Microstructure and Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of M3B2-Based Cermet Coatings
2.2. Characterization of M3B2-Based Cermet Coatings
3. Results
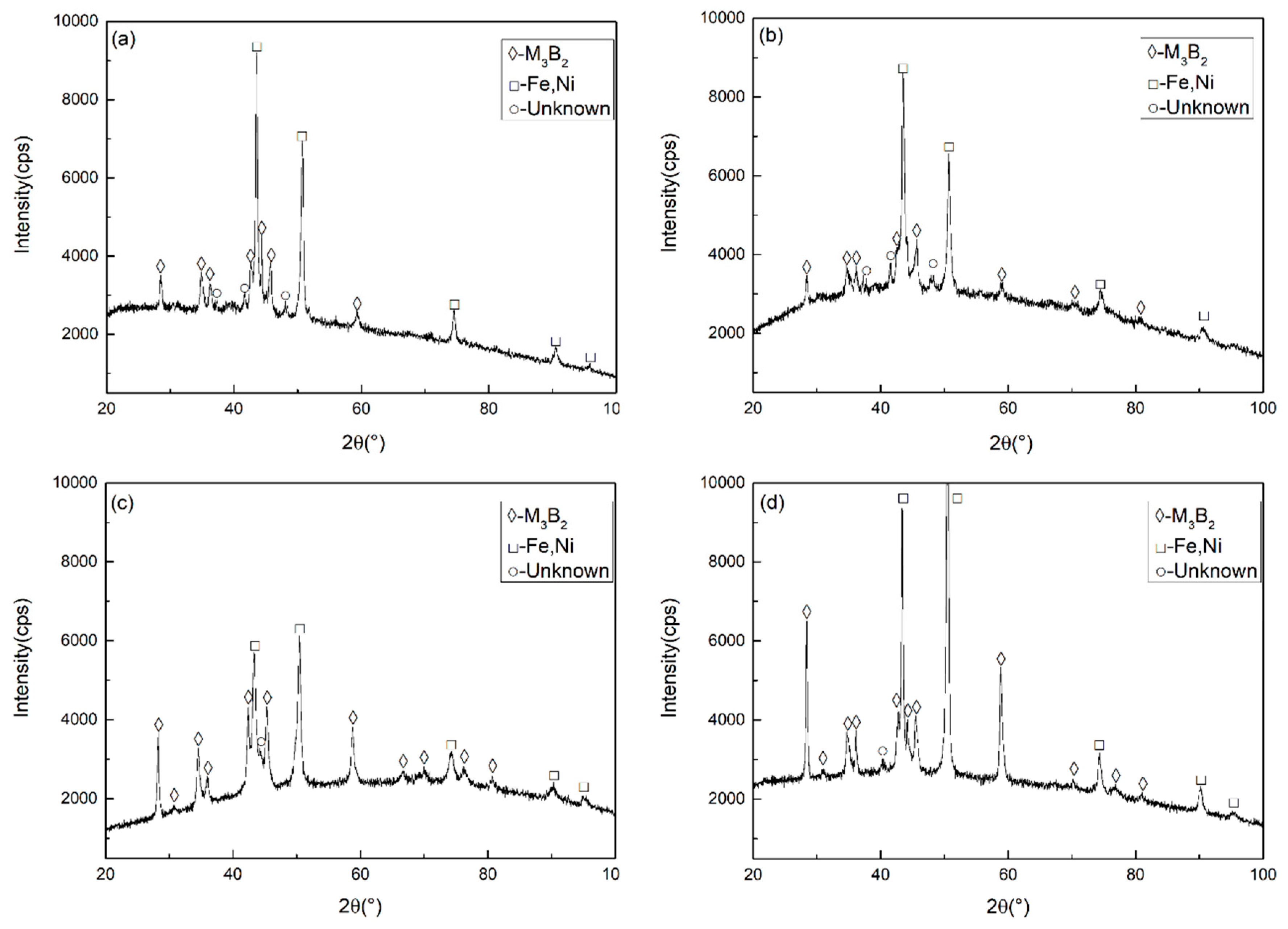
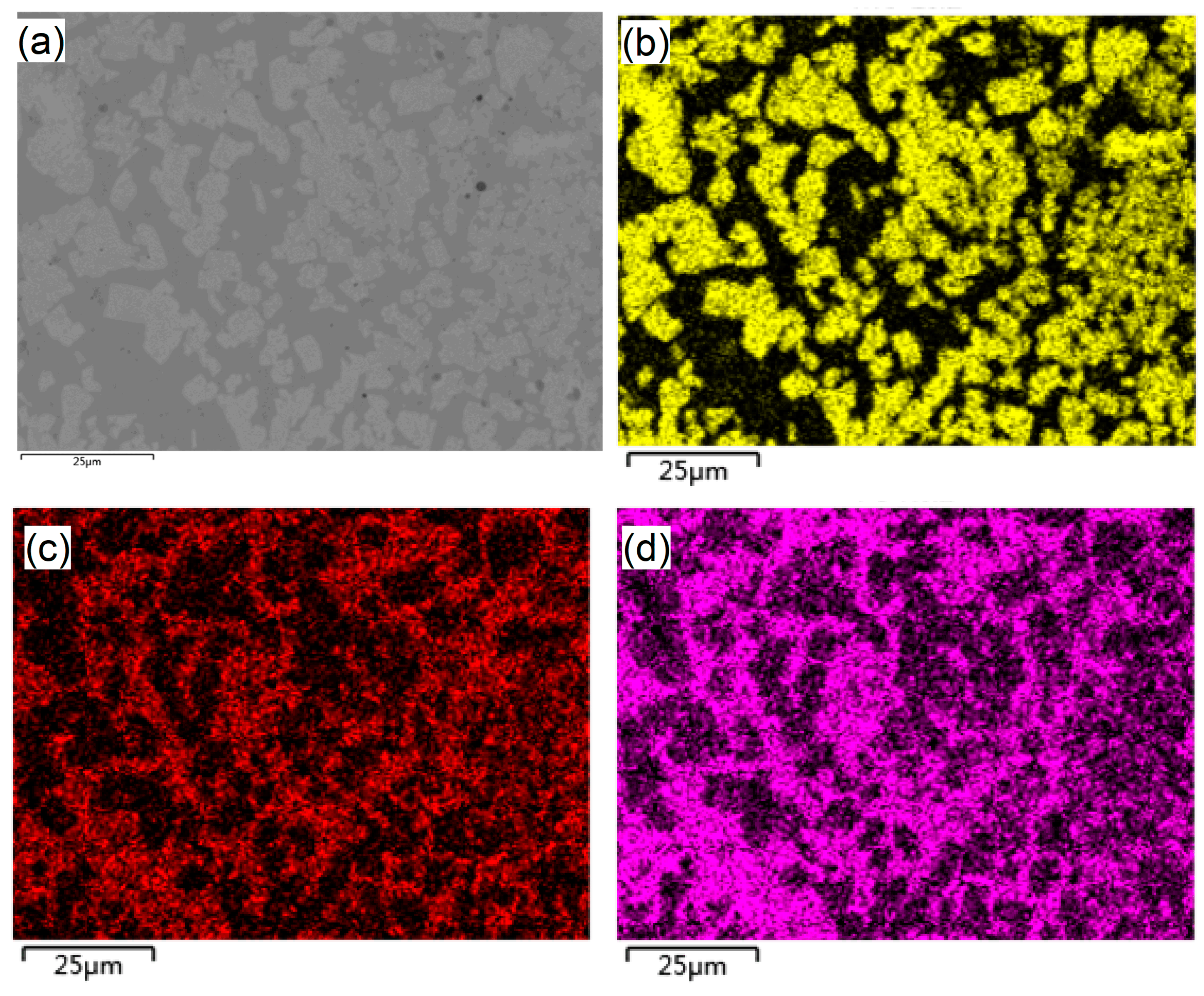
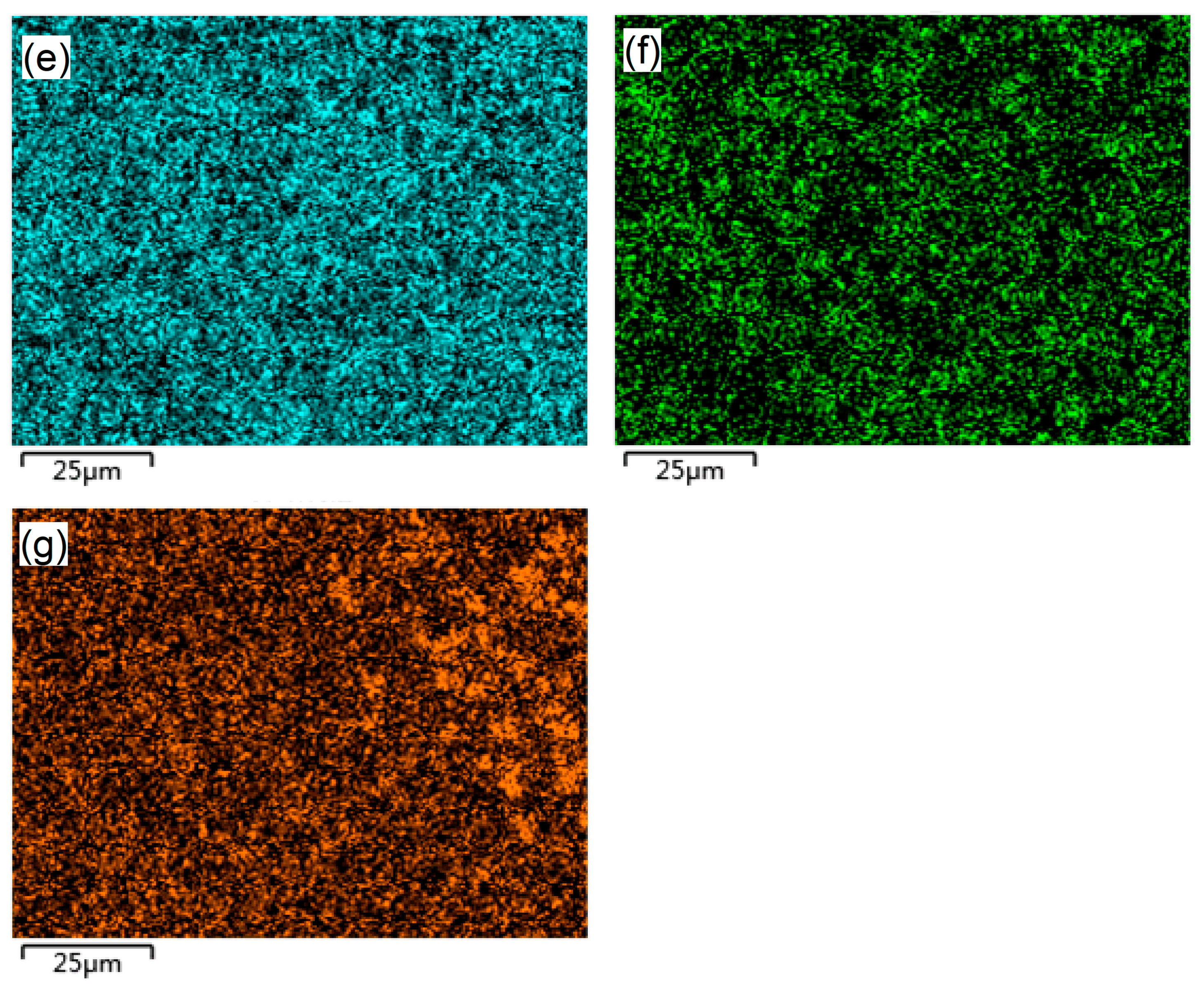
3.1. Microstructure and Composition of M3B2-Based Cermet Coatings
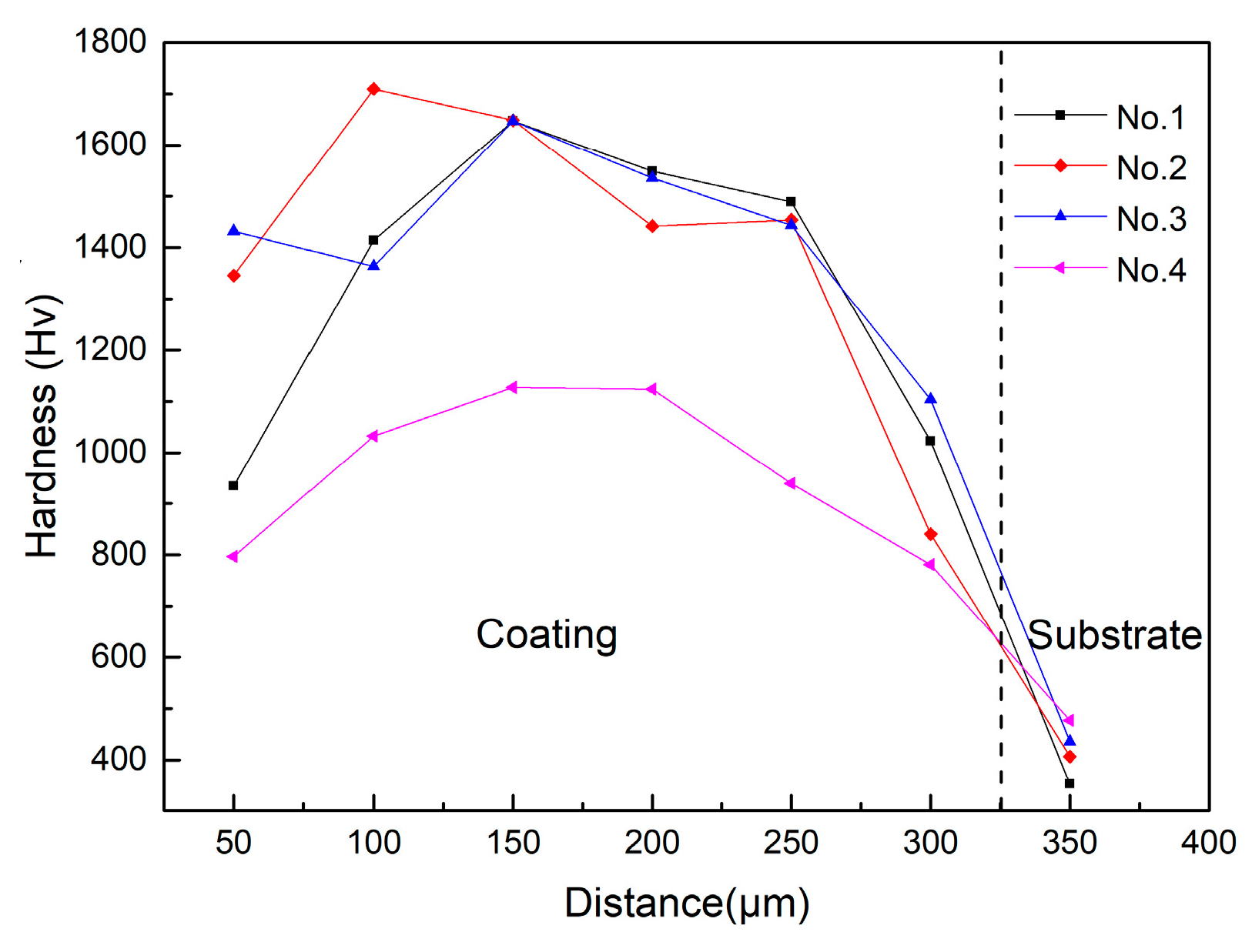
3.2. Hardness
3.3. Corrosion tests
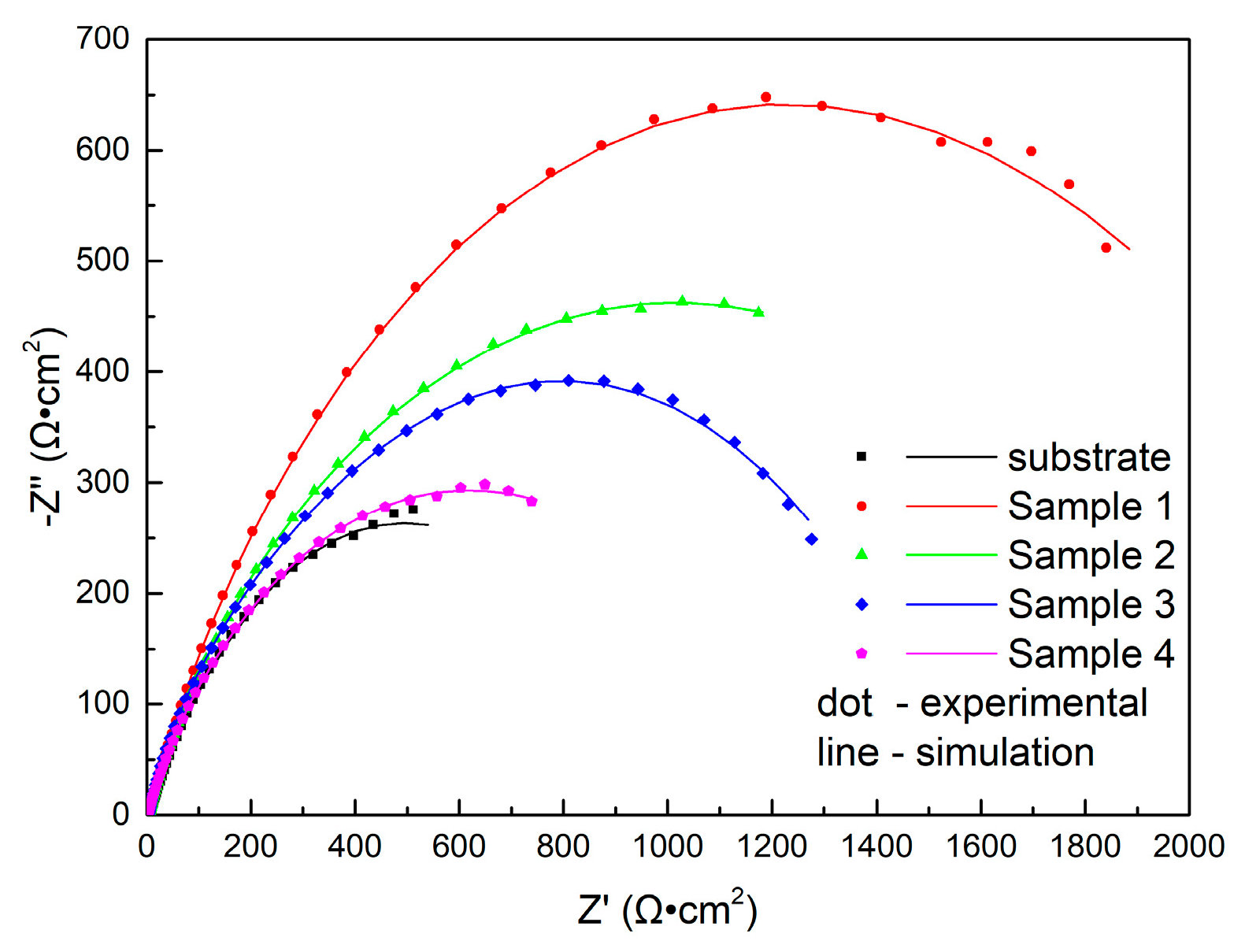
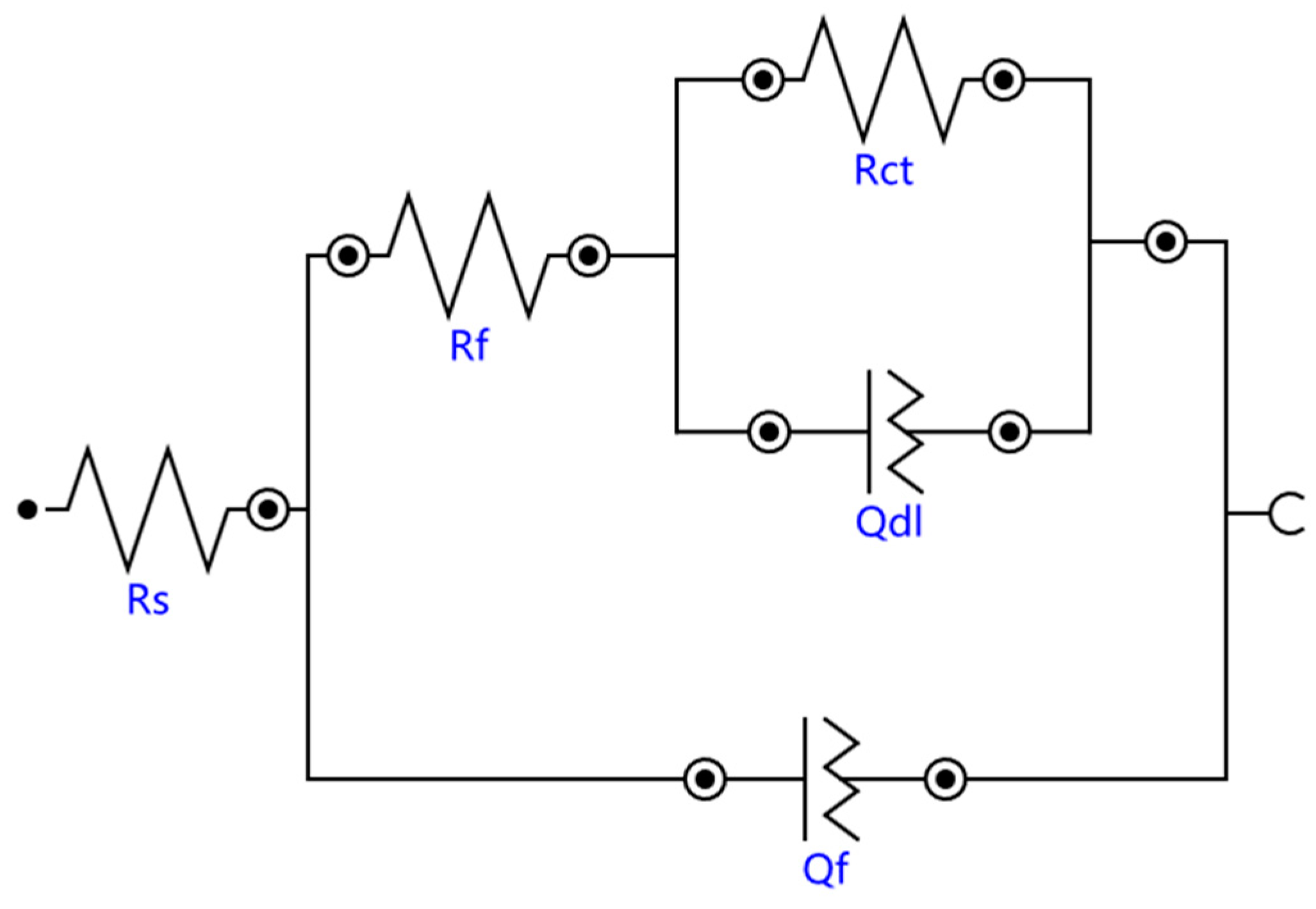
3.3.1. Electrochemical Impedance Spectroscopy
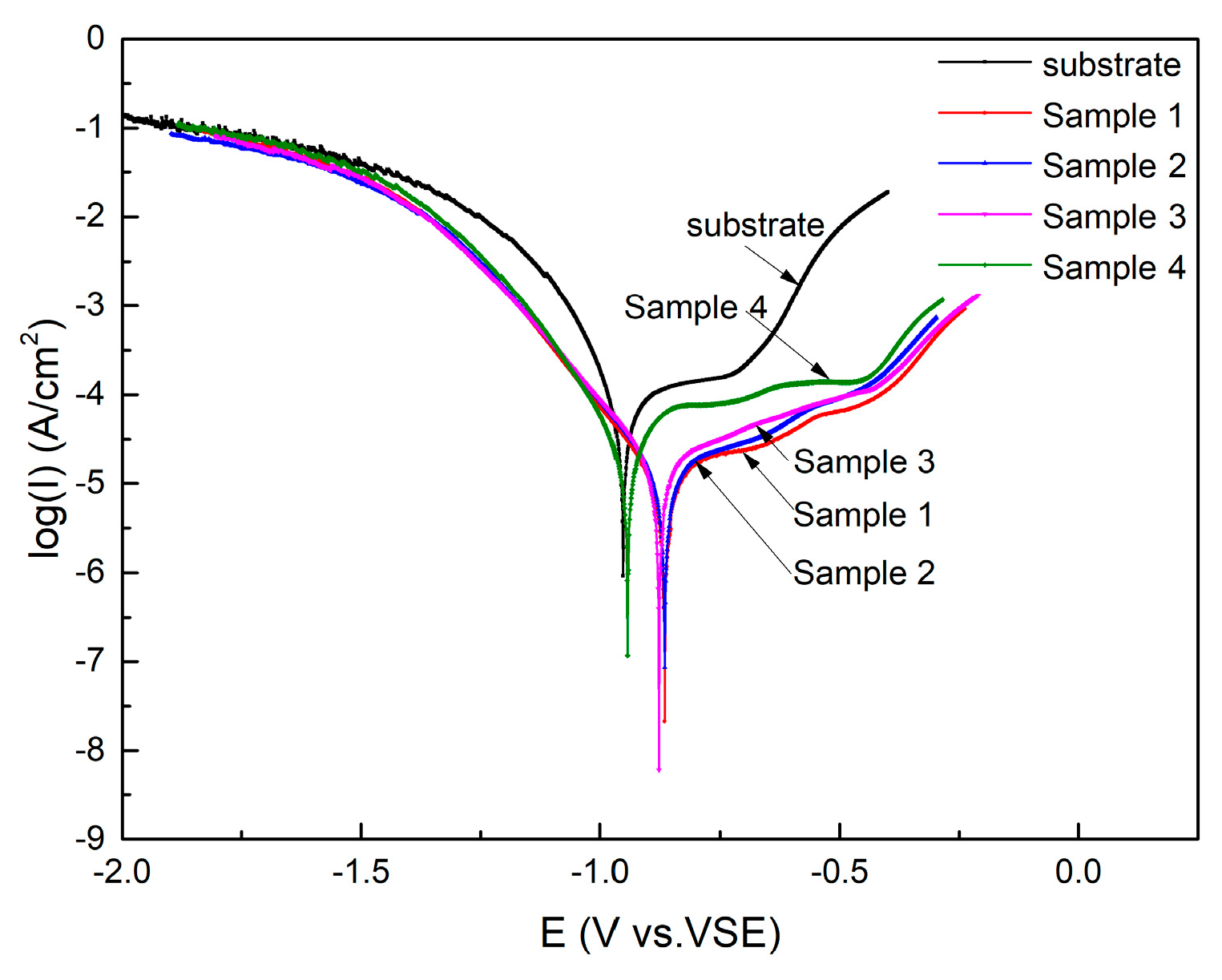
3.3.2. Polarization Curves
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.G.; Du, M.; Zhang, J.; Qiu, M. Electrochemical behavior of Q235 steel in saltwater saturated with carbon dioxide based on new imidazoline derivative inhibitor. Corros. Sci. 2009, 51, 102–109. [Google Scholar] [CrossRef]
- Han, E.; Chen, J.; Su, Y.; Liu, M. Corrosion protection techniques of marine engineering structure and ship equipment- current status and future trend. Mater. Chin. 2014, 33, 65–76. [Google Scholar] [CrossRef]
