Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Microcapsules
2.3. Preparation of Coatings
2.4. Test and Characterization
3. Results and Discussion
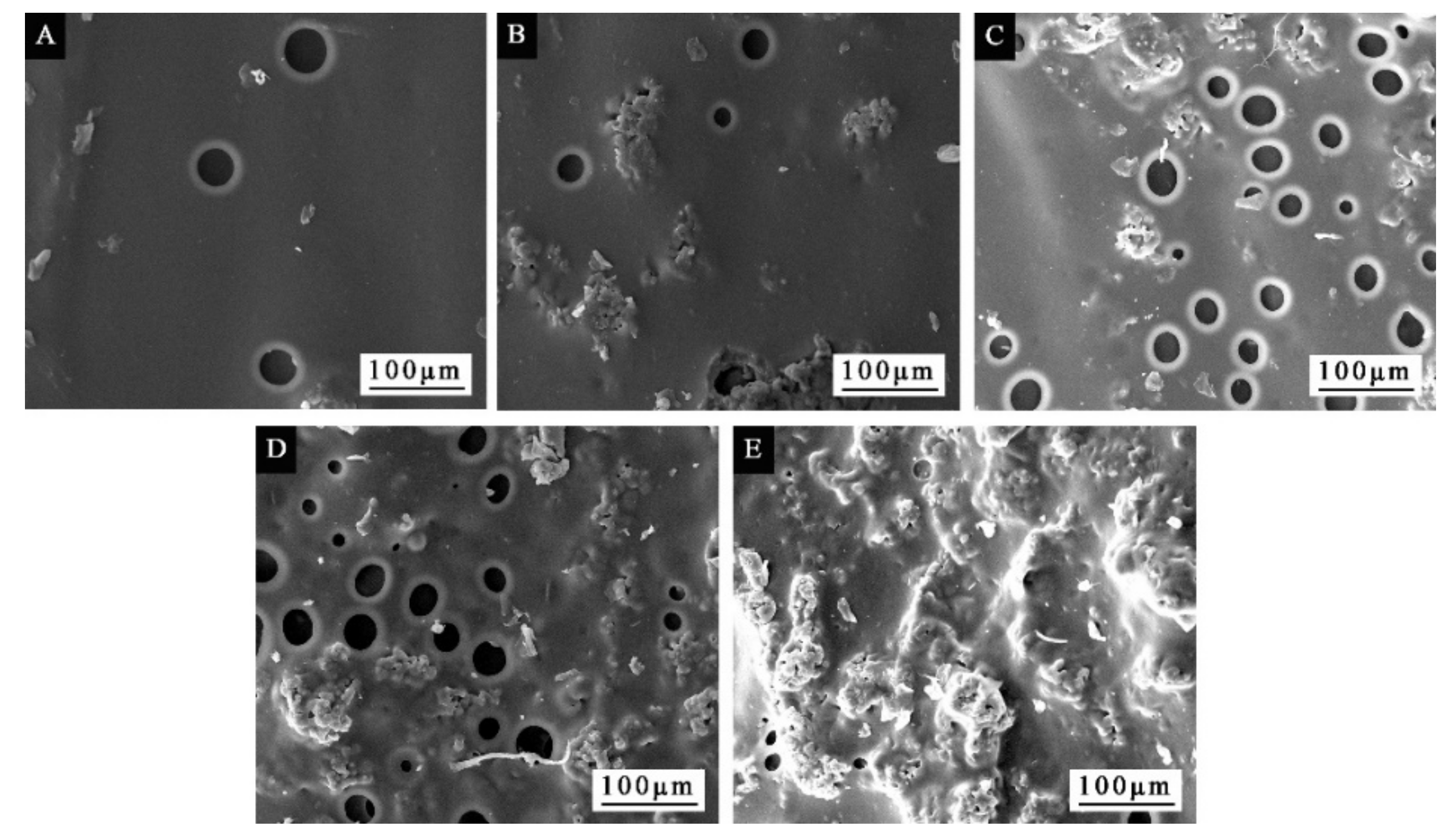
3.1. Morphological Analysis of Microcapsules
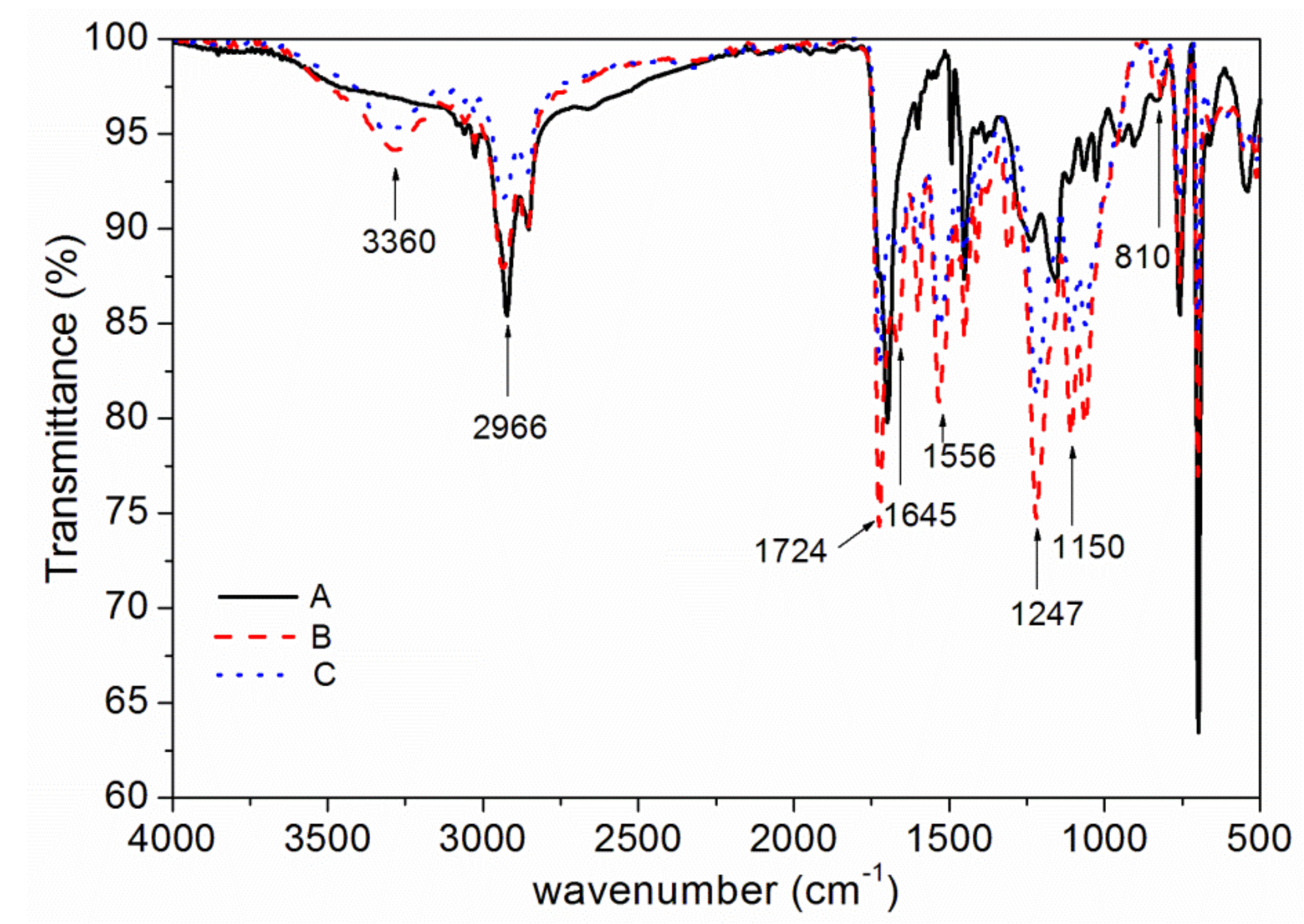
3.2. Chemical Composition Analysis
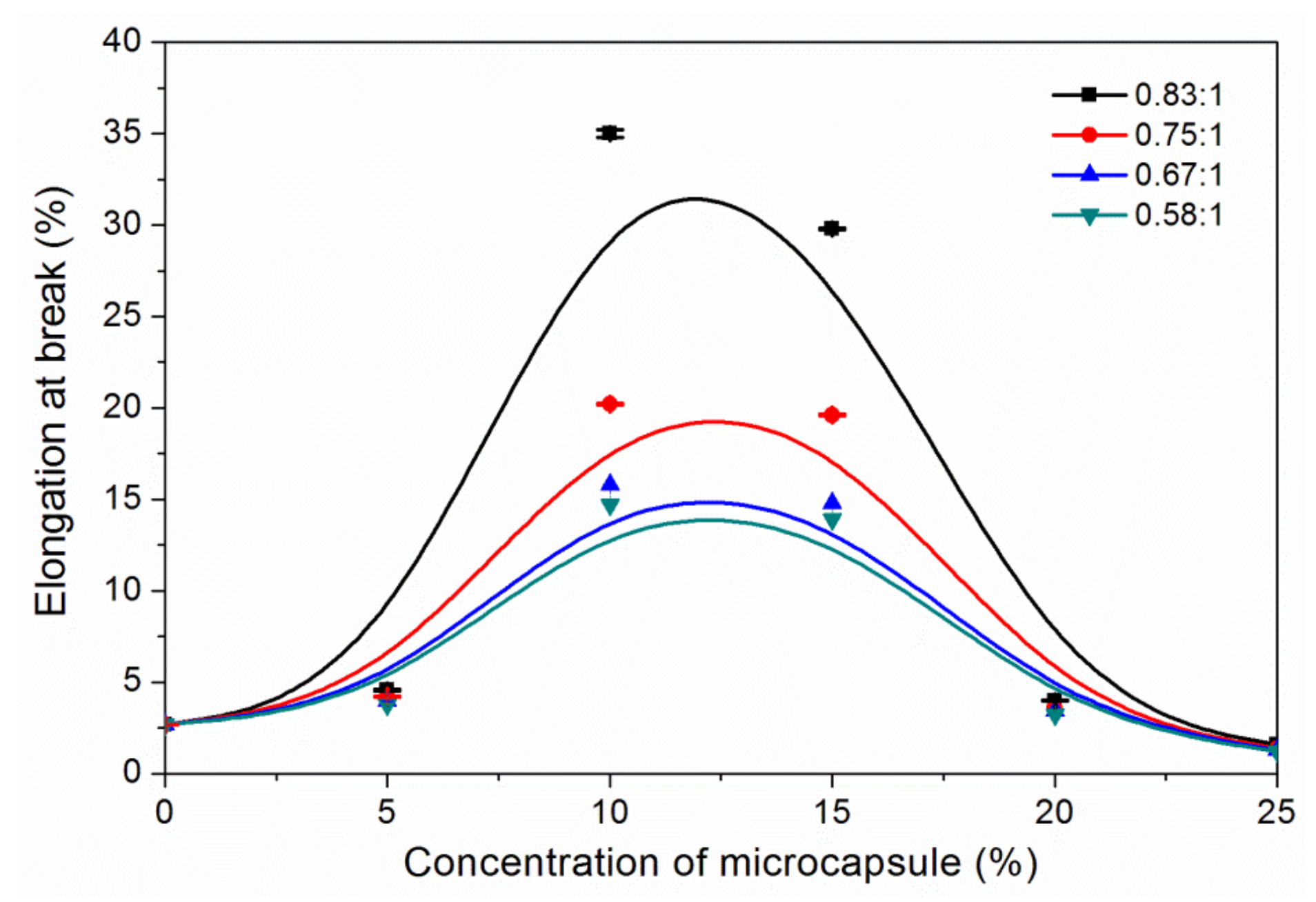
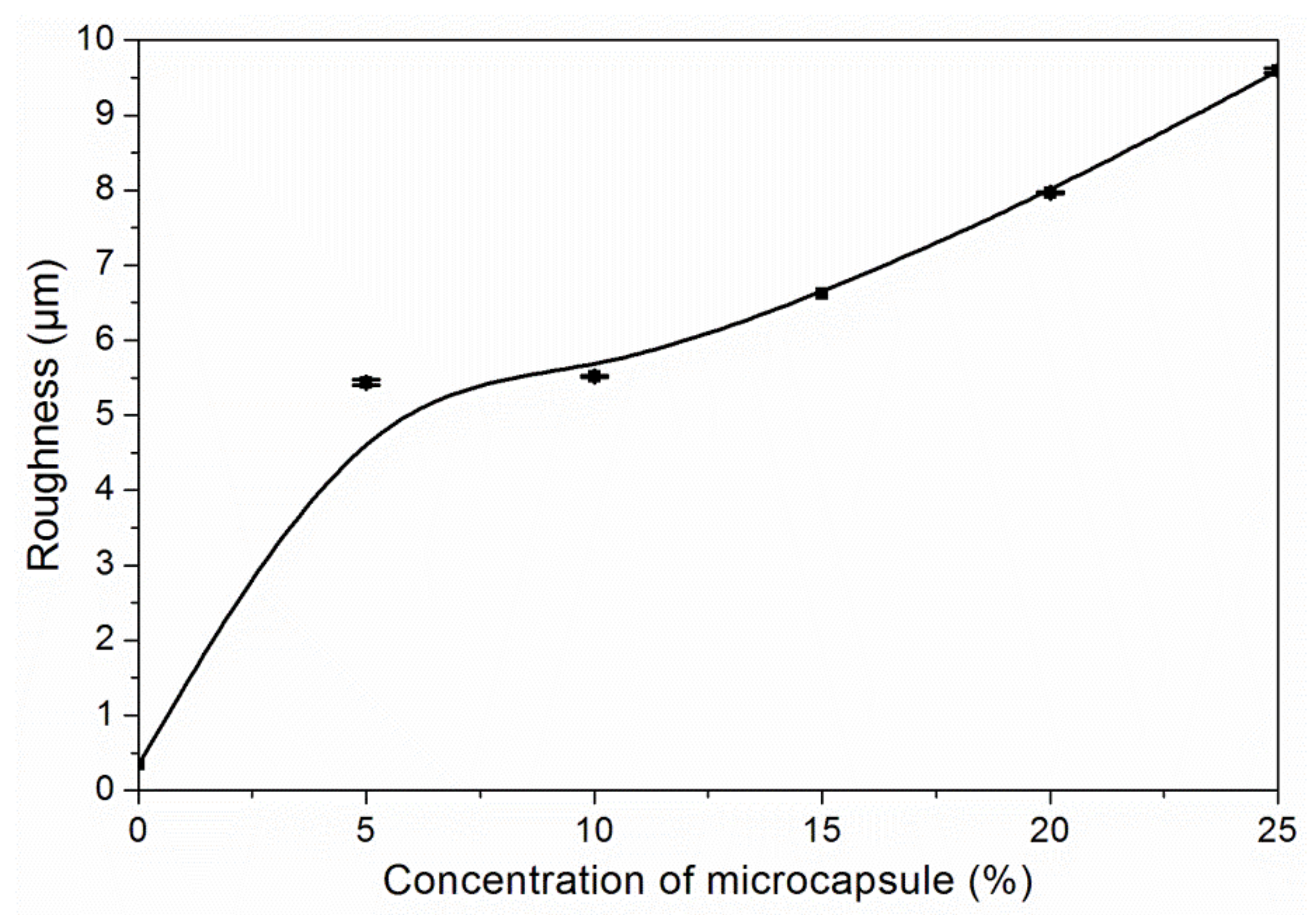
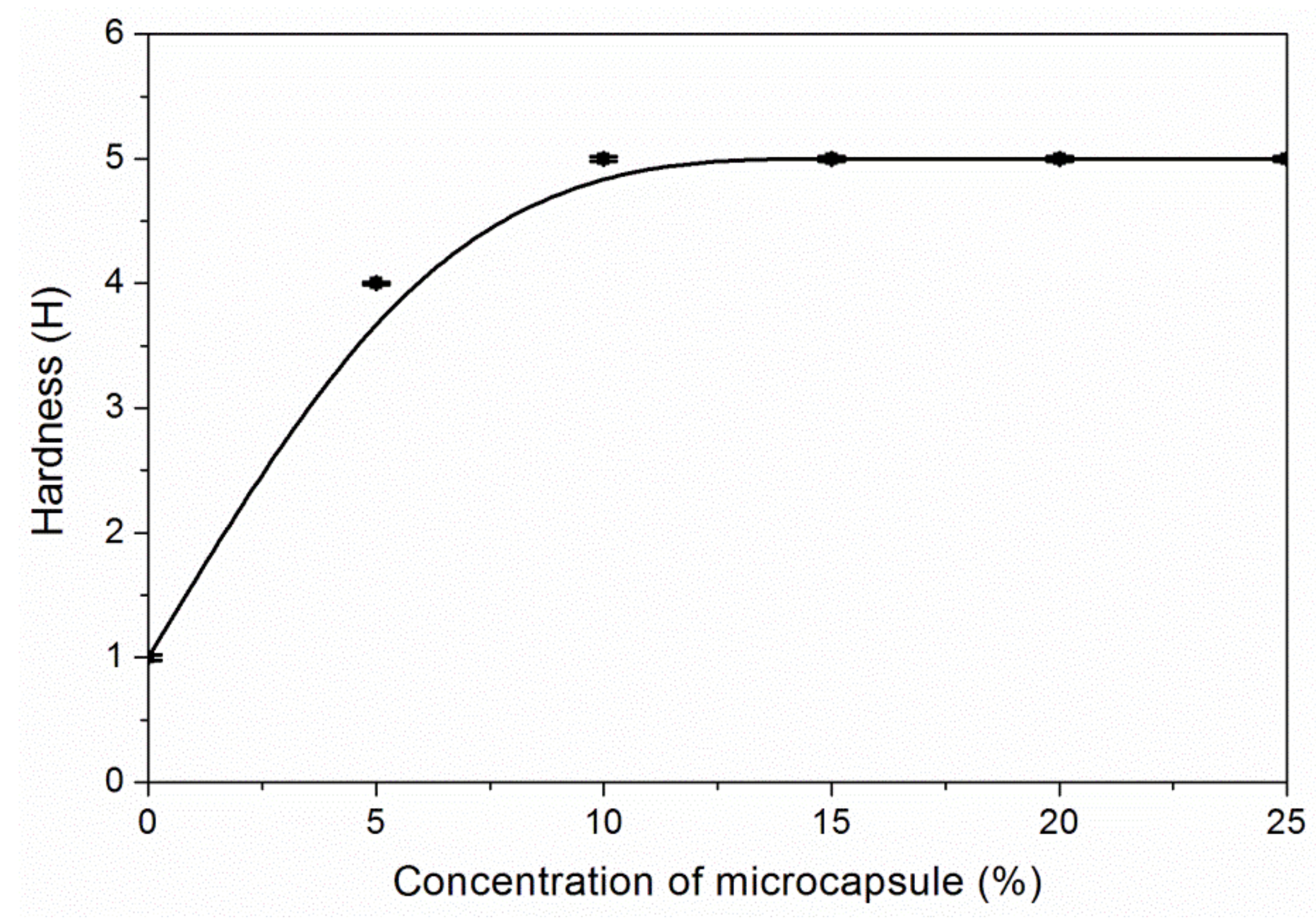
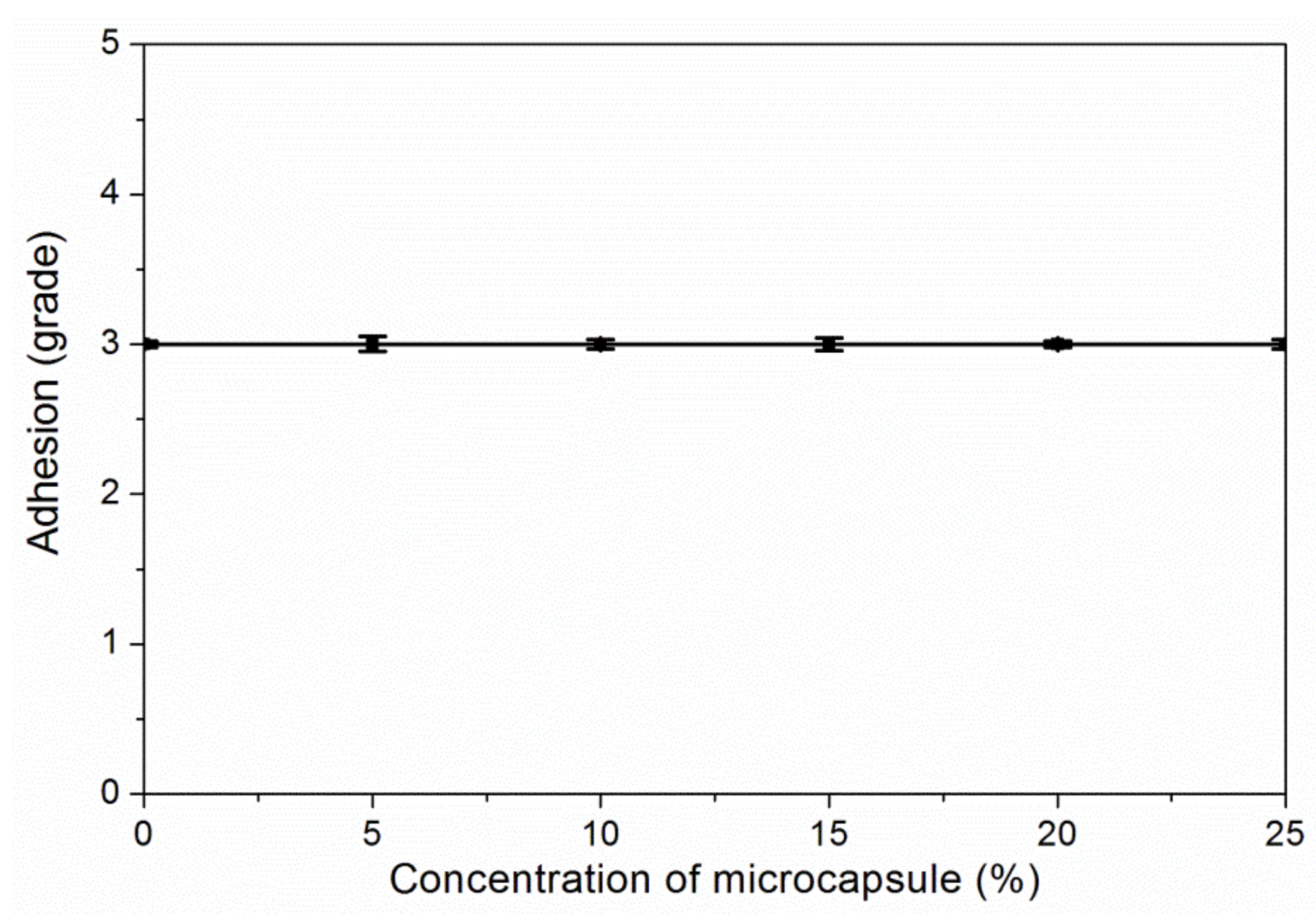
3.3. The Properties of Waterborne Coatings after Adding Microcapsules
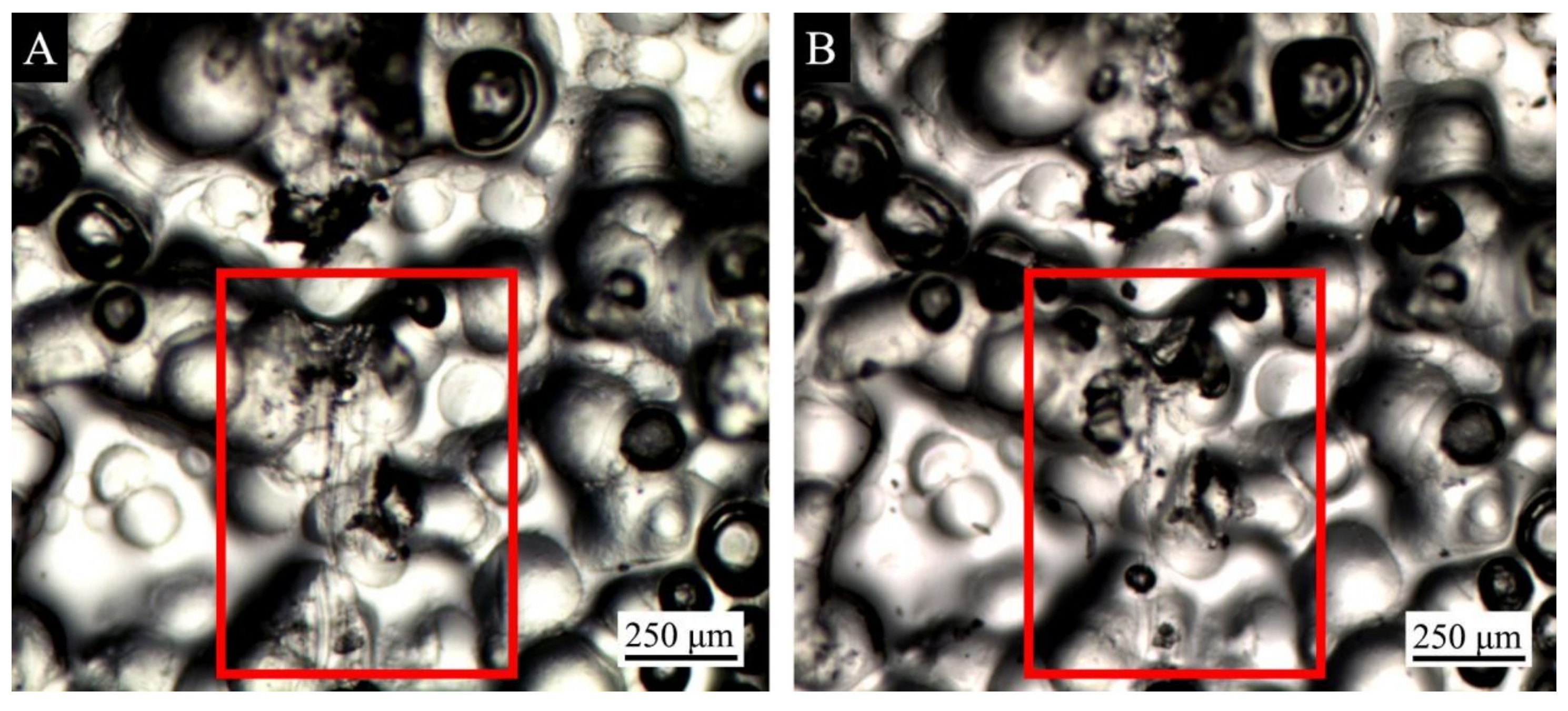
3.4. Repair Test of Waterborne Coating with the Microcapsule
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, W.; Wu, Z.H.; Zhang, J.L. Compressive creep and recovery behaviors of seat cushions in upholstered furniture. Wood Fiber Sci. 2015, 47, 431–444. [Google Scholar]
- Gu, Y.T.; Bian, H.Y.; Wei, L.Q.; Wang, R.B. Enhancement of hydrotropic fractionation of poplar wood using autohydrolysis and disk refining pretreatment: morphology and overall chemical characterization. Polymers 2019, 11, 685. