Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Silane Functionalized Graphene
2.3. Fabrication of the Composite Coatings
2.4. Characterization
3. Results and Discussion
3.1. Characterization of the PVSQ-GO Composite Material
3.2. Surface Properties and Section Morphology
3.3. Mechanical Performances
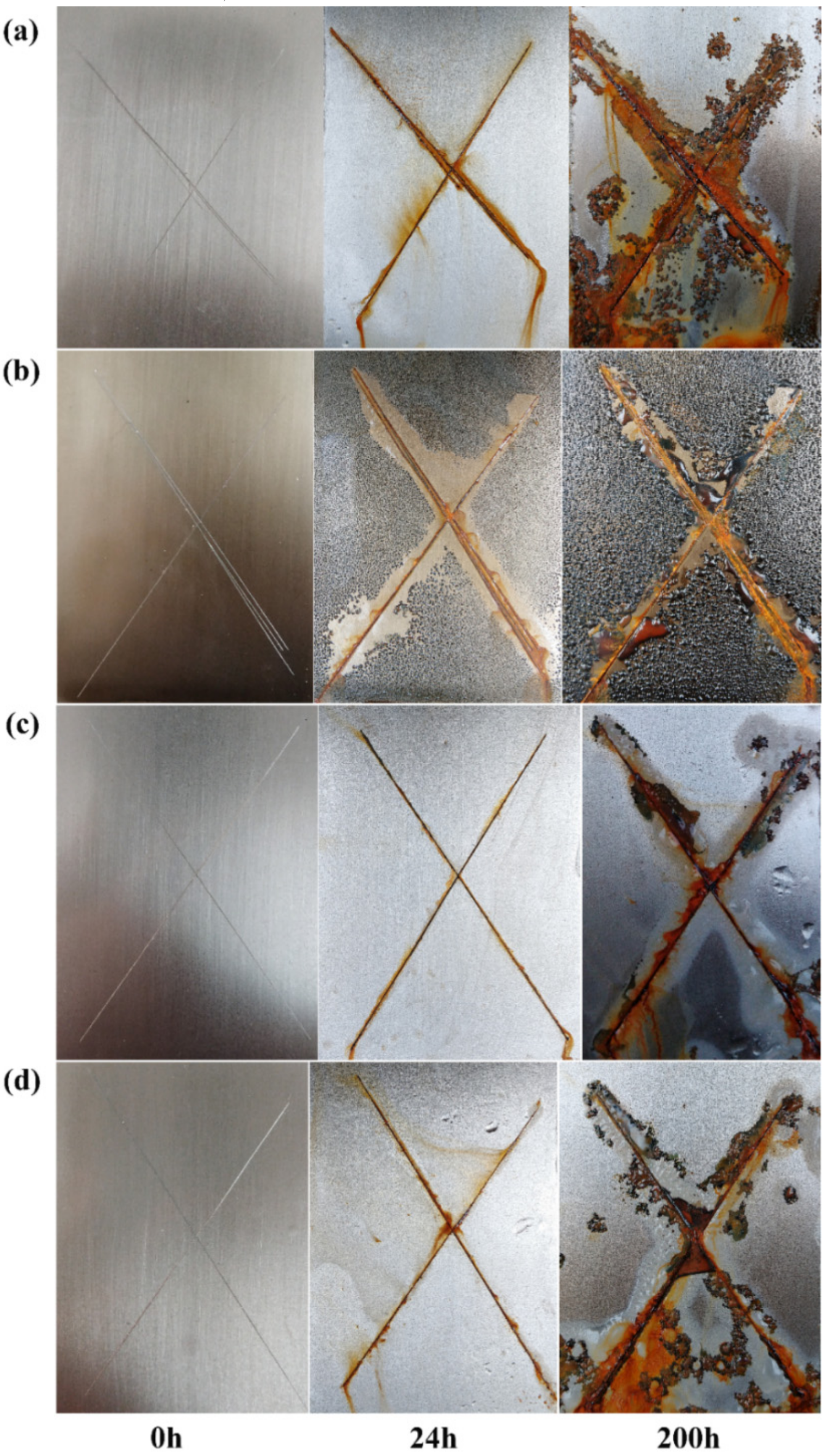
3.4. Anticorrosive Performances
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer grapheme. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Canal-Rodríguez, M.; Arenillas, A.A.; Rey-Raap, N.; Ramos-Fernández, G.; MartínGullón, I.; Menéndez, J.A. Graphene-doped carbon xerogel combining high electrical conductivity and surface area for optimized aqueous supercapacitors. Carbon 2017, 118, 291–298. [Google Scholar] [CrossRef]
- Wen, S.; Wang, Z.; Zheng, X.; Wang, X. Improved mechanical strength of porous chitosan scaffold by graphene coatings. Mater. Lett. 2017, 186, 17–20. [Google Scholar] [CrossRef]
- Dong, B.; Yuan, Y.; Luo, J.; Dong, L.; Liu, R.; Liu, X. Acryloyl-group functionalized graphene for enhancing thermal and mechanical properties of acrylated epoxidized soybean oil UV-curable based coatings. Prog. Org. Coat. 2018, 118, 57–65. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef]
- Chang, K.C.; Hsu, M.H.; Lu, H.I.; Lai, M.C.; Liu, P.J.; Hsu, C.H.; Ji, W.F.; Chuang, T.L.; Wei, Y.; Yeh, J.M.; et al. Room temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for coldrolled steel. Carbon 2014, 6, 144–153. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Z.T.; Shenoy, G.J.; Li, L.; Liu, H.T. Enhanced room-temperature corrosion of copper in the presence of grapheme. ACS Nano 2013, 7, 6939–6947. [Google Scholar] [CrossRef]
- Sahu, S.C.; Samantara, A.K.; Seth, M.; Parwaiz, S.; Singh, B.P.; Rath, P.C.; Jena, B.K. A facile electrochemical approach for development of highly corrosion protective coatings using graphene nanosheets. Electrochem. Commun. 2013, 32, 22–26. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Cai, W.; Borysiak, M.; Han, B.; Chen, D.; Piner, R.D.; Colombo, L.; Ruoff, R.S. Transfer of large-area graphene films for high-performance transparent conductive electrodes. Nano Lett. 2009, 9, 4359. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Karpan, V.M.; Van den, B.J.; Kelly, P.J. Doping graphene with metal contacts. Phys. Rev. Lett. 2008, 101, 026803. [Google Scholar] [CrossRef]
- Pu, N.W.; Shi, G.N.; Liu, Y.M.; Sun, X.; Chang, J.K.; Sun, C.L.; Ger, M.D.; Chen, C.Y.; Wang, P.C.; Peng, Y.Y. Graphene grown on stainless steel as a high performance and ecofriendly anti-corrosion coating for polymer electrolyte membrane fuel cell bipolar plates. J. Power Sources 2015, 282, 248–256. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Schriver, M.; Regan, W.; Gannett, W.J.; Zaniewski, A.M.; Crommie, M.F.; Zettl, A. Graphene as a long-term metal oxidation barrier: Worse than nothing. ACS Nano 2013, 7, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Berman, D. Inhibitor or promoter: Insights on the corrosion evolution in a graphene protected surface. Carbon 2018, 126, 225–231. [Google Scholar] [CrossRef]
