Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future
Abstract
:Simple Summary
Abstract
1. Introduction
2. Microbial Endophytes Present in Desert Plants
2.1. Bacterial Endophytes
2.2. Fungal Endophytes
2.3. Transfer of Endophytes between Desert Plants
3. Nutrient Acquisition
3.1. Nitrogen
3.2. Phosphorus
4. Effects on Abiotic Stress Resistance
4.1. Drought and Heat Tolerance
4.2. Salt Tolerance
5. Effects on Biotic Stress Resistance
5.1. Pathogen Tolerance
5.2. Pest Tolerance
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Livingston, B.E. The Relation of Desert Plants to Soil Moisture and to Evaporation; Carnegie Institution of Washington: Washington, DC, USA, 1906; Volume 50. [Google Scholar]
- West, N.; Klemmedson, J. Structural distribution of nitrogen in desert ecosystems. In Nitrogen in Desert Ecosystems; West, N.E., Skujin̦š, J., Eds.; Dowden, Hutchinson and Ross: Stroudsburg, PA, USA, 1978; pp. 1–16. [Google Scholar]
- Lajtha, K.; Schlesinger, W.H. The biogeochemistry of phosphorus cycling and phosphorus availability along a desert soil chronosequence. Ecology 1988, 69, 24–39. [Google Scholar] [CrossRef]
- Abrahams, A.D.; Parsons, A.J. Relation between infiltration and stone cover on a semiarid hillslope, southern Arizona. J. Hydrol. 1991, 122, 49–59. [Google Scholar] [CrossRef]
- Smith, S.; Herr, C.A.; Leary, K.; Piorkowski, J. Soil-plant water relations in a Mojave Desert mixed shrubcommunity: A comparison of three geomorphic surfaces. J. Arid Environ. 1995, 29, 339–351. [Google Scholar] [CrossRef]
- Warner, T.T. Desert Meteorology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Wilson, D. Endophyte: The evolution of a term, and clarification of its use and definition. Oikos 1995, 73, 274–276. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurek, T.; Handley, L.L.; Reinhold-Hurek, B.; Piché, Y. Azoarcus grass endophytes contribute fixed nitrogen to the plant in an unculturable state. Mol. Plant-Microbe Interact. 2002, 15, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.; Ibáñez, F.; Rosenblueth, M.; Etcheverry, M.; Martínez-Romero, E. Analysis of the bacterial diversity associated with the roots of maize (Zea mays L.) through culture-dependent and culture-independent methods. ISRN Ecol. 2011, 2011, 938546. [Google Scholar] [CrossRef] [Green Version]
- Baldani, V.D.; Baldani, J.I.; Döbereiner, J. Inoculation of rice plants with the endophytic diazotrophs Herbaspirillum seropedicae and Burkholderia spp. Biol. Fertil. Soils 2000, 30, 485–491. [Google Scholar] [CrossRef]
- Verma, S.K.; Kingsley, K.L.; Bergen, M.S.; Kowalski, K.P.; White, J.F. Fungal disease prevention in seedlings of rice (Oryza sativa) and other grasses by growth-promoting seed-associated endophytic bacteria from invasive Phragmites australis. Microorganisms 2018, 6, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturz, A.; Nowak, J. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops. Appl. Soil Ecol. 2000, 15, 183–190. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Van Bael, S.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.F.; Chang, X.; Kingsley, K.L.; Zhang, Q.; Chiaranunt, P.; Micci, A.; Velazquez, F.; Elmore, M.; Crane, S.; Li, S.; et al. Endophytic bacteria in grass crop growth promotion and biostimulation. Grass Res. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef] [Green Version]
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Wang, G. Agricultural drought in a future climate: Results from 15 global climate models participating in the IPCC 4th assessment. Clim. Dyn. 2005, 25, 739–753. [Google Scholar] [CrossRef]
- Christensen, O.; Christensen, J. Intensification of extreme European summer precipitation in a warmer climate. Glob. Planet. Change 2004, 44, 107–117. [Google Scholar] [CrossRef]
- Alfieri, L.; Burek, P.; Feyen, L.; Forzieri, G. Global warming increases the frequency of river floods in Europe. Hydrol. Earth Syst. Sci. 2015, 19, 2247. [Google Scholar] [CrossRef] [Green Version]
- Hallberg, G.R. Pesticides pollution of groundwater in the humid United States. Agric. Ecosyst. Environ. 1989, 26, 299–367. [Google Scholar] [CrossRef]
- Novotny, V. Diffuse pollution from agriculture-a worldwide outlook. Water Sci. Technol. 1999, 39, 1–13. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, L.; Yang, L.; Zhang, F.; Norse, D.; Zhu, Z. Agricultural non-point source pollution in China: Causes and mitigation measures. Ambio 2012, 41, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Donaldson, D. Organic Market Summary and Trends. Available online: https://www.ers.usda.gov/topics/natural-resources-environment/organic-agriculture/organic-market-summary-and-trends/ (accessed on 15 September 2021).