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.Q.; Bao, Y.L.; Guo, W.J.; Fang, L.; Wu, Z.H. Preparation and application of high performance corn starch glue in straw decorative panel. Wood Fiber Sci. 2018, 50, 88–95. [Google Scholar]
- Wu, J.M.; Wu, Y.; Yang, F.; Tang, C.Y.; Huang, Q.T.; Zhang, J.L. Impact of delignification on morphological, optical and mechanical properties of transparent wood. Compos. Part A: Appl. Sci. Manuf. 2019, 117, 324–331. [Google Scholar] [CrossRef]
- Liu, H.J.; Li, C.; Sun, X.S. Soy-oil-based waterborne polyurethane improved wet strength of soy protein adhesives on wood. Int. J. Adhes. Adhes. 2017, 73, 66–74. [Google Scholar] [CrossRef] [Green Version]
- Herrera, R.; Muszynska, M.; Krystofiak, T.; Labidi, J. Comparative evaluation of different thermally modified wood samples finishing with UV-curable and waterborne coatings. Appl. Surf. Sci. 2015, 357, 1444–1453. [Google Scholar] [CrossRef]
- Yan, X.X.; Qian, X.Y.; Lu, R.; Miyakoshi, T. Synergistic effect of addition of fillers on properties of interior waterborne UV-curing wood coatings. Coatings 2018, 8, 9. [Google Scholar] [CrossRef]
- Zhang, S.W.; Yu, A.X.; Song, X.Q.; Liu, X.Y. Synthesis and characterization of waterborne UV-curable polyurethane nanocomposites based on the macromonomer surface modification of colloidal silica. Prog. Org. Coat. 2013, 76, 1032–1039. [Google Scholar] [CrossRef]
- Yan, X.X.; Qian, X.Y.; Lu, R.; Miyakoshi, T. Comparison and optimization of reactive dyes and coating performance on Fraxinus mandshurica veneer. Polymers 2018, 10, 1302. [Google Scholar] [CrossRef]
- Jang, G.; Seo, S.; Lee, T.S. Electrostatically self-assembled microcapsule composed of conjugated polyelectrolytes and polypeptides for an emission color-changeable assay for trypsin. Sens. Actuators B 2015, 221, 1229–1235. [Google Scholar] [CrossRef]
- Liao, H.Z.; Zhang, B.; Huang, L.H.; Ma, D.; Jiao, Z.P.; Xie, Y.S.; Tan, S.Z.; Cai, X. The utilization of carbon nitride to reinforce the mechanical and thermal properties of UV-curable waterborne polyurethane acrylate coatings. Prog. Org. Coat. 2015, 89, 35–41. [Google Scholar] [CrossRef]
