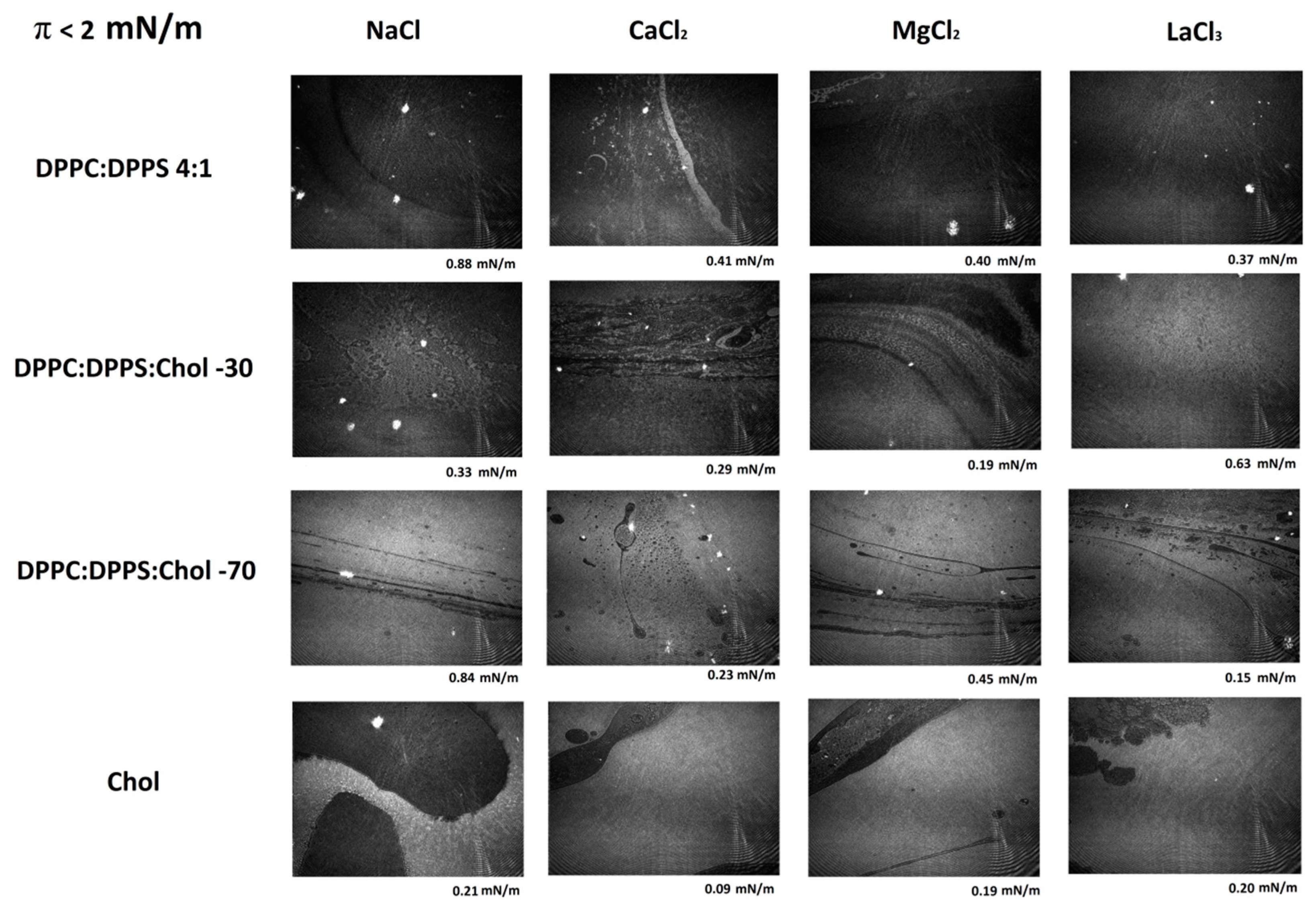
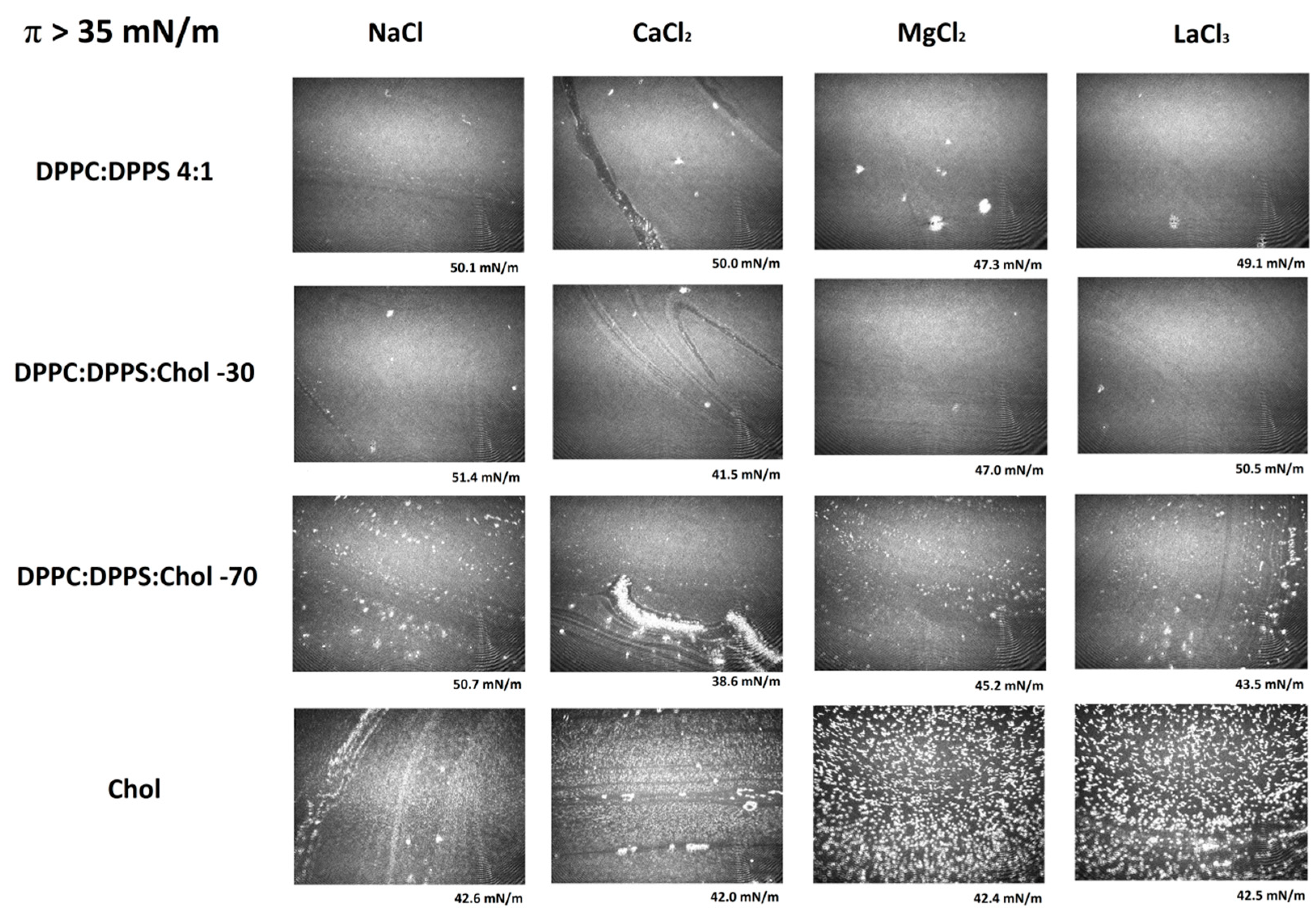
3.3. Micro-BAM Images
3.4. Effect of Ions
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alberts, B. Molecular Biology of the Cell; Garland Science: New York, NY, USA, 2015. [Google Scholar]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Levitan, I.; Barrantes, F.J. Cholesterol Regulation of Ion Channels and Receptors; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zhang, J.; Xue, R.; Ong, W.-Y.; Chen, P. Roles of cholesterol in vesicle fusion and motion. Biophys. J. 2009, 97, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Fonollosa, J.; De Pera, M.; Estelrich, J.; De La Maza, A.; Parra, J.L. Influence of cholesterol on liposome fluidity by EPR: Relationship with percutaneous absorption. J. Control. Release 2000, 68, 85–95. [Google Scholar] [CrossRef]
- Cabrera, I.; Abasolo, I.; Corchero, J.L.; Elizondo, E.; Gil, P.R.; Moreno, E.; Faraudo, J.; Sala, S.; Bueno, D.; González-Mira, E.; et al. α-galactosidase a loaded nanoliposomes with enhanced enzymatic activity and intracellular penetration. Adv. Healthc. Mater. 2016, 5, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Úbeda, M.; Rodríguez-Pulido, A.; Nogales, A.; Martín-Molina, A.; Aicart, E.; Junquera, E. Effect of lipid composition on the structure and theoretical phase diagrams of DC-Chol/DOPE-DNA lipoplexes. Biomacromolecules 2010, 11, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Paiva, D.; Martín-Molina, A.; Cardoso, I.; Quesada-Pérez, M.; do Carmo Pereira, M.; Rocha, S. The effect of a fluorinated cholesterol derivative on the stability and physical properties of cationic DNA vectors. Soft Matter 2013, 9, 401–409. [Google Scholar] [CrossRef]
- de Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef] [Green Version]
- Finegold, L. Cholesterol in Membrane Models; CRC Press: Boca Raton, FL, USA, 1993. [Google Scholar]
- Ege, C.; Ratajczak, M.K.; Majewski, J.; Kjaer, K.; Lee, K.Y.C. Evidence for lipid/cholesterol ordering in model lipid membranes. Biophys. J. 2006, 91, L01–L03. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Adhyapak, P.R.; Panchal, S.V.; Murthy, A.V.R. Cholesterol induced asymmetry in DOPC bilayers probed by AFM force spectroscopy. Biochim. Biophys. Acta Biomembr. 2018, 1860, 953–959. [Google Scholar] [CrossRef]
- Leathes, J.B. Croonian lectures on the rôle of fats in vital phenomena. Lancet 1925, 205, 853–856. [Google Scholar] [CrossRef]
