Study on Corrosion Resistance and Wear Resistance of Zn–Al–Mg/ZnO Composite Coating Prepared by Cold Spraying
Abstract
:1. Introduction
1.1. Cold Spray Technology Features
1.2. Cold Spray Coating on the Surface of Marine Equipment
2. Experimental Methods
2.1. Preparation of Coating
2.2. Coating Characteristics
2.3. Corrosion Behavior of the Composite Coatings
2.4. Photocatalytic Characteristics of the Composite Coatings
2.5. Composite Coating Friction and Wear Test
3. Results and Discussion
3.1. Preparation of Composite Coatings
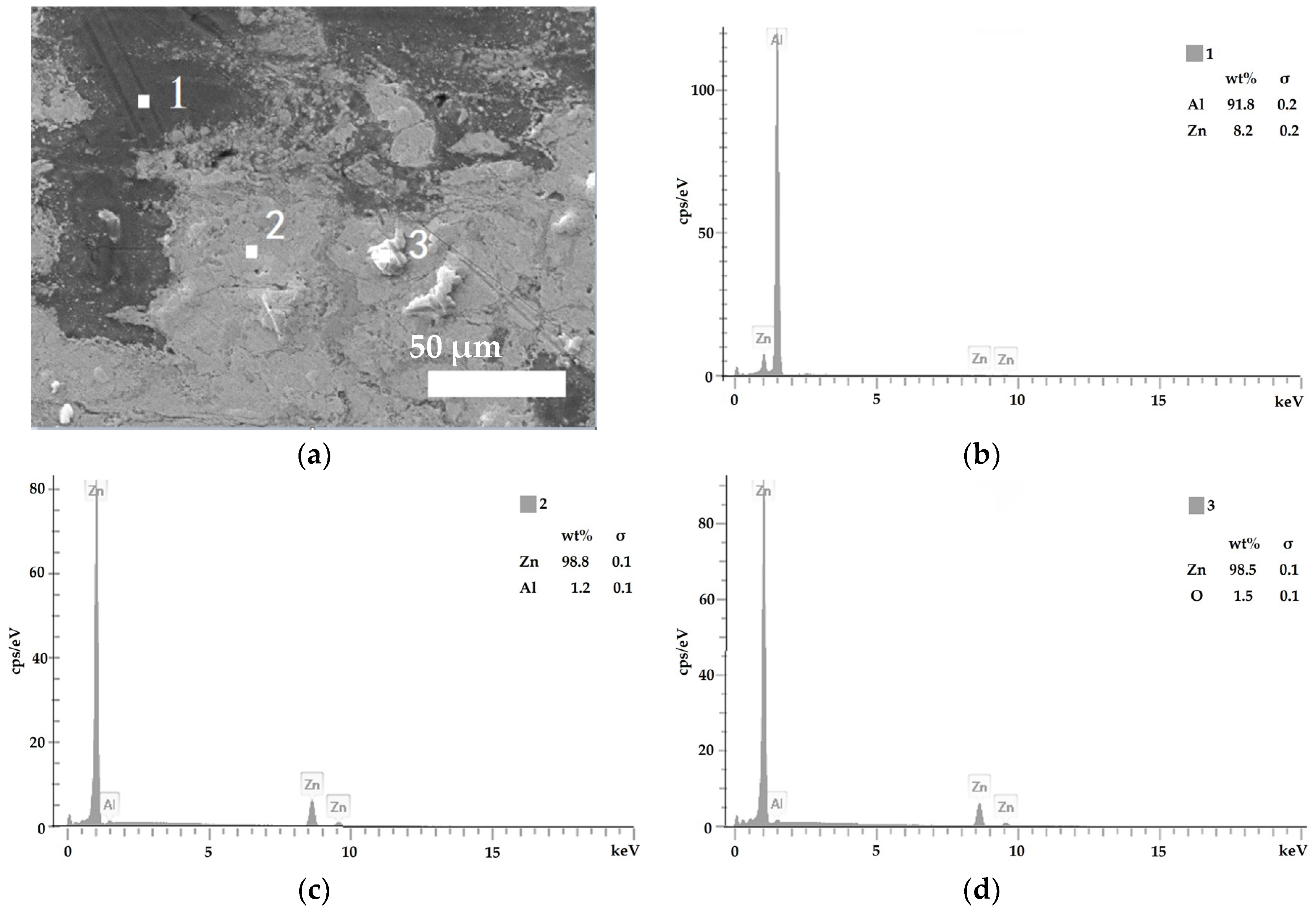
3.2. Composite Coating Morphology
3.3. Corrosion Resistance Test
3.3.1. Electrochemical Accelerated Corrosion Test
