Preparation of ZIF-67@PAN Nanofibers for CO2 Capture: Effects of Solvent and Time on Particle Morphology
Abstract
Highlights
- The synthesis conditions used, such as linker concentration, the time of synthesis, and the type of solvent, have a strong influence on ZIF-67 morphology, changing its shape (rhombic grains, flakes, or irregular grains), size (from ≈50 to 130 nm), and number of particles.
- The samples synthesized in the presence of methanol led to highly crystalline ZIF-67 particles that showed a CO2 adsorption of 0.40 mmol/g at 1.2 bar and 273 K.
- This work shows the paths for obtaining different structures of ZIF-67 that might be interesting for different applications, such as dye removal and gas separation.
- The knowledge obtained here can be used with different MOFs and to expand the field of in situ synthesis.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospinning
2.3. In Situ Growth
2.4. Characterization
3. Results and Discussion
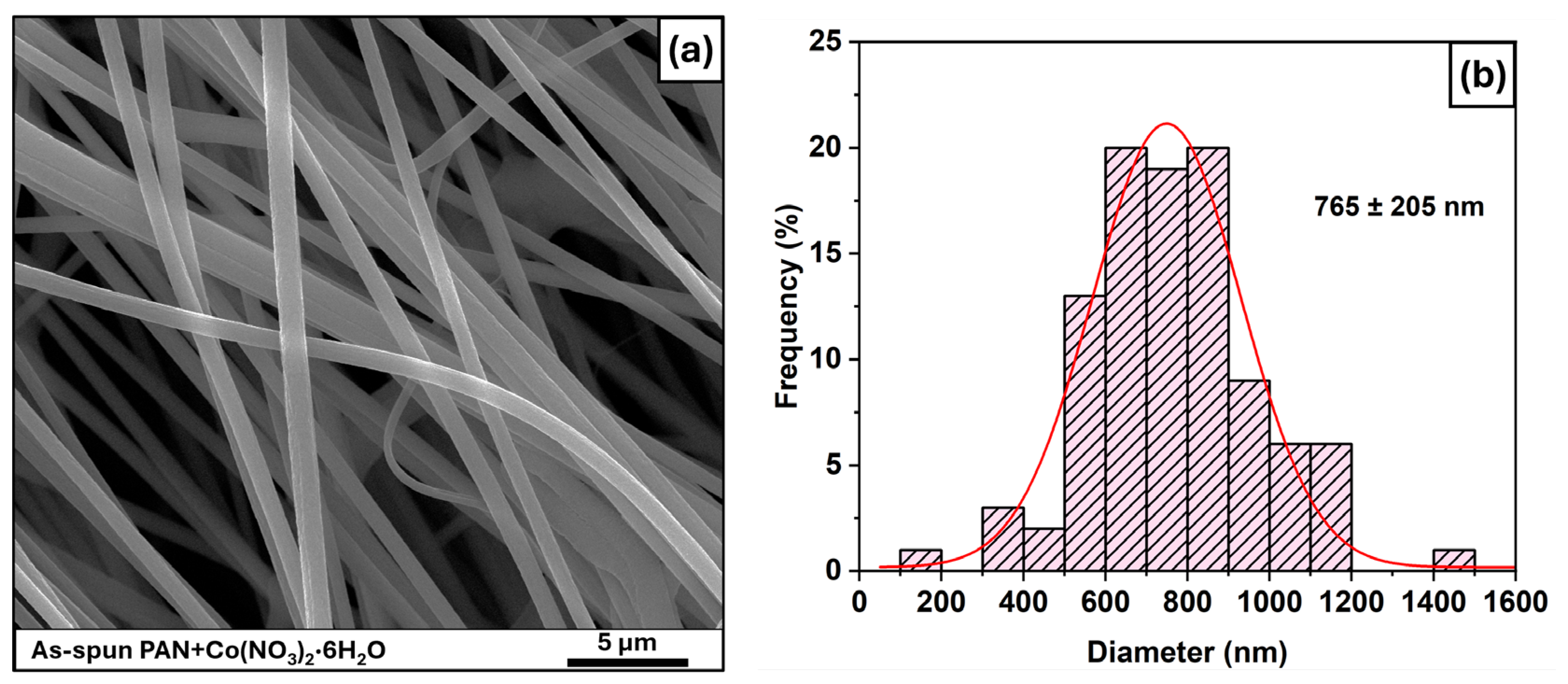
3.1. As-Spun Fibers
3.2. Influence of Time and Ligand Concentration
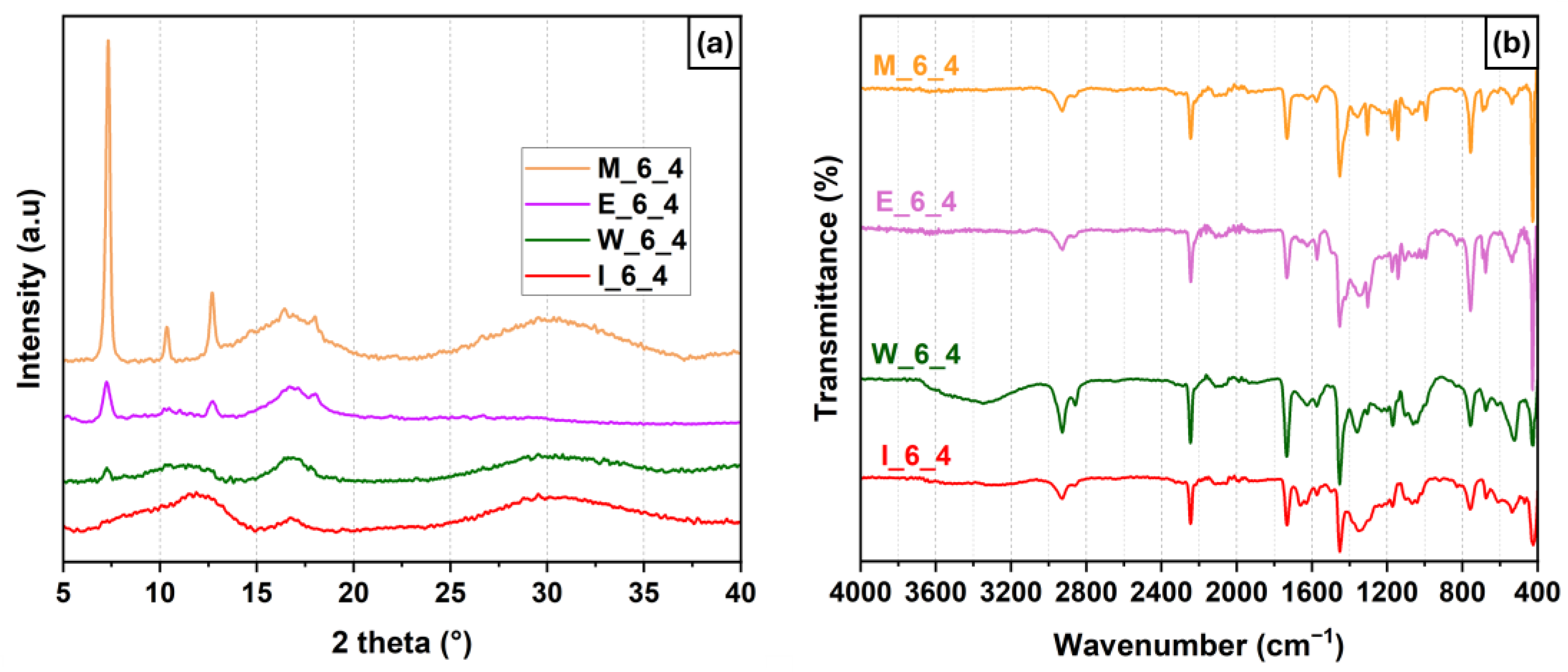
3.3. Influence of Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NOAA Research No Sign of Greenhouse Gases Increases Slowing in 2023. Available online: https://research.noaa.gov/no-sign-of-greenhouse-gases-increases-slowing-in-2023/ (accessed on 30 March 2025).
- National Grid What Is CCS and How Does It Work? Available online: https://www.nationalgrid.com/stories/energy-explained/what-is-ccs-how-does-it-work (accessed on 30 March 2025).
- Congressional Budget Office Carbon Capture and Storage in the United States. Available online: https://www.cbo.gov/publication/59832 (accessed on 30 March 2025).