- Dynarowicz-Łątka, P.; Hąc-Wydro, K. Interactions between phosphatidylcholines and cholesterol in monolayers at the air/water interface. Colloids Surf. B Biointerfaces 2004, 37, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Jurak, M. Thermodynamic aspects of cholesterol effect on properties of phospholipid monolayers: Langmuir and Langmuir–blodgett monolayer study. J. Phys. Chem. B 2013, 117, 3496–3502. [Google Scholar] [CrossRef] [PubMed]
- Korchowiec, B.; Paluch, M.; Corvis, Y.; Rogalska, E. A Langmuir film approach to elucidating interactions in lipid membranes: 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine/cholesterol/metal cation systems. Chem. Phys. Lipids 2006, 144, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Miñones, J.; Pais, S.; Miñones, J.; Conde, O.; Dynarowicz-Łatka, P. Interactions between membrane sterols and phospholipids in model mammalian and fungi cellular membranes—A Langmuir monolayer study. Biophys. Chem. 2009, 140, 69–77. [Google Scholar] [CrossRef]
- Sabatini, K.; Mattila, J.-P.; Kinnunen, P.K.J. Interfacial behavior of cholesterol, ergosterol, and lanosterol in mixtures with DPPC and DMPC. Biophys. J. 2008, 95, 2340–2355. [Google Scholar] [CrossRef] [PubMed]
- Savva, M.; Acheampong, S. The interaction energies of cholesterol and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine in spread mixed monolayers at the air-water interface. J. Phys. Chem. B 2009, 113, 9811–9820. [Google Scholar] [CrossRef]
- Zulueta Díaz, Y.D.L.M.; Mottola, M.; Vico, R.V.; Wilke, N.; Fanani, M.L. The rheological properties of lipid monolayers modulate the incorporation of I-ascorbic acid alkyl esters. Langmuir 2016, 32, 587–595. [Google Scholar] [CrossRef]
- Janicka, K.; Jastrzebska, I.; Petelska, A.D. Complex formation equilibria between cholesterol and diosgenin analogues in monolayers determined by the Langmuir method. Biointerphases 2018, 13, 061001. [Google Scholar] [CrossRef]
- Del Castillo-Santaella, T.; Maldonado-Valderrama, J.; Faraudo, J.; Martín-Molina, A. Specific ion effects in cholesterol monolayers. Materials 2016, 9, 340. [Google Scholar] [CrossRef]
- Gupta, R.K.; Suresh, K.A. AFM studies on Langmuir-Blodgett films of cholesterol. Eur. Phys. J. E 2004, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Nava, R.D.; Martin-Mirones, J.M.; Vázquez-Martínez, E.A.; Roca, J.A.; Ruiz-García, J. Direct observations of phase changes in Langmuir films of Cholesterol. Mexicana de Física 2006, 52, 32–40. [Google Scholar]