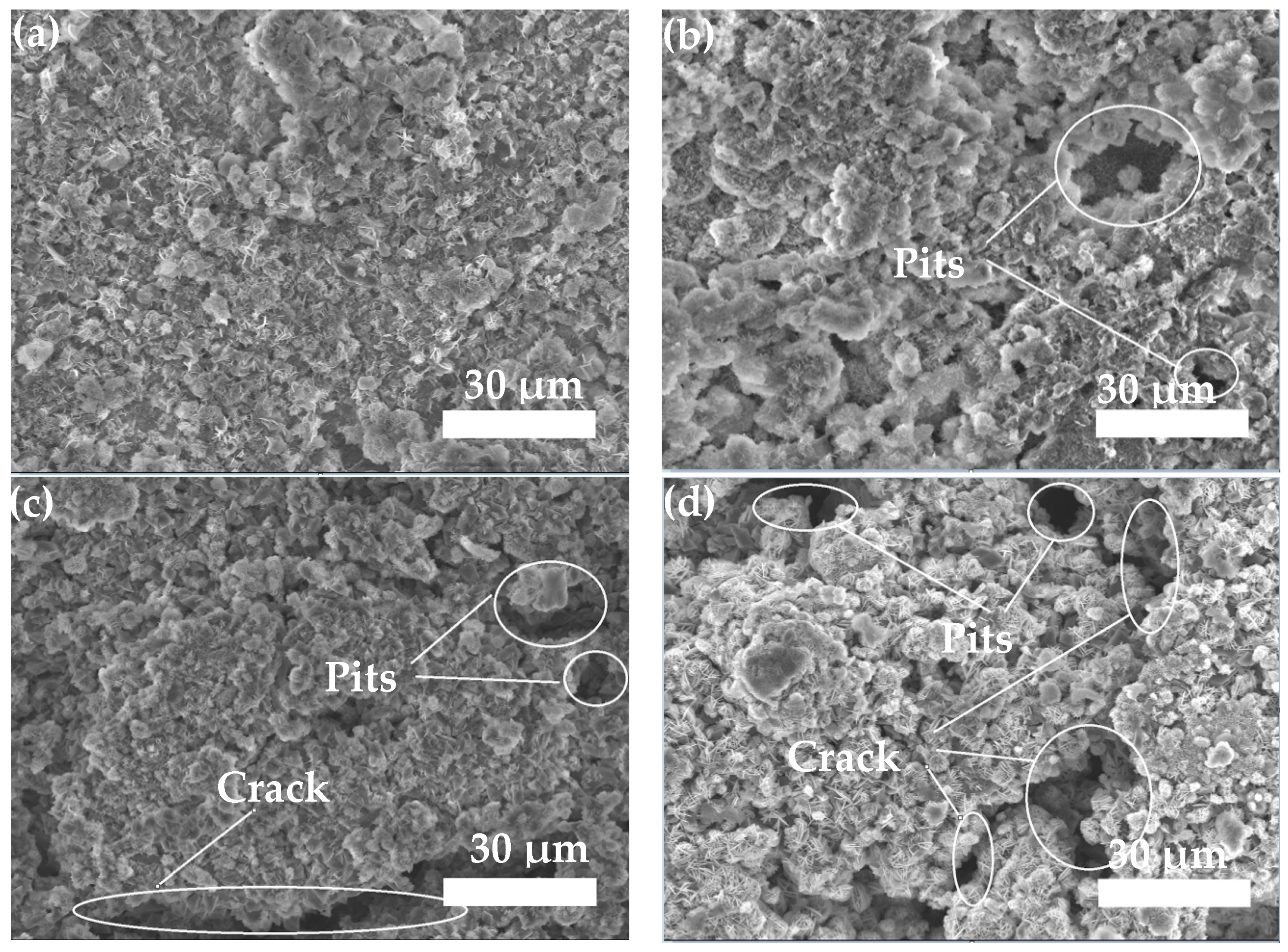
3.3.2. Neutral Salt Spray Test of Composite Coating
3.4. Photocatalytic Degradation of Methyl Blue
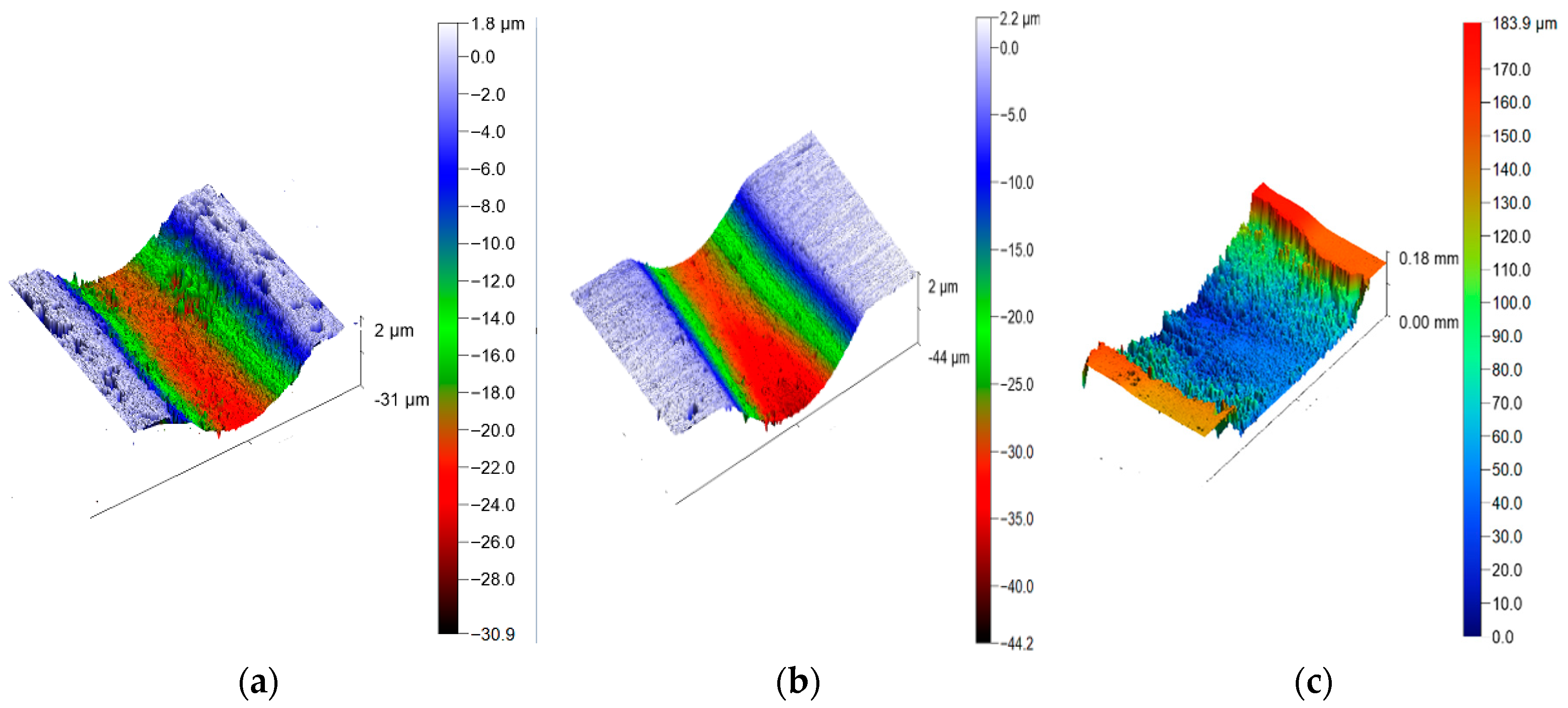
3.5. Friction and Wear Test of Composite Coatings
4. Conclusions
- Zn65Al15Mg5ZnO15 composite coating and Zn45Al35Mg5ZnO15 composite coating can be prepared on base steel Q235 by cold spray technique. The surface of the Zn45Al35Mg5ZnO15 composite coating is smoother and flatter than the Zn65Al15Mg5ZnO15 composite coating. The micromorphology of the two composite coatings prepared by cold spraying is dense, there are no defects such as voids inside the coating, and the Zn, Al, Mg, and ZnO elements inside the coating are uniformly distributed.
- The analysis of electrochemical workstation test data shows that both the Zn65Al15Mg5ZnO15 and Zn45Al35Mg5ZnO15 composite coatings have strong anodic oxidation cathodic protection characteristics, which can protect the matrix steel Q235 from corrosion. The open circuit potential and polarization current of the two composite coatings at different time periods show that the corrosion rate of the Zn65Al15Mg5ZnO15 composite coating is higher than that of the Zn45Al35Mg5ZnO15 composite coating, and the corrosion products produced by the oxidation of the composite coating have corrosion slowing characteristics during corrosion.