- Bhavsar, A.; Hingar, D.; Ostwal, S.; Thakkar, I.; Jadeja, S.; Shah, M. The Current Scope and Stand of Carbon Capture Storage and Utilization—A Comprehensive Review. Case Stud. Chem. Environ. Eng. 2023, 8, 100368. [Google Scholar] [CrossRef]
- Li, D.; Yadav, A.; Zhou, H.; Roy, K.; Thanasekaran, P.; Lee, C. Advances and Applications of Metal-Organic Frameworks (MOFs) in Emerging Technologies: A Comprehensive Review. Glob. Chall. 2024, 8, 2300244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-J.; Chen, F.-F. Microwave Synthesis of Nanomaterials. Encycl. Nanomater. 2023, 1, 48–63. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent Progress on Synthesis of ZIF-67-Based Materials and Their Application to Heterogeneous Catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Saeed, S.; Bashir, R.; Rehman, S.U.; Nazir, M.T.; ALOthman, Z.A.; Muteb Aljuwayid, A.; Abid, A.; Adnan, A. Synthesis and Characterization of ZIF-67 Mixed Matrix Nanobiocatalysis for CO2 Adsorption Performance. Front. Bioeng. Biotechnol. 2022, 10, 891549. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Zhang, X.-X.; Zhang, Z.; Tong, X.-S.; Jia, C.-Z.; Liu, B.; Sun, C.-Y.; Yang, L.-Y.; Chen, G.-J. Large-Scale Synthesis of ZIF-67 and Highly Efficient Carbon Capture Using a ZIF-67/Glycol-2-Methylimidazole Slurry. Chem. Eng. Sci. 2015, 137, 504–514. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Experimental Characterization of Electrospinning: The Electrically Forced Jet and Instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