- Jo, M.; Lee, H.C.; Lee, S.G.; Cho, K. Graphene as a metal passivation layer: Corrosion-accelerator and inhibitor. Carbon 2017, 116, 232–239. [Google Scholar] [CrossRef]
- Chang, C.H.; Huang, T.C.; Peng, C.W.; Yeh, T.C.; Lu, H.I.; Hung, W.I.; Weng, C.J.; Yang, T.I.; Yeh, J.M. Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 2012, 50, 5044–5051. [Google Scholar] [CrossRef]
- Chang, K.C.; Ji, W.F.; Lai, M.C.; Hsiao, Y.R.; Hsu, C.H.; Chuang, T.L.; Wei, Y.; Yeh, J.M.; Liu, W.R. Correction: Synergistic effects of hydrophobicity and gas barrier properties on the anticorrosion property of PMMA nanocomposite coatings embedded with graphene nanosheets. Polym. Chem. 2014, 5, 6865. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Wang, M.; Yang, Z.; Pan, Y.; Liu, G. Inhibiting the corrosion-promotion activity of graphene. Chem. Mater. 2015, 27, 2367–2373. [Google Scholar] [CrossRef]
- Sun, W.; Wang, L.; Wu, T.; Dong, C.; Liu, G. Tuning the functionalization degree of graphene: Determining critical conditions for inhibiting the corrosion promotion activity of graphene/epoxy nanocomposite coatings. Mater. Lett. 2019, 240, 262–266. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Q.; Yu, M.; Li, S.; Zhao, K.; Xue, B.; Zu, H. Silane modification of titanium dioxide-decorated graphene oxide nanocomposite for enhancing anticorrosion performance of epoxy coatings on AA-2024. J. Alloy. Compd. 2018, 744, 728–739. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Wei, M.; Zheng, Z.; Vu, D.D.; Bui, T.T.; Huang, X. Preparation, characterization, and properties of graphene oxide/urushiol-formaldehyde polymer composite coating. J. Coat. Technol. Res. 2018, 15, 1343–1356. [Google Scholar] [CrossRef]
- Wu, Z.J.; Xiang, H.; Kim, T.; Chun, M.S.; Lee, K. Surface properties of submicrometer silica spheres modified with aminopropyltriethoxysilane and phenyltriethoxysilane. J. Colloid Interface Sci. 2006, 304, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Reza-E-Rabby, M.; Jeelani, S.; Rangari, V.K. Structural Analysis of Polyhedral Oligomeric Silsesquioxane Coated SiC Nanoparticles and Their Applications in Thermoset Polymers. J. Nanomater. 2015, 2015, 894856. [Google Scholar] [CrossRef]
- Yadav, S.K.; Mahapatra, S.S.; Yoo, H.J.; Cho, J.W. Synthesis of multi-walled carbon nanotube/polyhedral oligomeric silsesquioxane nanohybrid by utilizing click chemistry. Nanoscale Res. Lett. 2011, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, Y.; Lu, F.; Qu, J.; Chen, H.; Dai, L. Functionalization of graphene oxide with polyhedral oligomeric silsesquioxane (POSS) for multifunctional applications. J. Phys. Chem. Lett. 2012, 3, 1607–1612. [Google Scholar] [CrossRef]