- The neutral salt spray test of the two composite coatings shows that the Zn45Al35Mg5ZnO15 composite coating experienced regular delamination corrosion with no pits or cracks defects but the Zn65Al15Mg5ZnO15 composite coating was unevenly corroded and had a deep corrosion, with defects such as pits and cracks, after 240 h of corrosion. The corrosion resistance test shows that the Zn45Al35Mg5ZnO15 composite coating has a better corrosion resistance and long-term stability than the Zn65Al15Mg5ZnO15 composite coating.
- By comparing the photocatalytic degradation data of the Zn65Al15Mg5ZnO15 composite coatings, Zn45Al35Mg5ZnO15 composite coatings, and Q235 matrix steel for methyl blue solution, the results show that the two composite coatings have good photocatalytic properties but that the photocatalytic properties of the Zn45Al35Mg5ZnO15 composite coatings are better.
- The results of the friction and wear tests show that both the Zn65Al15Mg5ZnO15 composite coating and the Zn45Al35Mg5ZnO15 composite coating have better friction and wear resistance than the base steel Q235 and that the friction and wear properties of the Zn45Al35Mg5ZnO15 composite coating are the best.
- The results of all the above experiments show that the Zn45Al35Mg5ZnO15 composite coating prepared by cold spraying has a superior deposition effect, high photocatalytic efficiency, better corrosion resistance, and wear resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Yan, S.K.; Song, G.L.; Li, Z.X.; Wang, H.N.; Zheng, D.J.; Cao, F.Y.; Horynova, H.; Dargusch, M.S.; Zhou, L. A state-of-the-art review on passivation and biofouling of Ti and its alloys in marine environments. J. Mater. Sci. Technol. 2018, 34, 421–435. [Google Scholar] [CrossRef]
- Chavan, N.M.; Kiran, B.; Jyothirmayi, A.; Phani, P.S.; Sundararajan, G. The corrosion behavior of cold sprayed zinc coatings on mild steel substrate. J. Therm. Spray Technol. 2013, 22, 463–470. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Doyoyo, M. Laser-assisted cold-sprayed corrosion-and wear-resistant coatings: A review. J. Therm. Spray Technol. 2014, 23, 765–785. [Google Scholar] [CrossRef]
- Cai, M.R.; Guo, R.S.; Zhou, F.; Liu, W.M. Lubricating a bright future: Lubrication contribution to energy saving and low carbon emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Cui, H.; Li, N.; Peng, J.; Yin, R.; Li, J.; Wu, Z. Investigation on the thermal performance of a novel spray tower with upward spraying and downward gas flow. Appl. Energy 2018, 231, 12–21. [Google Scholar] [CrossRef]
- Gkomoza, P.; Lampropoulos, G.S.; Vardavoulias, M.; Pantelis, D.I.; Karakizis, P.N.; Sarafoglou, C. Microstructural investigation of porous titanium coatings, produced by thermal spraying techniques, using plasma atomization and hydride-dehydride powders, for orthopedic implants. Surf. Coat. Technol. 2019, 357, 947–956. [Google Scholar] [CrossRef]
- Wu, Y.N.; Ke, P.L.; Wang, Q.M.; Sun, C.; Wang, F.H. High temperature properties of thermal barrier coatings obtained by detonation spraying. Corros. Sci. 2004, 46, 2925–2935. [Google Scholar] [CrossRef]
- Pawłowski, L. Strategic oxides for thermal spraying: Problems of availability and evolution of prices. Surf. Coat. Technol. 2013, 220, 14–19. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Xie, Y.; Aubry, E.; Ren, Z.; Ji, G.; Liao, H. Comparative investigation of microstructure and properties of Ni-coated FeSiAl soft magnetic composite coatings produced by cold spraying and HVOF. Surf. Coat. Technol. 2019, 371, 224–234. [Google Scholar] [CrossRef]