- Cramariuc, B.; Cramariuc, R.; Scarlet, R.; Manea, L.R.; Lupu, I.G.; Cramariuc, O. Fiber Diameter in Electrospinning Process. J. Electrostat. 2013, 71, 189–198. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Melo, G.H.F.; Sundararaj, U. Influence of Mixed Solvent in the Morphology and Hydrophobicity of Electrospun Polystyrene Porous Fibers. Macromol. Rapid Commun. 2024, 45, e2400403. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, Structures, and Materials. Macromol 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Ince Yardimci, A.; Yagmurcukardes, N.; Yagmurcukardes, M.; Capan, I.; Erdogan, M.; Capan, R.; Tarhan, O.; Acikbas, Y. Electrospun Polyacrylonitrile (PAN) Nanofiber: Preparation, Experimental Characterization, Organic Vapor Sensing Ability and Theoretical Simulations of Binding Energies. Appl. Phys. A 2022, 128, 173. [Google Scholar] [CrossRef]
- Zhao, R.-X.; Sun, P.; Liu, R.; Ding, Z.; Li, X.; Liu, X.; Zhao, X.; Gao, Z. Influence of Heating Procedures on the Surface Structure of Stabilized Polyacrylonitrile Fibers. Appl. Surf. Sci. 2018, 433, 321–328. [Google Scholar] [CrossRef]
- Melo, G.H.F.; Liu, Y.; Sundararaj, U. In Situ Growth of ZIF-67 Particles on PVDF Electrospun Nanofibers. Fibers Polym. 2024, 25, 3293–3306. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Li, Z. A Cobalt–Pyrrole Coordination Compound as High Performance Cathode Catalyst for Direct Borohydride Fuel Cells. RSC Adv. 2020, 10, 29119–29127. [Google Scholar] [CrossRef]
- Mashao, G.; Modibane, K.D.; Mdluli, S.B.; Iwuoha*, E.I.; Hato, M.J.; Makgopa, K.; Molapo, K.M. Polyaniline-Cobalt Benzimidazolate Zeolitic Metal-Organic Framework Composite Material for Electrochemical Hydrogen Gas Sensing. Electrocatalysis 2019, 10, 406–419. [Google Scholar] [CrossRef]
- Cetiner, S.; Sen, S.; Arman, B.; Sarac, A.S. Acrylonitrile/Vinyl Acetate Copolymer Nanofibers with Different Vinylacetate Content. J. Appl. Polym. Sci. 2013, 127, 3830–3838. [Google Scholar] [CrossRef]