- Willer, H.; Schaack, D. European Organic Market Grew to 40.7 Billion Euros in 2018. Available online: https://www.fibl.org/en/info-centre/news/european-organic-market-grew-to-40-7-billion-euros-in-2018 (accessed on 15 September 2021).
- Rana, J.; Paul, J. Consumer behavior and purchase intention for organic food: A review and research agenda. J. Retail. Consum. Serv. 2017, 38, 157–165. [Google Scholar] [CrossRef]
- McNeil, J.; Cotnoir, P.-A.; Leroux, T.; Laprade, R.; Schwartz, J.-L. A Canadian national survey on the public perception of biological control. BioControl 2010, 55, 445–454. [Google Scholar] [CrossRef]
- Koch, S.; Epp, A.; Lohmann, M.; Böl, G.-F. Pesticide residues in food: Attitudes, beliefs, and misconceptions among conventional and organic consumers. J. Food Prot. 2017, 80, 2083–2089. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.; Bearth, A.; Siegrist, M. How chemophobia affects public acceptance of pesticide use and biotechnology in agriculture. Food Qual. Prefer. 2021, 91, 104197. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [Green Version]
- Desgarennes, D.; Garrido, E.; Torres-Gomez, M.J.; Peña-Cabriales, J.J.; Partida-Martinez, L.P. Diazotrophic potential among bacterial communities associated with wild and cultivated Agave species. FEMS Microbiol. Ecol. 2014, 90, 844–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Schmid, M.; Hartmann, A.; Tripathi, A.K. Identification of diazotrophs in the culturable bacterial community associated with roots of Lasiurus sindicus, a perennial grass of Thar Desert, India. Microb. Ecol. 2007, 54, 82–90. [Google Scholar] [CrossRef]
- ALKahtani, M.D.; Fouda, A.; Attia, K.A.; Al-Otaibi, F.; Eid, A.M.; Ewais, E.E.-D.; Hijri, M.; St-Arnaud, M.; Hassan, S.E.-D.; Khan, N.; et al. Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy 2020, 10, 1325. [Google Scholar] [CrossRef]
- Cherif, H.; Marasco, R.; Rolli, E.; Ferjani, R.; Fusi, M.; Soussi, A.; Mapelli, F.; Blilou, I.; Borin, S.; Boudabous, A.; et al. Oasis desert farming selects environment-specific date palm root endophytic communities and cultivable bacteria that promote resistance to drought. Environ. Microbiol. Rep. 2015, 7, 668–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eida, A.A.; Alzubaidy, H.S.; de Zélicourt, A.; Synek, L.; Alsharif, W.; Lafi, F.F.; Hirt, H.; Saad, M.M. Phylogenetically diverse endophytic bacteria from desert plants induce transcriptional changes of tissue-specific ion transporters and salinity stress in Arabidopsis thaliana. Plant Sci. 2019, 280, 228–240. [Google Scholar] [CrossRef]
- Eke, P.; Kumar, A.; Sahu, K.P.; Wakam, L.N.; Sheoran, N.; Ashajyothi, M.; Patel, A.; Fekam, F.B. Endophytic bacteria of desert cactus (Euphorbia trigonas Mill) confer drought tolerance and induce growth promotion in tomato (Solanum lycopersicum L.). Microbiol. Res. 2019, 228, 126302. [Google Scholar] [CrossRef]
- Lopez, B.R.; Bashan, Y.; Bacilio, M. Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the southern Sonoran Desert. Arch. Microbiol. 2011, 193, 527–541. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, J.d.C.; Mora-Amutio, M.D.l.; Plascencia-Correa, L.A.; Audelo-Regalado, E.; Guardado, F.R.; Hernández-Sánchez, E.; Peña-Ramírez, Y.J.; Escalante, A.; Beltrán-García, M.J.; Ogura, T. Cultivable endophytic bacteria from leaf bases of Agave tequilana and their role as plant growth promoters. Braz. J. Microbiol. 2014, 45, 1333–1339. [Google Scholar] [CrossRef] [Green Version]