- Hunter, B.; Aldwell, B.; Jenkins, R.; Lupoi, R. A study on the feasibility of laser annealing to relieve residual stresses in cold spray coatings. Procedia CIRP 2018, 78, 91–96. [Google Scholar] [CrossRef]
- Xie, Y.C.; Chen, C.Y.; Planche, M.P.; Deng, S.H.; Huang, R.Z.; Ren, Z.M.; Liao, H.L. Strengthened peening effect on metallurgical bonding formation in cold spray additive manufacturing. J. Therm. Spray Technol. 2019, 28, 769–779. [Google Scholar] [CrossRef]
- Chen, C.Y.; Xie, Y.C.; Yin, Y.S.; Fukanuma, H.; Huang, R.Z.; Zhao, R.X.; Wang, J.; Ren, Z.G.; Liu, M.; Liao, H.L. Effect of hot isostatic pressing (HIP) on microstructure and mechanical properties of Ti6Al4V alloy fabricated by cold spray additive manufacturing. Addit. Manuf. 2019, 27, 595–605. [Google Scholar] [CrossRef]
- Kang, N.; Coddet, P.; Liao, H.L.; Coddet, C. The effect of heat treatment on microstructure and tensile properties of cold spray Zr base metal glass/Cu composite. Surf. Coat. Technol. 2015, 280, 64–71. [Google Scholar] [CrossRef]
- Coddet, P.; Verdy, C.; Coddet, C.; Debray, F. Effect of cold work, second phase precipitation and heat treatments on the mechanical properties of copper–silver alloys manufactured by cold spray. Mater. Sci. Eng. A 2015, 637, 40–47. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Duchoslav, J.; Steinberger, R.; Arndt, M.; Keppert, T.; Luckeneder, G.; Stellnberger, K.H.; Hagler, J.; Angeli, G.; Riener, C.K.; Stifter, D. Evolution of the surface chemistry of hot dip galvanized Zn–Mg–Al and Zn coatings on steel during short term exposure to sodium chloride containing environments. Corros. Sci. 2015, 91, 311–320. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Mechanisms of formation of slurry aluminide coatings from Al and Cr microparticles. Surf. Coat. Technol. 2019, 359, 323–333. [Google Scholar] [CrossRef]
- Li, C.G.; Li, S.; Zeng, M.; Sun, S.; Wang, F.F.; Wang, Y. Effect of high-frequency micro-vibration on microstructure and properties of laser cladding aluminum coatings. Int. J. Adv. Manuf. Technol. 2019, 103, 1633–1642. [Google Scholar] [CrossRef]
- La, P.; Ou, Y.; Han, S.; Wei, Y.; Zhu, D.; Feng, J. Effect of carbon content on morphology, size and phase of submicron tungsten carbide powders by salt-assisted combustion synthesis. Rare Metal Mater. Eng. 2016, 45, 853–857. [Google Scholar] [CrossRef]
- Li, H.X.; Li, X.B.; Sun, M.-X.; Wang, H.R.; Huang, G.S. Corrosion resistance of cold-sprayed Zn–50Al coatings in seawater. J. Chin. Soc. Corros. Prot. 2010, 30, 62–66. [Google Scholar] [CrossRef]
- Liang, Y.L.; Wang, Z.B.; Zhang, J.; Zhang, J.B.; Lu, K. Enhanced bonding property of cold-sprayed Zn–Al coating on interstitial-free steel substrate with a nanostructured surface layer. Appl. Sur. Sci. 2016, 385, 341–348. [Google Scholar] [CrossRef]
- Kumar, V.A.; Sammaiah, P. Comparison of process parameters influence on mechanical and metallurgical properties of zinc coating on mild steel and aluminium during mechanical process. Mater. Today Proc. 2018, 5, 3861–3866. [Google Scholar] [CrossRef]
- Mandal, G.K.; Das, S.K.; Balasubramaniam, R.; Mehrotra, S.P. Evolution of microstructures of galvanised and galvannealed coatings formed in 0.2 wt.% aluminium–zinc bath. Mater. Sci. Technol. 2011, 27, 1265–1270. [Google Scholar] [CrossRef]