- Hąc-Wydro, K.; Węder, K.; Mach, M.; Flasiński, M.; Wydro, P. The influence of cholesterol precursor-desmosterol—On artificial lipid membranes. Biochim. Biophys. Acta Biomembr. 2015, 1848, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Wydro, P. Sphingomyelin/phosphatidylcholine/cholesterol monolayers—Analysis of the interactions in model membranes and Brewster Angle Microscopy experiments. Colloids Surf. B Biointerfaces 2012, 93, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Daear, W.; Mahadeo, M.; Prenner, E.J. Applications of Brewster angle microscopy from biological materials to biological systems. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1749–1766. [Google Scholar] [CrossRef] [PubMed]
- Hac-Wydro, K.; Dynarowicz-Latka, P. Biomedical applications of the Langmuir monolayer technique. Ann. UMCS Chem. 2008, 63, 47–60. [Google Scholar] [CrossRef]
- Lopez, C.; Cheng, K.; Perez, J. Thermotropic phase behavior of milk sphingomyelin and role of cholesterol in the formation of the liquid ordered phase examined using SR-XRD and DSC. Chem. Phys. Lipids 2018, 215, 46–55. [Google Scholar] [CrossRef]
- Cheng, K.; Ropers, M.-H.; Lopez, C. The miscibility of milk sphingomyelin and cholesterol is affected by temperature and surface pressure in mixed Langmuir monolayers. Food Chem. 2017, 224, 114–123. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Lopez, C. Cholesterol decreases the size and the mechanical resistance to rupture of sphingomyelin rich domains, in lipid bilayers studied as a model of the milk fat globule membrane. Langmuir 2016, 32, 6757–6765. [Google Scholar] [CrossRef]
- Murthy, A.V.R.; Guyomarc’h, F.; Paboeuf, G.; Vié, V.; Lopez, C. Cholesterol strongly affects the organization of lipid monolayers studied as models of the milk fat globule membrane: Condensing effect and change in the lipid domain morphology. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2308–2316. [Google Scholar] [CrossRef]
- Wydro, P.; Flasiński, M.; Broniatowski, M. Does cholesterol preferentially pack in lipid domains with saturated sphingomyelin over phosphatidylcholine? A comprehensive monolayer study combined with grazing incidence X-ray diffraction and Brewster angle microscopy experiments. J. Colloid Interface Sci. 2013, 397, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mills, T.T.; Huang, J.; Feigenson, G.W.; Nagle, J.F. Effects of cholesterol and unsaturated DOPC lipid on chain packing of saturated gel-phase DPPC bilayers. Gen. Physiol. Biophys. 