Assessment of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Bone Metastases: An in Vitro Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Lines
2.2. PDT Exposure with 5-Aminolaevulinic Acid
2.3. Migration Assay
2.4. Viability Assessment
2.5. Nuclear Morphology Assessment
2.6. Senescence Assay
2.7. Statistical Analysis
3. Results
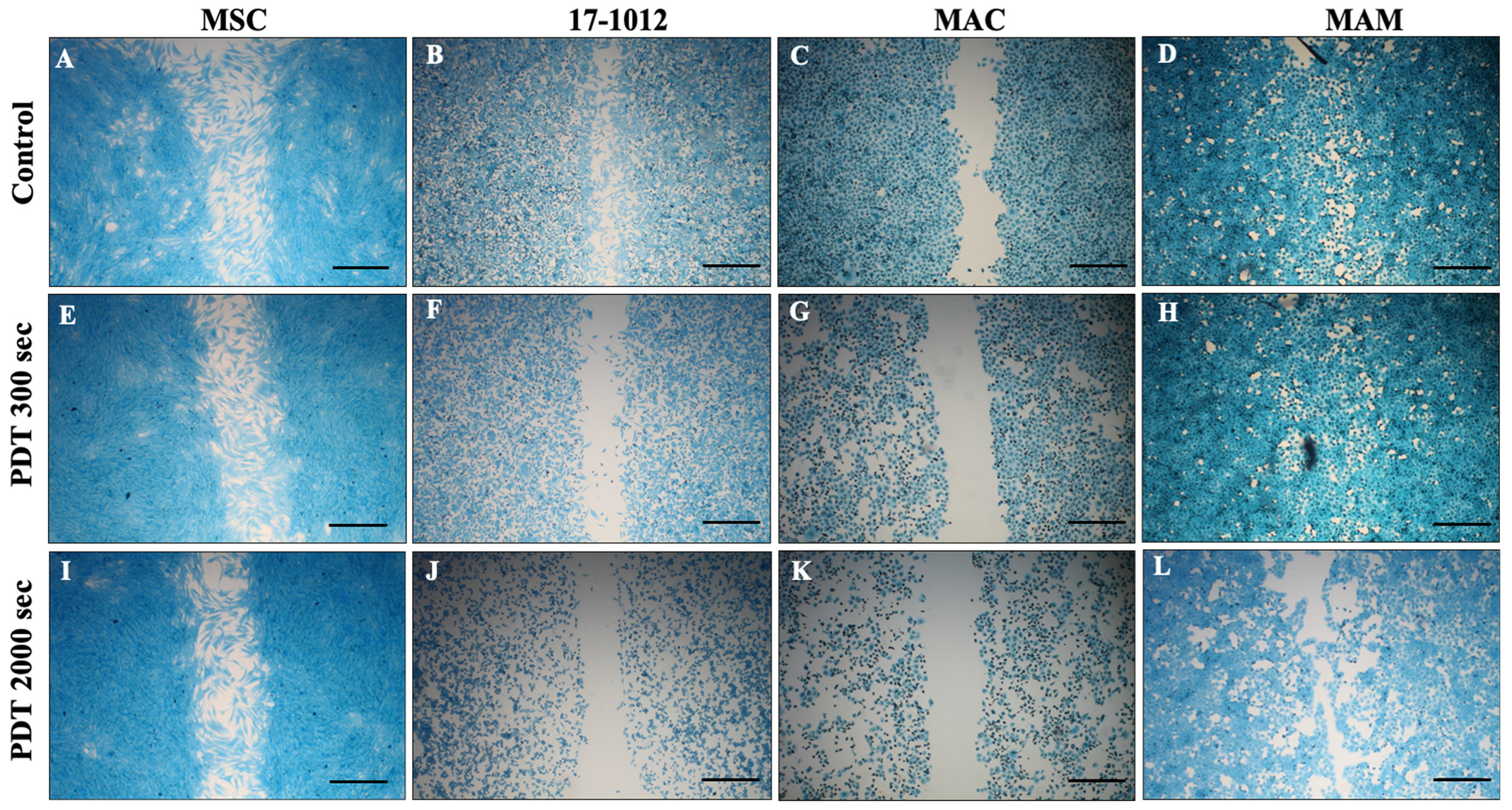
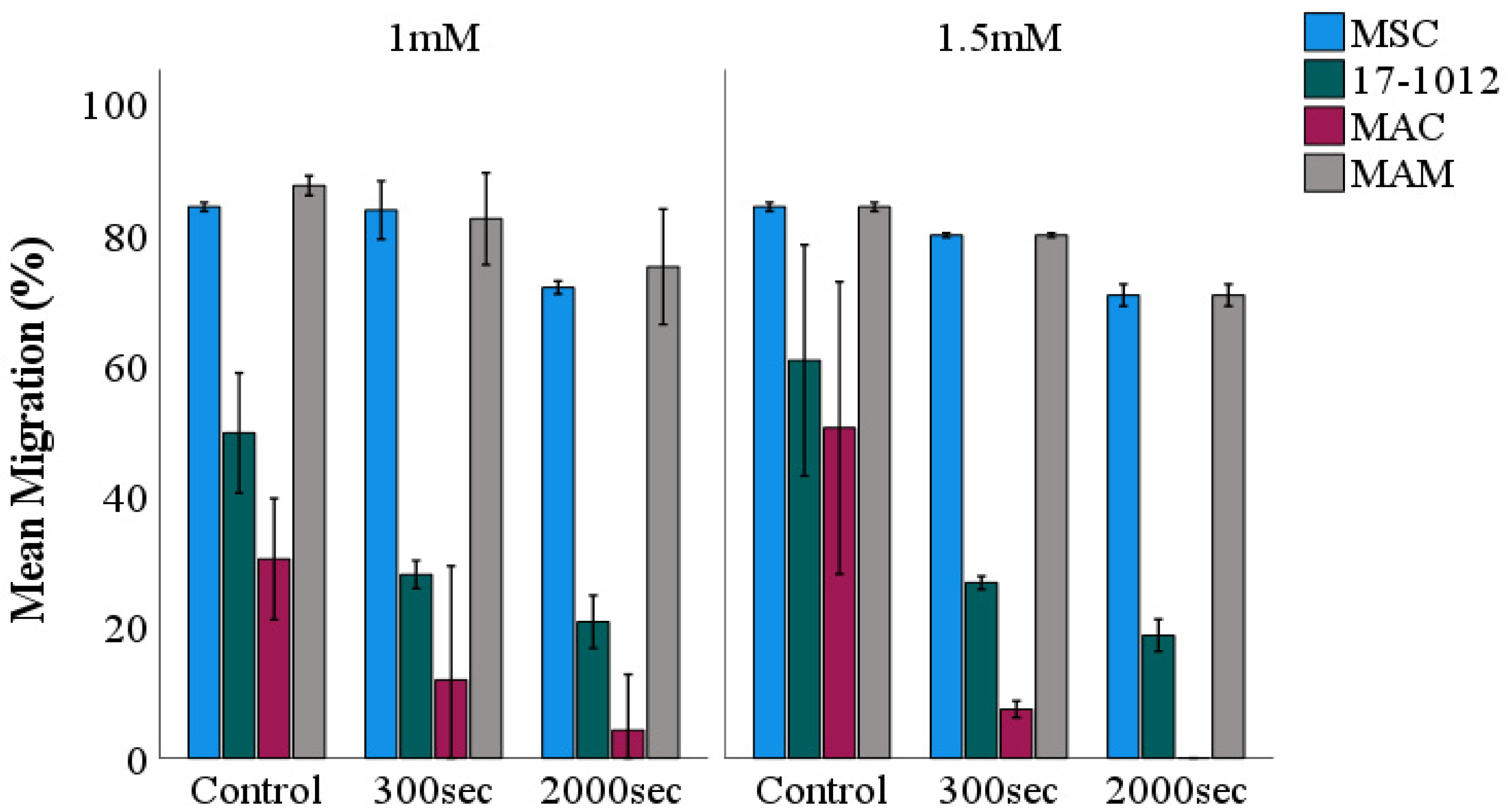
3.1. Migration Assay
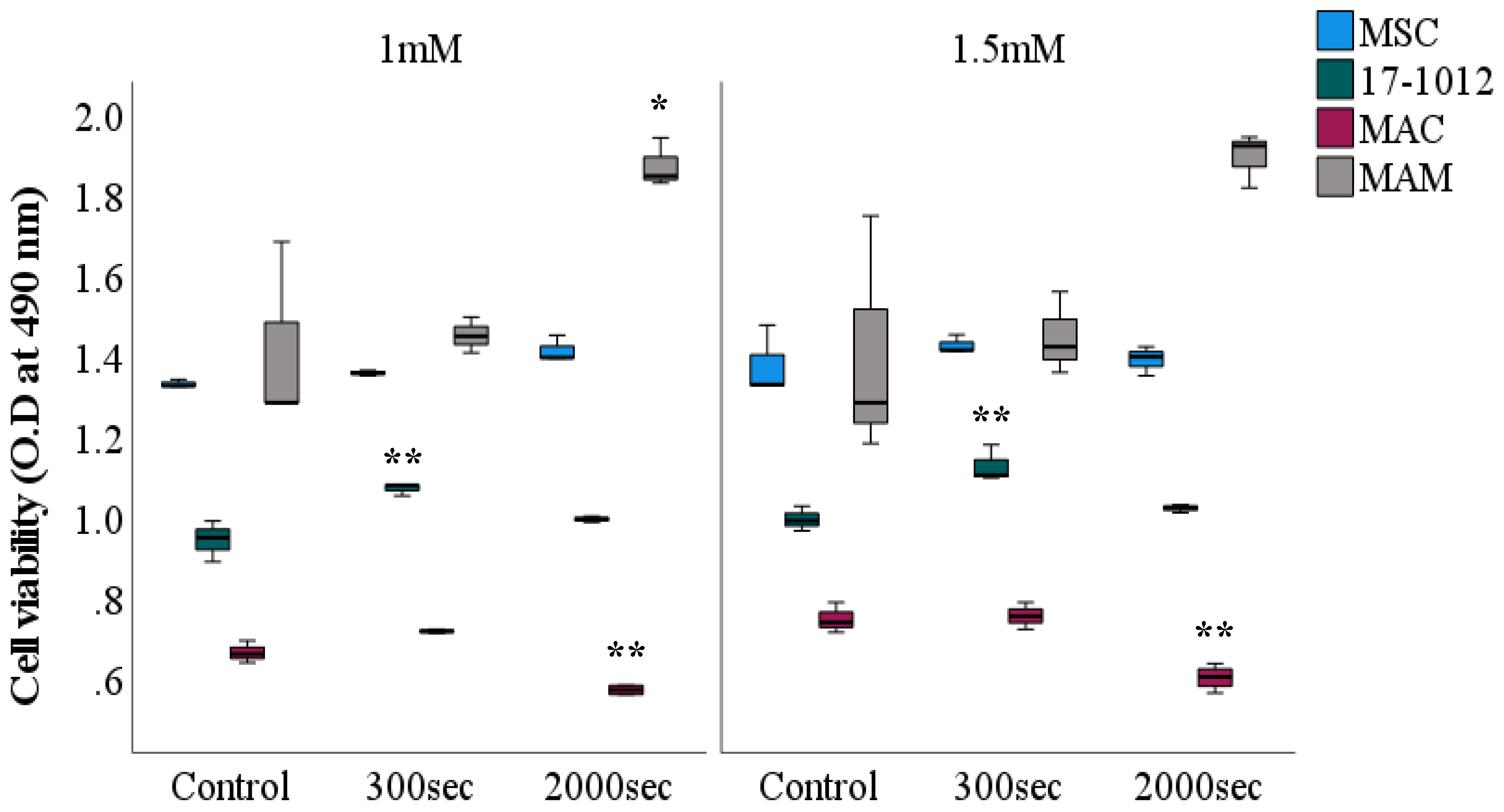
3.2. Viability Assessment
3.3. Fluorescence Assessment of Apoptosis
3.4. Cellular Senescence
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coleman, R.E. Metastatic bone disease: Clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001, 27, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Janjan, N.; Lutz, S.T.; Bedwinek, J.M.; Hartsell, W.F.; Ng, A.; Pieters, R.S., Jr.; Ratanatharathorn, V.; Silberstein, E.B.; Taub, R.J.; Yasko, A.W.; et al. Therapeutic guidelines for the treatment of bone metastasis: A report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J. Palliat. Med. 2009, 12, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Constine, L.S.; Okunieff, P. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin. Radiat. Oncol. 2007, 17, 131–140. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Jhingran, A.; Iyer, R.B.; Sun, C.C.; Eifel, P.J.; Soliman, P.T.; Ramirez, P.T.; Frumovitz, M.; Bodurka, D.C.; Sood, A.K. Pelvic fractures after radiotherapy for cervical cancer: Implications for survivors. Cancer 2010, 116, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.G.; Lee, B.R.; Choi, E.Y.; Kim, D.W.; Han, S.J. Photodynamic therapy for breast cancer in a BALB/c mouse model. J. Gynecol. Oncol. 2012, 23, 115–119. [Google Scholar] [CrossRef]
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic Enhancement of Carboplatin Efficacy with Photodynamic Therapy in a Three-Dimensional Model for Micrometastatic Ovarian Cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef] [Green Version]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Dougherty, T.J. Photosensitizers: Therapy and Detection of Malignant Tumors. Photochem. Photobiol. 1987, 45, 879–889. [Google Scholar] [CrossRef]