- Wang, H.Q.; Sun, J.S.; Li, C.N.; Geng, S.N.; Sun, H.G.; Wang, G.L. Microstructure and mechanical properties of molybdenum–iron–boron–chromium cladding using argon arc welding. Mater. Sci. Technol. 2016, 32, 1694–1701. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, H.; Zhang, C.; Zhang, R.; Xue, J.; Zheng, Q.; Qu, X. First-principles study on the mechanical properties and electronic structure of V doped WCoB and W2CoB2 ternary borides. Materials 2019, 12, 967. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, D.; Zheng, W.; Ma, Y.; Chen, C. Global structural optimization of tungsten borides. Phys. Rev. Lett. 2013, 110, 136403. [Google Scholar] [CrossRef]
- Bahrami-Karkevandi, M.; Ebrahimi-Kahrizsangi, R.; Nasiri-Tabrizi, B. Formation and stability of tungsten boride nanocomposites in WO3–B2O3–Mg ternary system: Mechanochemical effects. Int. J. Refract. Met. Hard Mater 2014, 46, 117–124. [Google Scholar] [CrossRef]
- Kadri, M.T.; Heciri, D.; Derradji, N.; Belfarhi, B.; Belkhir, H. Effects of Na, Mg and Al substitution in hypothetical superconducting Be2B. Phys. Status Solidi B 2008, 245, 2779–2785. [Google Scholar] [CrossRef]