- Naderizadeh, S.; Athanassiou, A.; Bayer, I.S. Interfacing superhydrophobic silica nanoparticle films with graphene and thermoplastic polyurethane for wear/abrasion resistance. J. Colloid Interface Sci. 2018, 519, 285–295. [Google Scholar] [CrossRef] [PubMed]
- ASTM B117 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2018; Volume 03.02.
- Kaminska, I.; Das, M.R.; Coffinier, Y.; Niedziolka-Jonsson, J.; Sobczak, J.; Woisel, P.; Lyskawa, J.; Opallo, M.; Boukherroub, R.; Szunerits, S. Reduction and functionalization of graphene oxide sheets using biomimetic dopamine derivatives in one step. ACS Appl. Mater. Interfaces 2012, 4, 1016–1020. [Google Scholar] [CrossRef]
- Das, A.K.; Srivastav, M.; Layek, R.K.; Uddin, M.E.; Jung, D.; Kim, N.H.; Lee, J.H. Iodide-mediated room temperature reduction of graphene oxide: A rapid chemical route for the synthesis of a bifunctional electrocatalyst. J. Mater. Chem. A 2014, 2, 1332–1340. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Ahmadi, A.; Mahdavian, M. Enhancement of the corrosion protection performance and cathodic delamination resistance of epoxy coating through treatment of steel substrate by a novel nanometric sol-gel based silane composite film filled with functionalized graphene oxide nanosheets. Corros. Sci. 2016, 109, 182–205. [Google Scholar] [CrossRef]
- Yu, Z.; Lv, L.; Ma, Y.; Di, H.; He, Y. Covalent modification of graphene oxide by metronidazole for reinforced anti-corrosion properties of epoxy coatings. RSC Adv. 2016, 6, 18217–18226. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.J.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Deshpande, R.R.; Eckert, H. Sol-gel preparation of mesoporous sodium aluminosilicate glasses: Mechanistic and structural investigation by solid state nuclear magnetic resonance. J. Mater. Chem. 2009, 19, 3419–3426. [Google Scholar] [CrossRef]
- Nguyen, T.; Hubbard, J.B.; Mcfadden, G.B.A. Mathematical model for the cathodic blistering of organic coatings on steel immersed in electrolytes. J. Coat. Technol. 1982, 54, 693–695. [Google Scholar]
- Cui, Z.; Liu, Z.; Wang, L.; Li, X.; Du, C.; Wang, X. Effect of plastic deformation on the electrochemical and stress corrosion cracking behavior of X70 steel in near-neutral pH environment. Mater. Sci. Eng. 2016, 677, 259–273. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Z.; Wu, W.; Li, X.; Du, C.; Jiang, B. Stress corrosion cracking behavior of ZK60 magnesium alloy under different conditions. Int. J. Hydrog. Energy 2017, 42, 2662–2674. [Google Scholar] [CrossRef]
- Hinderliter, B.R.; Croll, S.G.; Tallman, D.E.; Su, Q.; Bierwagen, G.P. Interpretation of EIS data from accelerated exposure of coated metals based on modeling of coating physical properties. Electrochim. Acta 2006, 51, 4505–4515. [Google Scholar] [CrossRef]
- Sarkar, N.; Sahoo, G.; Das, R.; Prusty, G.; Sahu, D.; Swain, S.K. Anticorrosion performance of three-dimensional hierarchical PANI@BN nanohybrids. Ind. Eng. Chem. Res. 2016, 55, 2921–2931. [Google Scholar] [CrossRef]