- Burch, S.; Bogaards, A.; Siewerdsen, J.; Moseley, D.; Yee, A.; Finkelstein, J.; Weersink, R.; Wilson, B.; Bisland, S. Photodynamic therapy for the treatment of metastatic lesions in bone: Studies in rat and porcine models. J. Biomed. Opt. 2005, 10, 034011. [Google Scholar] [CrossRef]
- Stewart, F.; Baas, P.; Star, W. What does photodynamic therapy have to offer radiation oncologists (or their cancer patients)? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1998, 48, 233–248. [Google Scholar] [CrossRef]
- Malik, Z.; Lugaci, H. Destruction of erythroleukaemic cells by photoactivation of endogenous porphyrins. Br. J. Cancer 1987, 56, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [Green Version]
- Dalton, J.T.; Yates, C.R.; Yin, D.; Straughn, A.; Marcus, S.L.; Golub, A.L.; Meyer, M.C. Clinical pharmacokinetics of 5-aminolevulinic acid in healthy volunteers and patients at high risk for recurrent bladder cancer. J. Pharmacol. Exp. Ther. 2002, 301, 507–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shetty, T.; Corson, T.W. Mitochondrial Heme Synthesis Enzymes as Therapeutic Targets in Vascular Diseases. Front. Pharmacol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Schwartz, C.; Rühm, A.; Tonn, J.-C.; Kreth, S.; Kreth, F.-W. SURG-25: Interstitial Photodynamic Therapy Of De-Novo Glioblastoma Multiforme WHO IV. Neuro Oncol. 2015, 17 (Suppl. 5), v219–v220. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Quidet, M.; Lecomte, F.; Lerhun, E.; Mordon, S.; Betrouni, N.; Reyns, N. Set-up of the first pilot study on intraopertive 5-ALA PDT: INDYGO trial. Photodiagnosis Photodyn. Ther. 2017, 100, A21. [Google Scholar] [CrossRef] [Green Version]
- Wiedmann, M.; Caca, K.; Berr, F.; Schiefke, I.; Tannapfel, A.; Wittekind, C.; Mössner, J.; Hauss, J.; Witzigmann, H. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: A phase II pilot study. Cancer 2003, 97, 2783–2790. [Google Scholar] [CrossRef]
- Sultan, S.M.; El-Doray, A.A.; Hofstetter, A.; Abdel-Gawad, O.; El-Mahdy Ael, D.; Khoder, W. Photodynamic selectivity of 5-aminolevulinic acid to prostate cancer cells. J. Egypt. Natl. Cancer Inst. 2006, 18, 382–386. [Google Scholar]