- Togano, K.; Badica, P.; Nakamori, Y.; Orimo, S.; Takeya, H.; Hirata, K. Superconductivity in metal rich Li-Pd-B ternary boride. Phys. Rev. Lett. 2004, 93, 247004. [Google Scholar] [CrossRef]
- Prakash, S.; Karacor, M.; Banerjee, S. Surface modification in microsystems and nanosystems. Surf. Sci. Rep. 2009, 64, 233–254. [Google Scholar] [CrossRef]
- Kayhan, M.; Hildebrandt, E.; Frotscher, M.; Senyshyn, A.; Hofmann, K.; Alff, L.; Albert, B. Neutron diffraction and observation of superconductivity for tungsten borides, WB and W2B4. Solid State Sci. 2012, 14, 1656–1659. [Google Scholar] [CrossRef]
- Moraes, V.; Riedl, H.; Fuger, C.; Polcik, P.; Bolvardi, H.; Holec, D.; Mayrhofer, P. Ab initio inspired design of ternary boride thin films. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Takagi, K.-I. Development and application of high strength ternary boride base cermets. J. Solid State Chem. 2006, 179, 2809–2818. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, G.-J.; Kan, Y.-M.; Wang, P.-L. Reactive synthesis and mechanical properties of Mo2NiB2 based hard alloy. Int. J. Refract. Met. Hard Mater. 2010, 28, 291–296. [Google Scholar] [CrossRef]