- Naghshbandi, Z.; Gholinejad, M.; Sansano, J.M.; Eskandari, M. Graphene Quantum Dots Incorporated ZIF-67 for Stabilization of Au Nanoparticles: Efficient Catalyst for A3-coupling and Nitroarenes Reduction Reactions. Appl. Organomet. Chem. 2024, 38, e7400. [Google Scholar] [CrossRef]
- Nagshbandi, Z.; Gholinejad, M.; Sansano, J.M. Novel Magnetic Zeolitic Imidazolate Framework for Room Temperature Enhanced Catalysis. Inorg. Chem. Commun. 2023, 150, 110463. [Google Scholar] [CrossRef]
- Frentzel-Beyme, L.; Kloß, M.; Pallach, R.; Salamon, S.; Moldenhauer, H.; Landers, J.; Wende, H.; Debus, J.; Henke, S. Porous Purple Glass—A Cobalt Imidazolate Glass with Accessible Porosity from a Meltable Cobalt Imidazolate Framework. J. Mater. Chem. A Mater. 2019, 7, 985–990. [Google Scholar] [CrossRef]
- Ma, M.; Zhou, A.; Hong, T.; Jia, X.; Liu, M. Tailored Porous Structure and CO2 Adsorption Capacity of Mg-MOF-74 via Solvent Polarity Regulation. Chem. Eng. J. 2023, 476, 146845. [Google Scholar] [CrossRef]
- Feng, X.; Carreon, M.A. Kinetics of Transformation on ZIF-67 Crystals. J. Cryst. Growth 2015, 418, 158–162. [Google Scholar] [CrossRef]
- Feng, X.; Wu, T.; Carreon, M.A. Synthesis of ZIF-67 and ZIF-8 Crystals Using DMSO (Dimethyl Sulfoxide) as Solvent and Kinetic Transformation Studies. J. Cryst. Growth 2016, 455, 152–156. [Google Scholar] [CrossRef]
- Venna, S.R.; Jasinski, J.B.; Carreon, M.A. Structural Evolution of Zeolitic Imidazolate Framework-8. J. Am. Chem. Soc. 2010, 132, 18030–18033. [Google Scholar] [CrossRef]
- Cantor, B. The Equations of Materials; Oxford University Press: Oxford, UK, 2020; ISBN 9780191886683. [Google Scholar]