- van Straten, D.; Mashayekhi, V.; de Bruijn, H.S.; Oliveira, S.; Robinson, D.J. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef]
- Fan, H.T.; Wang, L.; Zhang, P.; Liu, S.B. Photodynamic therapy in spinal metastases: A qualitative analysis of published results. Int. Surg. 2015, 100, 712–719. [Google Scholar] [CrossRef]
- Wise-Milestone, L.; Akens, M.K.; Lo, V.C.; Yee, A.J.; Wilson, B.C.; Whyne, C.M. Local treatment of mixed osteolytic/osteoblastic spinal metastases: Is photodynamic therapy effective? Breast Cancer Res. Treat. 2012, 133, 899–908. [Google Scholar] [CrossRef]
- Fisher, C.; Ali, Z.; Detsky, J.; Sahgal, A.; David, E.; Kunz, M.; Akens, M.; Chow, E.; Whyne, C.; Burch, S.; et al. Photodynamic Therapy for the Treatment of Vertebral Metastases: A Phase I Clinical Trial. Clin. Cancer Res. 2019, 25, 5766–5776. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Yen, C.-J.; Wu, B.-S.; Wong, T.-W. Recent Advances in Photodynamic Therapy for Deep-Seated Tumors with the Aid of Nanomedicine. Biomedicines 2021, 9, 69. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Wang, H.; Tang, Z.; Zuo, C.; He, M.; Bu, W. Photoelectron Transfer at ZnTPyP Self-Assembly/TiO(2) Interfaces for Enhanced Two-Photon Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Battula, V.L.; Treml, S.; Bareiss, P.M.; Gieseke, F.; Roelofs, H.; de Zwart, P.; Müller, I.; Schewe, B.; Skutella, T.; Fibbe, W.E.; et al. Isolation of functionally distinct mesenchymal stem cell subsets using antibodies against CD56, CD271, and mesenchymal stem cell antigen-1. Haematologica 2009, 94, 173–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C.P., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Mandelkow, R.; Gümbel, D.; Ahrend, H.; Kaul, A.; Zimmermann, U.; Burchardt, M.; Stope, M.B. Detection and Quantification of Nuclear Morphology Changes in Apoptotic Cells by Fluorescence Microscopy and Subsequent Analysis of Visualized Fluorescent Signals. Anticancer. Res. 2017, 37, 2239–2244. [Google Scholar] [CrossRef] [Green Version]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef] [Green Version]