- Takagi, K.-I.; Koike, W.; Momozawa, A.; Fujima, T. Effects of Cr on the properties of Mo2NiB2 ternary boride. Solid State Sci. 2012, 12, 1643–1647. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, Y.; Liu, W.; Zheng, J.; Xiong, W. Effect of V content on the microstructure and mechanical properties of Mo2FeB2 based cermets. Mater. Des. 2010, 31, 2680–2683. [Google Scholar] [CrossRef]
- Haizhou, Y.; Wenjun, L.; Ping, F.; Yong, Z. Synthesis and microstructure evolution during vacuum sintering of Mo2FeB2 based cermets. Int. J. Refract. Met. Hard Mater. 2014, 45, 48–52. [Google Scholar] [CrossRef]
- Magnani, M.; Suegama, P.; Espallargas, N.; Fugivara, C.S.; Dosta, S.; Guilemany, J.; Benedetti, A.V. Corrosion and wear studies of Cr3C2NiCr-HVOF coatings sprayed on AA7050 T7 under cooling. J. Therm. Spray Technol. 2009, 18, 353–363. [Google Scholar] [CrossRef]
- Keränen, J.; Stenberg, T.; Mäntylä, T.; Lepistö, T. Micro structural characterization of detonation gun-sprayed boride-based cermet coatings. Surf. Coat Technol. 1996, 82, 29–37. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Zhong, J.; Xiang, Y. Feasibility of preparing Mo2FeB2- based cermet coating by electrospark deposition on high speed steel. Surf. Coat. Technol. 2016, 296, 58–64. [Google Scholar] [CrossRef]