- Shirzad, K.; Viney, C. A Critical Review on Applications of the Avrami Equation beyond Materials Science. J. R. Soc. Interface 2023, 20, 20230242. [Google Scholar] [CrossRef]
- Van Vleet, M.J.; Weng, T.; Li, X.; Schmidt, J.R. In Situ, Time-Resolved, and Mechanistic Studies of Metal–Organic Framework Nucleation and Growth. Chem. Rev. 2018, 118, 3681–3721. [Google Scholar] [CrossRef]
- Rimer, J.D.; Tsapatsis, M. Nucleation of Open Framework Materials: Navigating the Voids. MRS Bull. 2016, 41, 393–398. [Google Scholar] [CrossRef]
- Akhundzadeh Tezerjani, A.; Halladj, R.; Askari, S. Different View of Solvent Effect on the Synthesis Methods of Zeolitic Imidazolate Framework-8 to Tuning the Crystal Structure and Properties. RSC Adv. 2021, 11, 19914–19923. [Google Scholar] [CrossRef]
- Choi, C.; Kadam, R.L.; Gaikwad, S.; Hwang, K.-S.; Han, S. Metal Organic Frameworks Immobilized Polyacrylonitrile Fiber Mats with Polyethyleneimine Impregnation for CO2 Capture. Microporous Mesoporous Mater. 2020, 296, 110006. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Wang, X.; Yu, J.; Ding, B. Ultrahigh Metal–Organic Framework Loading and Flexible Nanofibrous Membranes for Efficient CO2 Capture with Long-Term, Ultrastable Recyclability. ACS Appl. Mater. Interfaces 2018, 10, 34802–34810. [Google Scholar] [CrossRef]
- Mo, J.; Hu, D.; Liu, X.; Sun, Y.; Li, X.; Wang, C.; Chai, B.; Ding, D.; Yan, J.; Sun, L. Eco-Friendly ZIF-67/Rice Straw-Derived Cellulose Acetate Electrospun Nanofiber Mats for Efficient CO2 Capturing and Selectivity Removal of Methyl Orange Dye. J. Environ. Chem. Eng. 2022, 10, 108989. [Google Scholar] [CrossRef]