- Beauséjour, C. Bone marrow-derived cells: The influence of aging and cellular senescence. Handb. Exp. Pharmacol. 2007, 180, 67–88. [Google Scholar]
- Chen, X.; Zhao, P.; Chen, F.; Li, L.; Luo, R. Effect and mechanism of 5-aminolevulinic acid-mediated photodynamic therapy in esophageal cancer. Lasers Med. Sci. 2011, 26, 69–78. [Google Scholar] [CrossRef]
- Bacellar, I.O.; Tsubone, T.M.; Pavani, C.; Baptista, M.S. Photodynamic Efficiency: From Molecular Photochemistry to Cell Death. Int. J. Mol. Sci. 2015, 16, 20523–20559. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Amo, T.; Kawanishi, N.; Uchida, M.; Fujita, H.; Oyanagi, E.; Utsumi, T.; Ogino, T.; Inoue, K.; Shuin, T.; Utsumi, K.; et al. Mechanism of cell death by 5-aminolevulinic acid-based photodynamic action and its enhancement by ferrochelatase inhibitors in human histiocytic lymphoma cell line U937. Cell Biochem. Funct. 2009, 27, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Chelakkot, V.S.; Som, J.; Yoshioka, E.; Rice, C.P.; Rutihinda, S.G.; Hirasawa, K. Systemic MEK inhibition enhances the efficacy of 5-aminolevulinic acid-photodynamic therapy. Br. J. Cancer 2019, 121, 758–767. [Google Scholar] [CrossRef]
- Gordon, R.R.; Nelson, P.S. Cellular senescence and cancer chemotherapy resistance. Drug Resist. Updates Rev. Comment Antimicrob. Anticancer. Chemother. 2012, 15, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Shay, J.W.; Roninson, I.B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene 2004, 23, 2919–2933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Lee, J.S. Exploiting tumor cell senescence in anticancer therapy. BMB Rep. 2014, 47, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mosieniak, G.; Strzeszewska, A. The role of cellular senescence in carcinogenesis and antitumor therapy. Postepy Biochem. 2014, 60, 194–206. [Google Scholar] [PubMed]
- Coppé, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. The senescence-associated secretory phenotype: The dark side of tumor suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Fekrazad, R.; Asefi, S.; Khorsandi, K.; Nejatifard, M. Photo biostimulatory effect of low dose photodynamic therapy on human mesenchymal stem cells. Photodiagnosis Photodyn. Ther. 2020, 31, 101886. [Google Scholar] [CrossRef]