- Zhou, X.P.; Hu, X.B.; Xu, Y.S. The microstructure and properties of coating from Mo2FeB2 cermet on surface of H13 steel by reactive flame spraying. Adv. Mater. Res. 2010, 97–101, 1321–1327. [Google Scholar] [CrossRef]
- Zhuang, M.; Wei, W.; Zou, J.-F.; Dong, S.-Z.; Zhang, L.-Y.; Li, Z.-C. Preparation and properties of flame-sprayed Mo-FeB-Fe cermet coatings. Trans. Nonferrous Met. Soc. Chin. 2011, 21, 1314–1321. [Google Scholar] [CrossRef]
- Vencl, A.; Mrdak, M.; Banjac, M. Correlation of microstructures and tribological properties of ferrous coatings deposited by atmospheric plasma spraying on Al–Si cast alloy substrate. Metall. Mater. Trans. A 2009, 40, 398–405. [Google Scholar] [CrossRef]
- Manna, I.; Majumdar, J.D.; Chandra, B.R.; Nayak, S.; Dahotre, N.B. Laser surface cladding of Fe–B–C, Fe–B–Si and Fe–BC–Si–Al–C on plain carbon steel. Surf. Coat. Technol. 2006, 201, 434–440. [Google Scholar] [CrossRef]
- Sexton, L.; Lavin, S.; Byrne, G.; Kennedy, A. Laser cladding of aerospace materials. J. Mater. Process. Technol. 2002, 122, 63–68. [Google Scholar] [CrossRef]