- Hanna, A.L.; Youssef, H.H.; Amer, W.M.; Monib, M.; Fayez, M.; Hegazi, N.A. Diversity of bacteria nesting the plant cover of north Sinai deserts, Egypt. J. Adv. Res. 2013, 4, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, D.; Maymon, M.; Agapakis, C.M.; Lee, A.; Wang, A.; Prigge, B.A.; Volkogon, M.; Hirsch, A.M. A survey of the microbial community in the rhizosphere of two dominant shrubs of the Negev Desert highlands, Zygophyllum dumosum (Zygophyllaceae) and Atriplex halimus (Amaranthaceae), using cultivation-dependent and cultivation-independent methods. Am. J. Bot. 2013, 100, 1713–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Rock-degrading endophytic bacteria in cacti. Environ. Exp. Bot. 2009, 66, 389–401. [Google Scholar] [CrossRef]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting desert plant Bacillus endophytic strains for their potential to enhance plant stress tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef]
- El-Deeb, B.; Fayez, K.; Gherbawy, Y. Isolation and characterization of endophytic bacteria from Plectranthus tenuiflorus medicinal plant in Saudi Arabia desert and their antimicrobial activities. J. Plant Interact. 2013, 8, 56–64. [Google Scholar] [CrossRef]
- Rodriguez, R.; White, J.F., Jr.; Arnold, A.E.; Redman, A.R. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Massimo, N.C.; Devan, M.N.; Arendt, K.R.; Wilch, M.H.; Riddle, J.M.; Furr, S.H.; Steen, C.; U’Ren, J.M.; Sandberg, D.C.; Arnold, A.E. Fungal endophytes in aboveground tissues of desert plants: Infrequent in culture, but highly diverse and distinctive symbionts. Microb. Ecol. 2015, 70, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Loro, M.; Valero-Jiménez, C.; Nozawa, S.; Márquez, L. Diversity and composition of fungal endophytes in semiarid Northwest Venezuela. J. Arid Environ. 2012, 85, 46–55. [Google Scholar] [CrossRef]
- Fisher, P.; Sutton, B.; Petrini, L.; Petrini, O. Fungal endophytes from Opuntia stricta: A first report. Nova Hedwig. Ger. 1994, 59, 195–200. [Google Scholar]
- Silva-Hughes, A.F.; Wedge, D.E.; Cantrell, C.L.; Carvalho, C.R.; Pan, Z.; Moraes, R.M.; Madoxx, V.L.; Rosa, L.H. Diversity and antifungal activity of the endophytic fungi associated with the native medicinal cactus Opuntia humifusa (Cactaceae) from the United States. Microbiol. Res. 2015, 175, 67–77. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Raghavan, S.; Garcia, M.; Sinsabaugh, R.L.; Natvig, D.O.; Lowrey, T.K. Endophytic fungal symbionts associated with gypsophilous plants. Botany 2014, 92, 295–301. [Google Scholar] [CrossRef]
- Gehlot, P.; Bohra, N.; Purohit, D. Endophytic mycoflora of inner bark of Prosopis cineraria—A key stone tree species of Indian desert. Am. Eur. J. Bot 2008, 1, 01–04. [Google Scholar]
- González-Teuber, M.; Vilo, C.; Bascuñán-Godoy, L. Molecular characterization of endophytic fungi associated with the roots of Chenopodium quinoa inhabiting the Atacama Desert, Chile. Genom. Data 2017, 11, 109–112. [Google Scholar] [CrossRef]
- Bezerra, J.D.; Santos, M.G.; Barbosa, R.N.; Svedese, V.M.; Lima, D.M.; Fernandes, M.J.S.; Gomes, B.S.; Paiva, L.M.; Almeida-Cortez, J.S.; Souza-Motta, C.M. Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: A first study. Symbiosis 2013, 60, 53–63. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Wittlinger, S.K.; Faeth, S.H. Endophytic fungi associated with cacti in Arizona. Mycol. Res. 2005, 109, 635–639. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, Q.; Lu, X.; Okane, I.; Kakishima, M. Endophytic fungal community in stems and leaves of plants from desert areas in China. Mycol. Prog. 2012, 11, 781–790. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [Green Version]
- Newsham, K.; Upson, R.; Read, D. Mycorrhizas and dark septate root endophytes in polar regions. Fungal Ecol. 2009, 2, 10–20. [Google Scholar] [CrossRef] [Green Version]