2009, 28, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Wydro, P. The interactions between cholesterol and phospholipids located in the inner leaflet of humane erythrocytes membrane (DPPE and DPPS) in binary and ternary films—The effect of sodium and calcium ions. Colloids Surf. B Biointerfaces 2011, 82, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Vargas, S.; Martín-Molina, A.; Quesada-Pérez, M.; Barnadas-Rodríguez, R.; Estelrich, J.; Callejas-Fernández, J. Aggregation of liposomes induced by calcium: A structural and kinetic study. Phys. Rev. E 2007, 75, 021912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roldán-Vargas, S.; Barnadas-Rodríguez, R.; Martín-Molina, A.; Quesada-Pérez, M.; Estelrich, J.; Callejas-Fernández, J. Growth of lipid vesicle structures: from surface fractals to mass fractals. Phys. Rev. E 2008, 78, 10902. [Google Scholar] [CrossRef] [PubMed]
- Martín-Molina, A.; Rodríguez-Beas, C.; Faraudo, J. Charge reversal in anionic liposomes: Experimental demonstration and molecular origin. Phys. Rev. Lett. 2010, 104, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Wilke, N.; Maggio, B. Electrostatic field effects on membrane domain segregation and on lateral diffusion. Biophys. Rev. 2011, 3, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Molina, A.; Rodríguez-Beas, C.; Faraudo, J. Effect of calcium and magnesium on phosphatidylserine membranes: experiments and all-atomic simulations. Biophys. J. 2012, 102, 2095–2103. [Google Scholar] [CrossRef]
- Stolarczyk, U.E.; Sidoryk, K.; Cybulski, M.; Kubiszewski, M.; Stolarczyk, K. Design of therapeutic self-assembled monolayers of thiolated abiraterone. Nanomaterials 2018, 8, 1018. [Google Scholar] [CrossRef]
- Martín-Molina, A.; Lue, L.; Quesada-Pérez, M.; Bohinc, K. Interaction between charged lipid vesicles and point- or rod-like trivalent ions. Colloids Surf. B Biointerfaces 2019, 178, 525–529. [Google Scholar] [CrossRef]
- Vashchenko, O.V.; Kasian, N.A.; Budianska, L.V.; Brodskii, R.Y.; Bespalova, I.I.; Lisetski, L.N. Adsorption of ions on model phospholipid membranes. J. Mol. Liq. 2019, 275, 173–177. [Google Scholar] [CrossRef]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Luque-Caballero, G.; Maldonado-Valderrama, J.; Quesada-Pérez, M.; Martín-Molina, A. Interaction of DNA with likely-charged lipid monolayers: An experimental study. Colloids Surf. B Biointerfaces 2019, 178, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tristram-Nagle, S.; Nagle, J.F. Effect of cholesterol on structural and mechanical properties of membranes depends on lipid chain saturation. Phys. Rev. E 2009, 80, 21931. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Steinem, C.; Galla, H.-J.; Janshoff, A. Visualization of chemical and physical properties of calcium-induced domains in DPPC/DPPS Langmuir-Blodgett layers. Langmuir 2001, 17, 2437–2445. [Google Scholar] [CrossRef]