- Agarwal, A.; Dahotre, N.B. Laser surface engineering of steel for hard refractory ceramic composite coating. Int. J. Refract. Met. Hard Mater 1999, 17, 283–293. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhong, N.; Wang, G. Microstructure and properties laser-clad Mo2NiB2 cermet coating on steel substrate. Steel Res. Int. 2014, 85, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G.; Wu, W.; Qiao, Q.; Li, Y.; Li, X. Effect of pH and chloride on the micro-mechanism of pitting corrosion for high strength pipeline steel in aerated NaCl solutions. Appl. Surf. Sci. 2015, 349, 746–756. [Google Scholar] [CrossRef]
- Quej-Aké, L.; Contreras, A.; Aburto, J. Electrochemical study on corrosion inhibition of X52 steel by non-ionic surfactant in substitute ocean water. Int. J. Electrochem. Sci. 2018, 12, 7416–7431. [Google Scholar] [CrossRef]
- Xie, F.; Guo, Z.; Wang, D.; Li, R.; Wu, M.; Zong, Y.; Wang, Y. Synergistic effect between chloride and sulfate reducing bacteria in corrosion inhibition of X100 pipeline steel in marine environment. Int. J. Electrochem. Sci. 2019, 14, 2693–2704. [Google Scholar] [CrossRef]