- Song, J.H.; Cui, X.F.; Liu, Z.; Jin, G.; Liu, E.B.; Zhang, D.; Gao, Z.H. Advanced microcapsules for self-healing conversion coating on magnesium alloy in Ce(NO3)3 solution with microcapsules containing La(NO3)3. Surf. Coat. Technol. 2016, 307, 500–505. [Google Scholar] [CrossRef]
- Guo, W.C.; Jia, Y.; Tian, K.S.; Xu, Z.P.; Jiao, J.; Li, R.F.; Wu, Y.H.; Cao, L.; Wang, H.Y. UV-Triggered self-healing of a single robust SiO2 microcapsule based on cationic polymerization for potential application in aerospace coatings. ACS Appl. Mater. Interfaces 2016, 8, 21046–21054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.R.; Zhou, Q.X. Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent. Prog. Org. Coat. 2018, 125, 403–410. [Google Scholar] [CrossRef]
- Li, W.T.; Zhu, X.J.; Zhao, N.; Jiang, Z.W. Preparation and properties of melamine urea-formaldehyde microcapsules for self-healing of cementitious materials. Materials 2016, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, M.; Kumar, D.; Roy, P.K. Microencapsulation of reactive amine by interfacially engineered epoxy microcapsules for smart applications. Iran. Polym. J. 2017, 26, 489–497. [Google Scholar] [CrossRef]
- Cai, X.L.; Fu, D.T.; Qu, A.L. Effects of processing conditions on the properties of epoxy resin microcapsule. J. Wuhan Univ. Technol. 2015, 30, 689–694. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L.; Qian, X.Y. Effect of urea-formaldehyde-coated epoxy microcapsule modification on gloss, toughness and chromatic distortion of acrylic copolymers waterborne coating. Coatings 2019, 9, 239. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.P.; Liu, W.L.; Guo, F.C.; Pei, J.Z.; Zhu, C.Z.; Zhang, W.W. Preparation and characterization of self-healing microcapsules embedding waterborne epoxy resin and curing agent for asphalt materials. Constr. Build. Mater. 2018, 183, 384–394. [Google Scholar] [CrossRef]
- Hillewaere, X.K.D.; Teixeira, R.F.A.; Nguyen, L.T.T.; Ramos, J.A.; Rahier, H.; Du Prez, F.E. Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry. Adv. Funct. Mater. 2014, 24, 5575–5583. [Google Scholar] [CrossRef]