- Li, C.; He, N.; Zhao, X.; Zhang, X.; Li, W.; Zhao, X.; Qiao, Y. Chitosan/ZIF-8 Composite Beads Fabricated by In Situ Growth of MOFs Crystals on Chitosan Beads for CO2 Adsorption. ChemistrySelect 2022, 7, e202103927. [Google Scholar] [CrossRef]
- Gaikwad, R.; Gaikwad, S.; Han, S. Piperazine-Loaded Electrospun Fiber Mats with MIL-101(Cr, Mg) Metal Organic Framework for CO2 Capture. J. Environ. Chem. Eng. 2023, 11, 111592. [Google Scholar] [CrossRef]


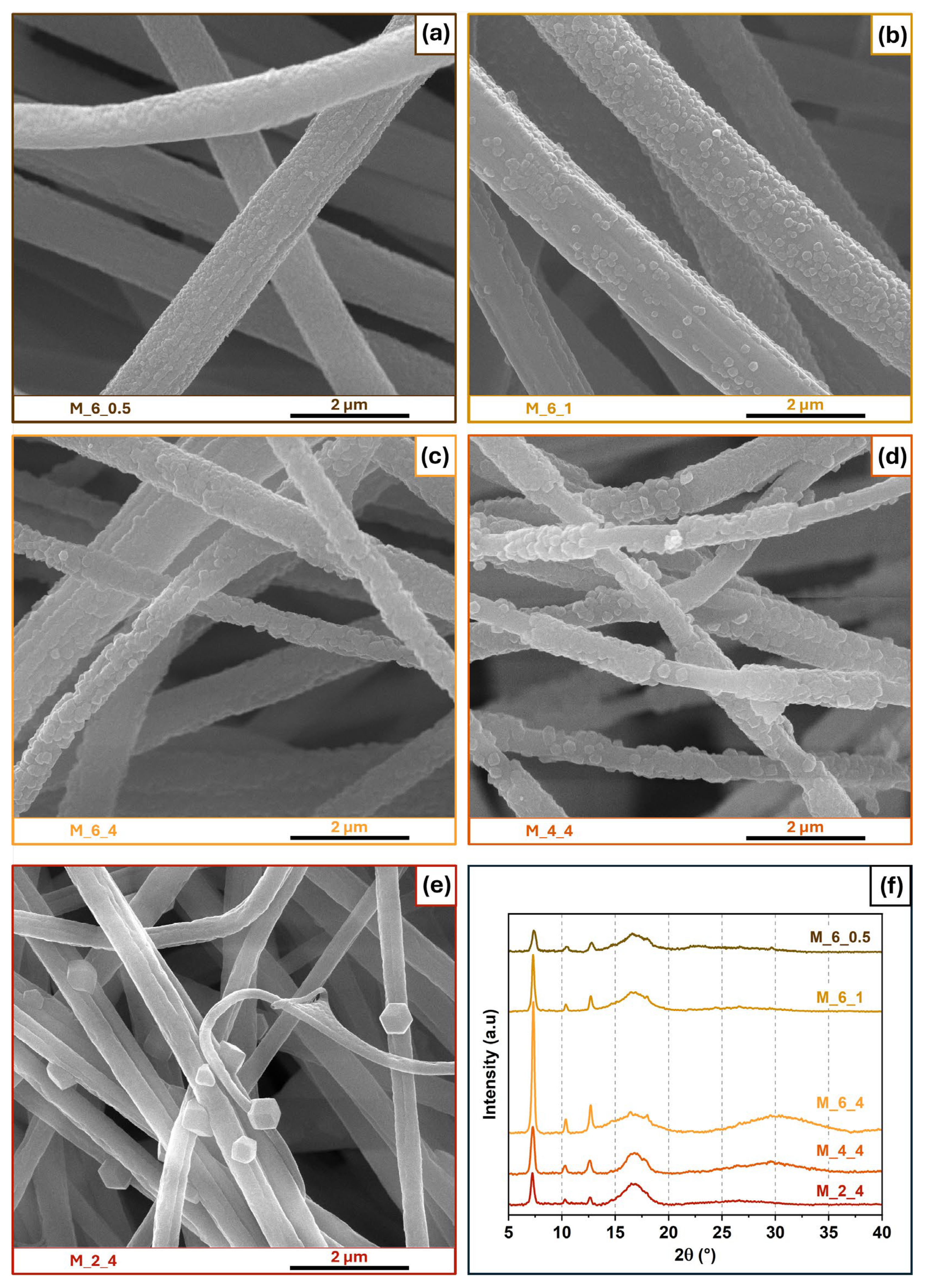
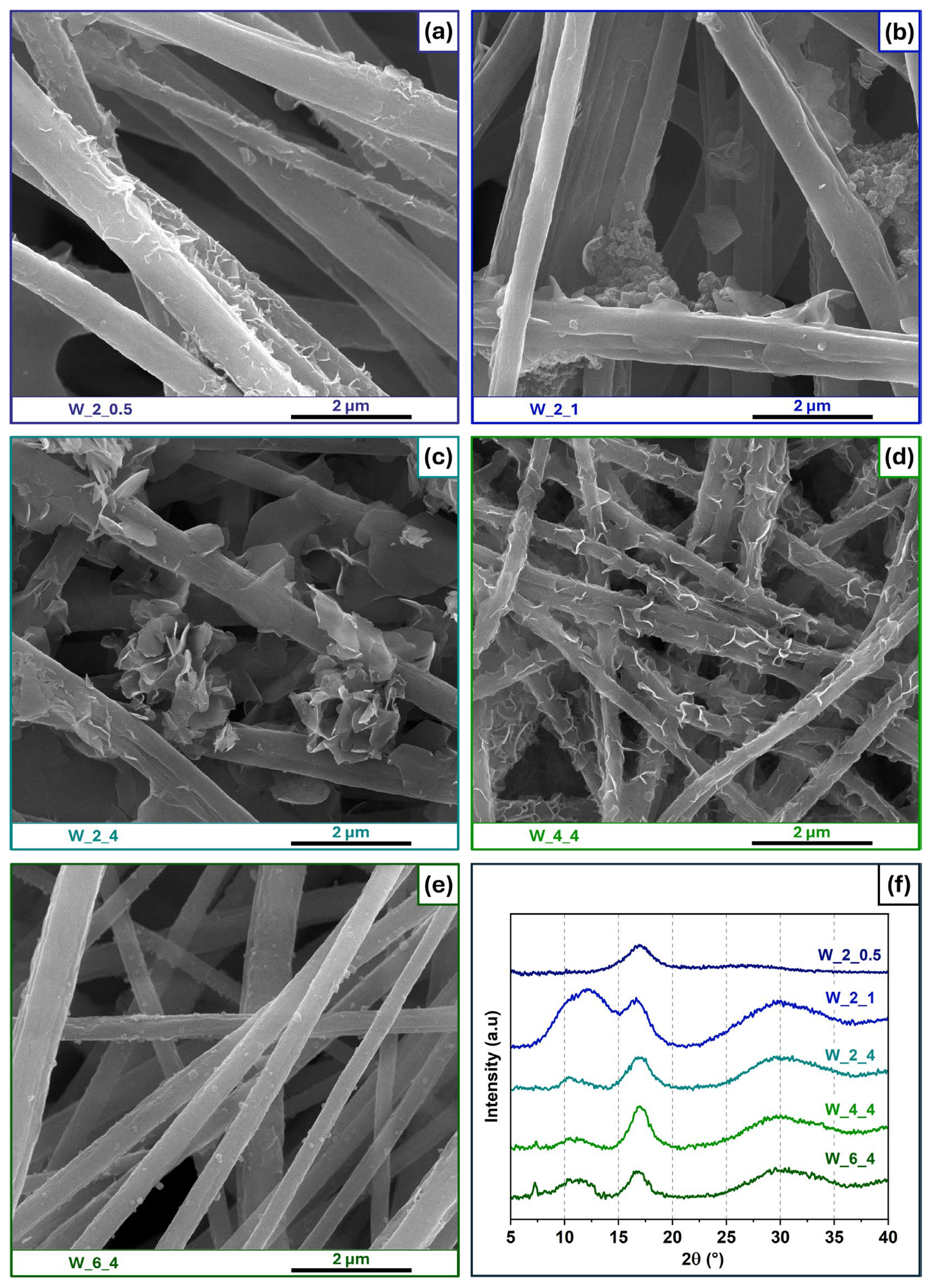


| Solvent | 2-MeIm Concentration (mg/mL) | Time (h) | Sample Name |
|---|---|---|---|
| Methanol | 6 | 0.5 | M_6_0.5 |
| Methanol | 6 | 1 | M_6_1 |
| Methanol | 6 | 4 | M_6_4 |
| Methanol | 4 | 4 | M_4_4 |
| Methanol | 2 | 4 | M_2_4 |
| Water | 2 | 0.5 | W_2_0.5 |
| Water | 2 | 1 | W_2_1 |
| Water | 2 | 4 | W_2_4 |
| Water | 4 | 4 | W_4_4 |
| Water | 6 | 4 | W_6_4 |
| Ethanol | 6 | 4 | E_6_4 |
| Isopropanol | 6 | 4 | I_6_4 |
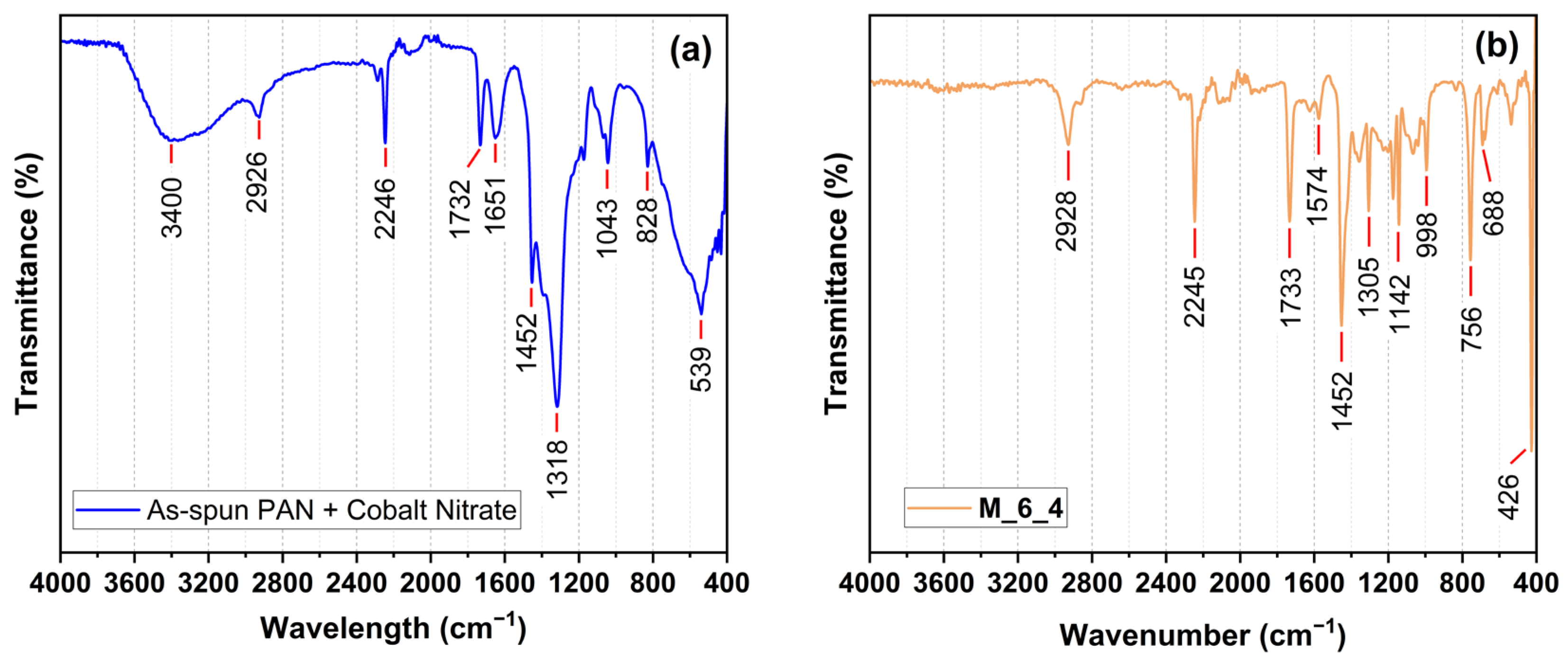
| As-Spun Fiber | ZIF-67@PAN | |||
|---|---|---|---|---|
| Frequency (cm−1) | Bond | Assignment | Bond | Assignment |
| 3400 | O-H | H2O | - | - |
| 2926 | C-H | PAN–comonomer | C-H | PAN–comonomer |
| 2246 | C≡N | PAN | C≡N | PAN |
| 1732 | C=O | Comonomer | C=O | Comonomer |
| 1651 | N=O | Cobalt nitrate | - | - |
| 1572 | - | - | C=N | ZIF-67 |
| 1452 | C-H | PAN | C-H | PAN |
| 1392 | N=O | Cobalt nitrate | - | - |
| 1318 | C=O | Comonomer | C=O | Comonomer |
| 1170 | C-O | Comonomer | C-O | Comonomer |
| 1141 | - | - | Imidazole ring | ZIF-67 |
| 1043 | C-O-C | PAN–comonomer | C-O-C | PAN–comonomer |
| 998 | - | - | Imidazole ring | ZIF-67 |
| 828 | N-O | Cobalt nitrate | - | - |
| 756 | - | - | Imidazole ring | ZIF-67 |
| 688 | - | - | Imidazole ring | ZIF-67 |
| 539 | Co-O | Cobalt nitrate | Co-O | Cobalt nitrate |