- Brytan, Z.; Niagaj, J. Corrosion studies using potentiodynamic and EIS electrochemical techniques of welded lean duplex stainless steel UNS S82441. Appl. Surf. Sci. 2016, 388, 160–168. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 365–381. ISBN 0-471-64749-7. [Google Scholar]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Characterisation of passive films formed on low carbon steel in borate buffer solution(pH 9.2) by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2005, 252, 1510–1519. [Google Scholar] [CrossRef]
- Toor, I.-U.-H. Effect of Mn content and solution annealing temperature on the corrosion resistance of stainless steel alloys. J. Chem. 2014, 388, 1–18. [Google Scholar] [CrossRef]





| Element | Mo | Ni | B | Cr | Fe | Si | W | C |
|---|---|---|---|---|---|---|---|---|
| wt % | 61.72 | 18.88 | 6.95 | 5.06 | 4.87 | 1.3 | 0.97 | 0.26 |
| Sample No. | Presetting Method | Laser Power (W) | Scanning Speed (mm/s) | Lapping Rate (%) | Remelting Power (W) | Remelting Speed (mm/s) |
|---|---|---|---|---|---|---|
| 1 | Polyvinyl butyral (PVB) binder | 800 | 3 | 30 | NO | NO |
| 2 | PVB binder | 800 | 3 | 30 | 1000 | 4 |
| 3 | Squash | 800 | 3 | 30 | NO | NO |
| 4 | Squash | 800 | 3 | 30 | 1000 | 4 |
| Sample No. | Rs (Ω·cm2) | Rf (Ω·cm2) | Qf, Y0 (m·Ω−1·cm−2·sn) | Rct (Ω·cm2) | Qdl, Y0 (m·Ω−1·cm−2·sn) |