- Luque-Caballero, G.; Martín-Molina, A.; Sánchez-Treviño, A.Y.; Rodríguez-Valverde, M.A.; Cabrerizo-Vílchez, M.A.; Maldonado-Valderrama, J. Using AFM to probe the complexation of DNA with anionic lipids mediated by Ca2+: The role of surface pressure. Soft Matter 2014, 10, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Luque-Caballero, G.; Maldonado-Valderrama, J.; Quesada-Pérez, M.; Martín-Molina, A. Atomic force microscopy as a tool to study the adsorption of DNA onto lipid interfaces. Microsc. Res. Tech. 2017, 80, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, J.M.; Davis-Harrison, R.L.; Clay, M.C.; Nieuwkoop, A.J.; Ohkubo, Y.Z.; Tajkhorshid, E.; Morrissey, J.H.; Rienstra, C.M. Atomic view of calcium-induced clustering of phosphatidylserine in mixed lipid bilayers. Biochemistry 2011, 50, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Lehrmann, R.; Seelig, J. Adsorption of Ca2+ and La3+ to bilayer-membranes—Measurement of the adsorption enthalpy and binding constant with titration calorimetry. Biochim. Biophys. Acta Biomembr. 1994, 1189, 89–95. [Google Scholar] [CrossRef]
- Pandit, S.A.; Chiu, S.-W.; Jakobsson, E.; Grama, A.; Scott, H.L. Cholesterol packing around lipids with saturated and unsaturated chains: A simulation study. Langmuir 2008, 24, 6858–6865. [Google Scholar] [CrossRef]
- Kučerka, N.; Perlmutter, J.D.; Pan, J.; Tristram-Nagle, S.; Katsaras, J.; Sachs, J.N. The effect of cholesterol on short- and long-chain monounsaturated lipid bilayers as determined by molecular dynamics simulations and X-ray scattering. Biophys. J. 2008, 95, 2792–2805. [Google Scholar] [CrossRef] [PubMed]
- Ermilova, I.; Lyubartsev, A.P. Cholesterol in phospholipid bilayers: Positions and orientations inside membranes with different unsaturation degrees. Soft Matter 2019, 15, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Himeno, H.; Shimokawa, N.; Komura, S.; Andelman, D.; Hamada, T.; Takagi, M. Charge-induced phase separation in lipid membranes. Soft Matter 2014, 10, 7959–7967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casper, C.B.; Verreault, D.; Adams, E.M.; Hua, W.; Allen, H.C. Surface potential of DPPC monolayers on concentrated aqueous salt solutions. J. Phys. Chem. B 2016, 120, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Schultz, Z.D.; Pazos, I.M.; McNeil-Watson, F.K.; Lewis, E.N.; Levin, I.W. Magnesium-induced lipid bilayer microdomain reorganizations: Implications for membrane fusion. J. Phys. Chem. B 2009, 113, 9932–9941. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Silvius, J.R. Effects of cholesterol on the divalent cation-mediated interactions of vesicles containing amino and choline phospholipids. Biochim. Biophys. Acta Biomembr. 1987, 905, 81–90. [Google Scholar] [CrossRef]