| 426 | - | - | Co-N | ZIF-67 |
| MOF | Polymer | Weight Content | Integration Method | CO2 Capacity (mmol/g) | Temperature [K] | Reference |
|---|---|---|---|---|---|---|
| ZIF-67 | PAN | 2% | In situ growth on nanofibers | 0.40 | 273 | This work |
| ZIF-67 | PSU | 3% | Solution casting | 0.50 | 273 | [8] |
| ZIF-8 | PAN | 60% | Direct electrospinning | 0.55 | 298 | [36] |
| HKUST-1 | PAN | 60% | Direct electrospinning | 2.55 | 298 | [36] |
| HKUST-1 | PAN | 82% | Direct electrospinning + Secondary growth | 3.90 | 298 | [37] |
| ZIF-67 | RSCA | - | In situ growth on nanofibers | 1.33 | 273 | [38] |
| ZIF-8 | Chitosan | - | In situ growth on beads | ≈0.70 | 275 | [39] |
| MIL-101 | PAN | 70% | Direct electrospinning | 1.00 | 298 | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, G.H.F.; Yau, T.; Liu, Y.; Sundararaj, U. Preparation of ZIF-67@PAN Nanofibers for CO2 Capture: Effects of Solvent and Time on Particle Morphology. Fibers 2025, 13, 50. https://doi.org/10.3390/fib13050050
Melo GHF, Yau T, Liu Y, Sundararaj U. Preparation of ZIF-67@PAN Nanofibers for CO2 Capture: Effects of Solvent and Time on Particle Morphology. Fibers. 2025; 13(5):50. https://doi.org/10.3390/fib13050050
Chicago/Turabian StyleMelo, Guilherme Henrique Franca, Tiffany Yau, Yuxin Liu, and Uttandaraman Sundararaj. 2025. "Preparation of ZIF-67@PAN Nanofibers for CO2 Capture: Effects of Solvent and Time on Particle Morphology" Fibers 13, no. 5: 50. https://doi.org/10.3390/fib13050050
APA StyleMelo, G. H. F., Yau, T., Liu, Y., & Sundararaj, U. (2025). Preparation of ZIF-67@PAN Nanofibers for CO2 Capture: Effects of Solvent and Time on Particle Morphology. Fibers, 13(5), 50. https://doi.org/10.3390/fib13050050