- Grigalavicius, M.; Juraleviciute, M.; Kwitniewski, M.; Juzeniene, A. The influence of photodynamic therapy with 5-aminolevulinic acid on senescent skin cancer cells. Photodiagnosis Photodyn. Ther. 2017, 17, 29–34. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis Photodyn. Ther. 2005, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.R.; Matthews, W.; Mitchell, J.B.; Russo, A.; Evans, S.; Pass, H.I. Sensitivity of different human lung cancer histologies to photodynamic therapy. Cancer Res. 1990, 50, 4272–4276. [Google Scholar]
- Wickman, G.; Julian, L.; Olson, M.F. How apoptotic cells aid in the removal of their own cold dead bodies. Cell Death Differ. 2012, 19, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Schulze-Osthoff, K.; Walczak, H.; Dröge, W.; Krammer, P.H. Cell nucleus and DNA fragmentation are not required for apoptosis. J. Cell Biol. 1994, 127, 15–20. [Google Scholar] [CrossRef]
- Tong, Z.; Singh, G.; Rainbow, A.J. Sustained activation of the extracellular signal-regulated kinase pathway protects cells from photofrin-mediated photodynamic therapy. Cancer Res. 2002, 62, 5528–5535. [Google Scholar]
- Casas, A.; Di Venosa, G.; Hasan, T.; Al, B. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Bae, S.M.; Battogtokh, G.; Bang, H.J.; Ahn, W.S. Synergistic anti-tumor effects of combination of photodynamic therapy and arsenic compound in cervical cancer cells: In vivo and in vitro studies. PLoS ONE 2012, 7, e38583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, C.A.; Szeimies, R.-M.; Basset-Séguin, N.; Calzavara-Pinton, P.G.; Gilaberte, Y.; Hædersdal, M.; Hofbauer, G.F.L.; Hunger, R.E.; Karrer, S.; Piaserico, S.; et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 2: Emerging indications—Field cancerization, photorejuvenation and inflammatory/infective dermatoses. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the in vivo and in vitro effects of photodynamic therapy on breast cancer by using a sensitizer, sinoporphyrin sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Huxley, J. Biological aspects of cancer: Harcourt, Brace. Science 1958, 127, 1440–1441. [Google Scholar]