- Sun, D.W.; Zhang, H.; Tang, X.Z.; Yang, J.L. Water resistant reactive microcapsules for self-healing coatings in harsh environments. Polymer 2016, 91, 33–40. [Google Scholar] [CrossRef]
- Bolimowski, P.A.; Kozera, R.; Boczkowska, A. Poly (urea-formaldehyde) microcapsules-synthesis and influence of stirring speed on capsules size. Polimery 2018, 63, 339–346. [Google Scholar] [CrossRef]
- Safaei, F.; Khorasani, S.N.; Rahnama, H.; Neisiany, R.E.; Koochaki, M.S. Single microcapsules containing epoxy healing agent used for development in the fabrication of cost efficient self-healing epoxy coating. Prog. Org. Coat. 2018, 114, 40–46. [Google Scholar] [CrossRef]
- Sadrabadi, T.E.; Allahkaram, S.R.; Staab, T.; Towhidi, N. Preparation and characterization of durable micro/nanocapsules for use in self-healing anticorrosive coatings. Polym. Sci. Ser. B 2017, 59, 281–291. [Google Scholar] [CrossRef]
- Comlekci, G.K.; Ulutan, S. Encapsulation of linseed oil and linseed oil based on alkyd resin by urea formaldehyde shell for self-healing systems. Prog. Org. Coat. 2018, 121, 190–200. [Google Scholar] [CrossRef]
- Fereidoon, A.; Ahangari, M.G.; Jahanshahi, M. Effect of nanoparticles on the morphology and thermal properties of self-healing poly(urea-formaldehyde) microcapsules. J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
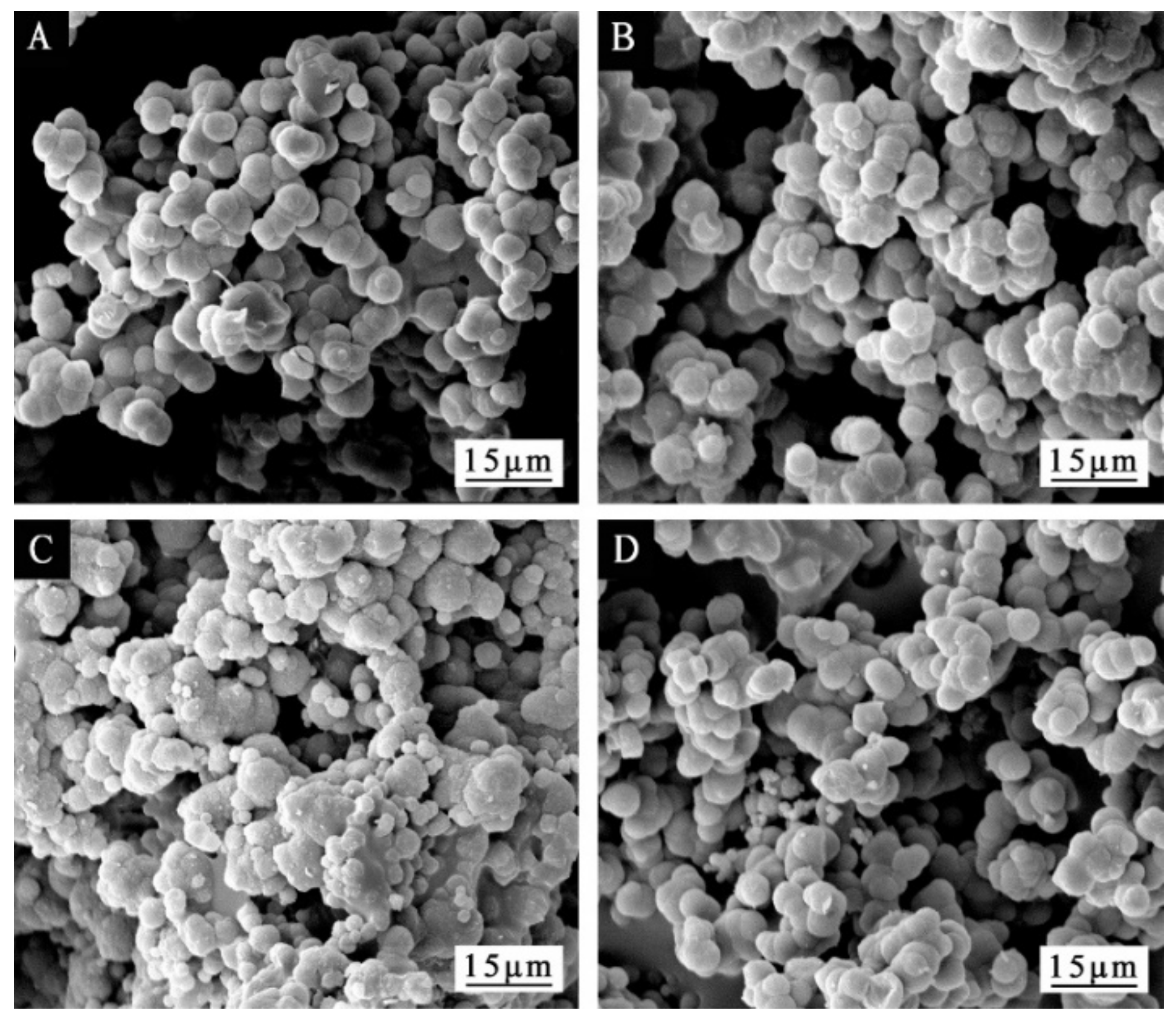




| Sample | Sodium Dodecyl Benzene Sulfonate/g | Deionized Water/g | Epoxy Resin/g | Urea/g | Formaldehyde Solution/g | Mass Ratio of Core to Wall Material |
|---|---|---|---|---|---|---|
| 1 | 1.95 | 193.05 | 25.0 | 20.0 | 34.0 | 0.83:1 |
| 2 | 1.76 | 174.24 | 22.5 | 20.0 | 34.0 | 0.75:1 |
| 3 | 1.56 | 154.44 | 20.0 | 20.0 | 34.0 | 0.67:1 |
| 4 | 1.37 | 135.63 | 17.5 | 20.0 | 34.0 | 0.58:1 |
| Sample | Concentration (%) | Microcapsules (g) | Waterborne Coatings (g) |
|---|---|---|---|
| 1 | 0 | 0 | 100.0 |
| 2 | 5.0 | 5.0 | 95.0 |
| 3 | 10.0 | 10.0 | 90.0 |
| 4 | 15.0 | 15.0 | 85.0 |
| 5 | 20.0 | 20.0 | 80.0 |
| 6 | 25.0 | 25.0 | 75.0 |
| Peak (cm−1) | Assignment |
|---|---|
| 3360 | absorption of N–H |
| 2966 | absorption of C–H |
| 1645,1724 | absorption of C=O |
| 1556 | absorption of C–N |
| 1247 | symmetrical vibration absorption of epoxy group |
| 810 | absorption of C=C–H |
| 1150 | absorption of C–O–C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Qian, X.; Chang, Y. Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings. Coatings 2019, 9, 475. https://doi.org/10.3390/coatings9080475
Yan X, Qian X, Chang Y. Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings. Coatings. 2019; 9(8):475. https://doi.org/10.3390/coatings9080475
Chicago/Turabian StyleYan, Xiaoxing, Xingyu Qian, and Yijuan Chang. 2019. "Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings" Coatings 9, no. 8: 475. https://doi.org/10.3390/coatings9080475
APA StyleYan, X., Qian, X., & Chang, Y. (2019). Preparation and Characterization of Urea Formaldehyde @ Epoxy Resin Microcapsule on Waterborne Wood Coatings. Coatings, 9(8), 475. https://doi.org/10.3390/coatings9080475