| Sample | DPPC | DPPS | CHOL | DPPC:DPPS:CHOL |
|---|---|---|---|---|
| DPPC:DPPS 4:1 | 0.8 | 0.2 | 0 | 0.8:0.2:0 |
| DPPC:DPPS:CHOL30 | 0.56 | 0.14 | 0.3 | 0.56:0.14:0.3 |
| DPPC:DPPS:CHOL70 | 0.24 | 0.06 | 0.7 | 0.24:0.06:0.7 |
| CHOL | 0 | 0 | 1 | 0:0:1 |
| Sample | LMA | πcoll | ε0 (mN/m) |
|---|---|---|---|
| DPPC:DPPS 4:1 | 52 ± 5 | 59 ± 5 | 134 ± 20 |
| DPPC:DPPS:CHOL-30 | 48 ± 5 | 59 ± 3 | 150 ± 10 |
| DPPC:DPPS:CHOL-70 | 40 ± 2 | 53 ± 4 | 180 ± 25 |
| CHOL | 40 ± 2 | 44 ± 2 | 245 ± 20 |
| Sample | LMA | πcoll | ε0 (mN/m) |
|---|---|---|---|
| DPPC:DPPS 4:1 | 55 ± 1 | 52 ± 1 | 190 ± 10 |
| DPPC:DPPS:CHOL-30 | 48 ± 2 | 42 ± 2 | 150 ± 15 |
| DPPC:DPPS:CHOL-70 | 45 ± 2 | 38 ± 2 | 210 ± 15 |
| CHOL | 40 ± 2 | 46 ± 1 | 230 ± 15 |
| Sample | LMA | πcoll | ε0 (mN/m) |
|---|---|---|---|
| DPPC:DPPS 4:1 | 50 ± 5 | 64 ± 1 | 174 ± 20 |
| DPPC:DPPS:CHOL-30 | 55 ± 5 | 50 ± 1 | 180 ± 20 |
| DPPC:DPPS:CHOL-70 | 56 ± 5 | 50 ± 2 | 80 ± 20 |
| CHOL | 45 ± 1 | 46 ± 1 | 140 ± 20 |
| Sample | LMA | πcoll | ε0 (mN/m) |
|---|---|---|---|
| DPPC:DPPS 4:1 | 85 ± 3 | 47 ± 5 | 115 ± 20 |
| DPPC:DPPS:CHOL-30 | 64 ± 1 | 50 ± 5 | 100 ± 20 |
| DPPC:DPPS:CHOL-70 | 45 ± 2 | 45 ± 5 | 170 ± 20 |
| CHOL | 40 ± 1 | 45 ± 1 | 150 ± 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-Molina, A.; Del Castillo-Santaella, T.; Yang, Y.; Maldonado-Valderrama, J. Condensation of Model Lipid Films by Cholesterol: Specific Ion Effects. Coatings 2019, 9, 474. https://doi.org/10.3390/coatings9080474
Martín-Molina A, Del Castillo-Santaella T, Yang Y, Maldonado-Valderrama J. Condensation of Model Lipid Films by Cholesterol: Specific Ion Effects. Coatings. 2019; 9(8):474. https://doi.org/10.3390/coatings9080474
Chicago/Turabian StyleMartín-Molina, Alberto, Teresa Del Castillo-Santaella, Yan Yang, and Julia Maldonado-Valderrama. 2019. "Condensation of Model Lipid Films by Cholesterol: Specific Ion Effects" Coatings 9, no. 8: 474. https://doi.org/10.3390/coatings9080474
APA StyleMartín-Molina, A., Del Castillo-Santaella, T., Yang, Y., & Maldonado-Valderrama, J. (2019). Condensation of Model Lipid Films by Cholesterol: Specific Ion Effects. Coatings, 9(8), 474. https://doi.org/10.3390/coatings9080474