- Darvish, A.; Naderi, R.; Attar, M.R.M. Improvement in polyurethane coating performance through zinc aluminium phosphate pigment. Pigment. Resin Technol. 2016, 45, 419–425. [Google Scholar] [CrossRef]
- La, J.H.; Lee, S.Y.; Hong, S.J. Synthesis of Zn–Mg coatings using unbalanced magnetron sputtering and theirs corrosion resistance. Surf. Coat. Technol. 2014, 259, 56–61. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Shi, Z.C.; Guo, M.Q.; Lu, F.; Sun, Z.H.; Tang, Z.Z.; Yu, B. Morphology and properties of cold sprayed Zn–Ni composite coatings. Therm. Spray. Technol. 2014, 6, 6–13. (In Chinese) [Google Scholar] [CrossRef]
- Conde, A.; Arenas, M.A.; De Damborenea, J.J. Electrodeposition of Zn–Ni coatings as Cd replacement for corrosion protection of high strength steel. Corros. Sci. 2011, 534, 1489–1497. [Google Scholar] [CrossRef]
- Gayathri, P.V.; Yesodharan, S.; Yesodharan, E.P. Microwave/Persulphate assisted ZnO mediated photocatalysis (MW/PS/UV/ZnO) as an efficient advanced oxidation process for the removal of RhB dye pollutant from water. J. Environ. Chem. Eng. 2019, 7, 103122. [Google Scholar] [CrossRef]
- Singh, N.K.; Koutu, V.; Malik, M.M. Enhancement of room temperature ferromagnetic behavior of Co-doped ZnO nanoparticles synthesized via sol–gel technique. J. Sol-Gel Sci. Technol. 2019, 91, 324–334. [Google Scholar] [CrossRef]
- Goel, S.; Kumar, B. Lead-free high Tc ferroelectric material: Hierarchical Dy-doped ZnO architectures co-assembled by 1D nanorods and 2D nanosheets. J. Alloy. Compd. 2019, 801, 626–639. [Google Scholar] [CrossRef]










| Samples | Times/h | Ecorr (V) | Icorr (A·cm−2) |
|---|---|---|---|
| Q235 | 1 | −0.95 ± 0.02 | 6.58 × 10−4 |
| Zn45Al35Mg5ZnO15 | 1 | −1.22 ± 0.02 | 2.11 × 10−5 |
| 120 | −1.42 ± 0.02 | 1.08 × 10−5 | |
| 360 | −1.27 ± 0.02 | 1.28 × 10−4 | |
| 480 | −1.20 ± 0.02 | 8.23 × 10−5 | |
| Zn65Al15Mg5ZnO15 | 1 | −1.41 ± 0.02 | 3.03 × 10−5 |
| 120 | −1.50 ± 0.02 | 1.63 × 10−5 | |
| 360 | −1.32 ± 0.02 | 3.54 × 10−4 | |
| 480 | −1.40 ± 0.02 | 1.08 × 10−4 |
| Samples | Methyl Blue Absorbance | Methyl Blue Degradation Solution |
|---|---|---|
| Zn45Al35Mg5ZnO15 | 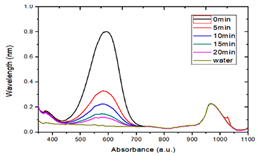 |  |
| Zn65Al15Mg5ZnO15 | 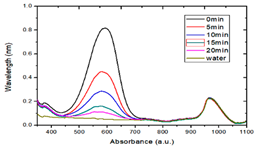 |  |
| Q235 | 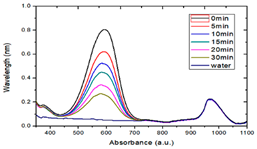 |  |
| Samples | m1 (g) | m2 (g) | Δm (g) | f |
|---|---|---|---|---|
| Zn45Al35Mg5ZnO15 | 2.6735 | 2.6700 | 0.0035 | 0.181 |
| Zn65Al15Mg5ZnO15 | 2.6420 | 2.6370 | 0.0050 | 0.231 |
| Q235 | 1.7734 | 1.7623 | 0.0111 | 0.358 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Wang, S.; Xiong, T.; Wen, D.; Wang, G.; Du, H. Study on Corrosion Resistance and Wear Resistance of Zn–Al–Mg/ZnO Composite Coating Prepared by Cold Spraying. Coatings 2019, 9, 505. https://doi.org/10.3390/coatings9080505
Lu X, Wang S, Xiong T, Wen D, Wang G, Du H. Study on Corrosion Resistance and Wear Resistance of Zn–Al–Mg/ZnO Composite Coating Prepared by Cold Spraying. Coatings. 2019; 9(8):505. https://doi.org/10.3390/coatings9080505
Chicago/Turabian StyleLu, Xinqiang, Shouren Wang, Tianying Xiong, Daosheng Wen, Gaoqi Wang, and Hao Du. 2019. "Study on Corrosion Resistance and Wear Resistance of Zn–Al–Mg/ZnO Composite Coating Prepared by Cold Spraying" Coatings 9, no. 8: 505. https://doi.org/10.3390/coatings9080505
APA StyleLu, X., Wang, S., Xiong, T., Wen, D., Wang, G., & Du, H. (2019). Study on Corrosion Resistance and Wear Resistance of Zn–Al–Mg/ZnO Composite Coating Prepared by Cold Spraying. Coatings, 9(8), 505. https://doi.org/10.3390/coatings9080505