- Li, J.; Cui, J.; Yang, J.; Li, Y.; Qiu, H.; Yang, J. Reinforcement of graphene and its derivatives on the anticorrosive properties of waterborne polyurethane coatings. Compos. Sci. Technol. 2016, 129, 30–37. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2010, 14, 381–391. [Google Scholar] [CrossRef]
- Visser, P.; Meeusen, M.; Garcia, Y.G.; Terryn, H.; Mol, J.M.C. Electrochemical evaluation of corrosion inhibiting layers formed in a defect from lithium-leaching organic coatings. J. Electrochem. Soc. 2017, 164, 396–406. [Google Scholar] [CrossRef]
- Visser, P.; Garcia, Y.G.; Mol, J.M.C.; Terryn, H. Mechanism of passive layer formation on AA2024-T3 from alkaline lithium carbonate solutions in the presence of sodium chloride. J. Electrochem. Soc. 2018, 165, 60–70. [Google Scholar] [CrossRef]
- Huang, T.C.; Su, Y.A.; Yeh, T.C.; Huang, H.Y.; Wu, C.P.; Huang, K.Y.; Chou, Y.C.; Yeh, J.M.; Wei, Y. Advanced anticorrosive coatings prepared from electroactive epoxy-SiO2 hybrid nanocomposite materials. Electrochim. Acta 2011, 56, 6142–6149. [Google Scholar] [CrossRef]
- Yeh, T.C.; Huang, T.C.; Huang, H.Y.; Huang, Y.P.; Cai, Y.T.; Lin, S.T.; Wei, Y.; Yeh, J.M. Electrochemical investigations on anticorrosive and electrochromic properties of electroactive polyurea. Polym. Chem. 2012, 3, 2209–2216. [Google Scholar] [CrossRef]
















| Elements | Fe | C | Mn | Si | S | P |
|---|---|---|---|---|---|---|
| Content (wt.%) | 99.03 | 0.16 | 0.42 | 0.30 | 0.050 | 0.045 |
| Sample | Ecorr (V) | Icorr (A/cm2) | ba (mV/dec) | bc (mV/dec) | Rp (Ω·cm2) | IE (%) | vcorr (mm/year) |
|---|---|---|---|---|---|---|---|
| Bare steel | −0.791 | 7.921 × 10−5 | 143.46 | −198.25 | 4.56 × 102 | — | 0.921 |
| Pure WPU | −0.614 | 2.404 × 10−7 | 246.74 | −180.31 | 1.88 × 105 | 99.69 | 2.79 × 10−3 |
| 0.5 wt.% GO/WPU | −0.539 | 4.366 × 10−7 | 194.64 | −236.84 | 1.06 × 105 | 99.44 | 5.07 × 10−3 |
| 0.5 wt.% PVSQ-GO/WPU | −0.325 | 7.079 × 10−9 | 233.41 | −160.39 | 5.83 × 106 | 99.99 | 8.96 × 10−5 |
| 1.0 wt.% PVSQ-GO/WPU | −0.442 | 4.875 × 10−8 | 216.25 | −184.56 | 8.87 × 105 | 99.93 | 5.76 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Wei, S.; Xiang, B.; Wang, B.; Wang, Y.; Liang, Y.; Yuan, Y. Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings. Coatings 2019, 9, 587. https://doi.org/10.3390/coatings9090587
Chen C, Wei S, Xiang B, Wang B, Wang Y, Liang Y, Yuan Y. Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings. Coatings. 2019; 9(9):587. https://doi.org/10.3390/coatings9090587
Chicago/Turabian StyleChen, Chao, Shicheng Wei, Bin Xiang, Bo Wang, Yujiang Wang, Yi Liang, and Yue Yuan. 2019. "Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings" Coatings 9, no. 9: 587. https://doi.org/10.3390/coatings9090587
APA StyleChen, C., Wei, S., Xiang, B., Wang, B., Wang, Y., Liang, Y., & Yuan, Y. (2019). Synthesis of Silane Functionalized Graphene Oxide and Its Application in Anti-Corrosion Waterborne Polyurethane Composite Coatings. Coatings, 9(9), 587. https://doi.org/10.3390/coatings9090587