|---|---|---|---|---|---|
| Substrate | 3.63 | 6.25 | 1.54 | 980 | 1.72 |
| 1 | 4.86 | 174 | 0.31 | 2460 | 0.34 |
| 2 | 4.51 | 710 | 0.96 | 1420 | 1.61 |
| 3 | 3.42 | 451 | 0.41 | 1100 | 0.94 |
| 4 | 1.17 | 2.32 | 1.16 | 1360 | 0.77 |
| Sample No. | Open Circuit Potential (OCP) (V) | Ecorr (V) | icorr (μA) | jcorr (μA/cm2) |
|---|---|---|---|---|
| Substrate | −0.59 | −0.95 | 43.76 | 43.76 |
| 1 | −0.52 | −0.88 | 6.35 | 6.35 |
| 2 | −0.55 | −0.87 | 8.30 | 8.30 |
| 3 | −0.50 | −0.89 | 10.05 | 10.05 |
| 4 | −0.53 | −0.95 | 14.42 | 14.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Li, W.; Zhao, Y. Microstructure and Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis. Coatings 2019, 9, 476. https://doi.org/10.3390/coatings9080476
Hu Z, Li W, Zhao Y. Microstructure and Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis. Coatings. 2019; 9(8):476. https://doi.org/10.3390/coatings9080476
Chicago/Turabian StyleHu, Zhaowei, Wenge Li, and Yuantao Zhao. 2019. "Microstructure and Properties of M3B2-Type Boride-Based Cermet Coatings Prepared by Laser Cladding Synthesis" Coatings 9, no. 8: 476. https://doi.org/10.3390/coatings9080476