- Alizadeh, A.A.; Aranda, V.; Bardelli, A.; Blanpain, C.; Bock, C.; Borowski, C.; Caldas, C.; Califano, A.; Doherty, M.; Elsner, M. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 2015, 21, 846–853. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Z.; Gu, X.-y.; Xiang, S.-y.; Gong, D.-d.; Man, C.-f.; Fan, Y. Research and application of single-cell sequencing in tumor heterogeneity and drug resistance of circulating tumor cells. Biomark. Res. 2020, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Barron, G.A.; Moseley, H.; Woods, J.A. Differential sensitivity in cell lines to photodynamic therapy in combination with ABCG2 inhibition. J. Photochem. Photobiol. B Biol. 2013, 126, 87–96. [Google Scholar] [CrossRef]
- Choi, B.-h.; Ryoo, I.-g.; Kang, H.C.; Kwak, M.-K. The Sensitivity of Cancer Cells to Pheophorbide a-Based Photodynamic Therapy Is Enhanced by NRF2 Silencing. PLoS ONE 2014, 9, e107158. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sachsenmaier, S.M.; Traub, F.; Cykowska, A.; Riester, R.; Wülker, N.; Walter, C.; Danalache, M. Assessment of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Bone Metastases: An in Vitro Study. Biology 2021, 10, 1020. https://doi.org/10.3390/biology10101020
Sachsenmaier SM, Traub F, Cykowska A, Riester R, Wülker N, Walter C, Danalache M. Assessment of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Bone Metastases: An in Vitro Study. Biology. 2021; 10(10):1020. https://doi.org/10.3390/biology10101020
Chicago/Turabian StyleSachsenmaier, Saskia Magdalen, Frank Traub, Anna Cykowska, Rosa Riester, Nikolaus Wülker, Christian Walter, and Marina Danalache. 2021. "Assessment of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Bone Metastases: An in Vitro Study" Biology 10, no. 10: 1020. https://doi.org/10.3390/biology10101020
APA StyleSachsenmaier, S. M., Traub, F., Cykowska, A., Riester, R., Wülker, N., Walter, C., & Danalache, M. (2021). Assessment of 5-Aminolevulinic Acid-Mediated Photodynamic Therapy on Bone Metastases: An in Vitro Study. Biology, 10(10), 1020. https://doi.org/10.3390/biology10101020