- Green, L.E.; Porras-Alfaro, A.; Sinsabaugh, R.L. Translocation of nitrogen and carbon integrates biotic crust and grass production in desert grassland. J. Ecol. 2008, 96, 1076–1085. [Google Scholar] [CrossRef]
- Shearin, Z.R.; Filipek, M.; Desai, R.; Bickford, W.A.; Kowalski, K.P.; Clay, K. Fungal endophytes from seeds of invasive, non-native Phragmites australis and their potential role in germination and seedling growth. Plant Soil 2018, 422, 183–194. [Google Scholar] [CrossRef]
- Hubbard, M.; Germida, J.; Vujanovic, V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Nobel, P.S. Water relations of flowering of Agave deserti. Bot. Gaz. 1977, 138, 1–6. [Google Scholar] [CrossRef]
- Faeth, S.H.; Hammon, K.E. Fungal endophytes in oak trees: Long-term patterns of abundance and associations with leafminers. Ecology 1997, 78, 810–819. [Google Scholar] [CrossRef]
- Faeth, S.H.; Fagan, W.F. Fungal endophytes: Common host plant symbionts but uncommon mutualists. Integr. Comp. Biol. 2002, 42, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Fracchia, S.; Krapovickas, L.; Aranda-Rickert, A.; Valentinuzzi, V. Dispersal of arbuscular mycorrhizal fungi and dark septate endophytes by Ctenomys cf. knighti (Rodentia) in the northern Monte Desert of Argentina. J. Arid Environ. 2011, 75, 1016–1023. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Tijana, G.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat. Commun. 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Hoffman, M.T.; Arnold, A.E. Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycol. Res. 2008, 112, 331–344. [Google Scholar] [CrossRef]
- Heaton, T.H. Isotopic studies of nitrogen pollution in the hydrosphere and atmosphere: A review. Chem. Geol. Isot. Geosci. Sect. 1986, 59, 87–102. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831–849. [Google Scholar] [CrossRef] [PubMed]
- Correll, D.L. The role of phosphorus in the eutrophication of receiving waters: A review. J. Environ. Qual. 1998, 27, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Glibert, P.M.; Seitzinger, S.; Heil, C.A.; Burkholder, J.M.; Parrow, M.W.; Codispoti, L.A.; Kelly, V. Eutrophication. Oceanography 2005, 18, 198. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, A.H.; Patz, J.A. Reactive nitrogen and human health: Acute and long-term implications. Ambio J. Hum. Environ. 2002, 31, 120–125. [Google Scholar] [CrossRef]
- Scholz, R.W.; Ulrich, A.E.; Eilittä, M.; Roy, A. Sustainable use of phosphorus: A finite resource. Sci. Total Environ. 2013, 461, 799–803. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Doty, S.L.; Oakley, B.; Xin, G.; Kang, J.W.; Singleton, G.; Khan, Z.; Vajzovic, A.; Staley, J.T. Diazotrophic endophytes of native black cottonwood and willow. Symbiosis 2009, 47, 23–33. [Google Scholar] [CrossRef]
- Asis, C., Jr.; Adachi, K. Isolation of endophytic diazotroph Pantoea agglomerans and nondiazotroph Enterobacter asburiae from sweetpotato stem in Japan. Lett. Appl. Microbiol. 2004, 38, 19–23. [Google Scholar] [CrossRef]
- Ladha, J.; Barraquio, W.; Revilla, L. Isolation of endophytic diazotrophic bacteria from wetland rice. In Opportunities for Biological Nitrogen Fixation in Rice and Other Non-Legumes; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1997; pp. 15–24. [Google Scholar]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef]
- Hino, S.; Wilson, P. Nitrogen fixation by a facultative bacillus. J. Bacteriol. 1958, 75, 403. [Google Scholar] [CrossRef] [Green Version]
- Seldin, L.; Van Elsas, J.; Penido, E. Bacillus azotofixans sp. nov., a nitrogen-fixing species from Brazilian soils and grass roots. Int. J. Syst. Evol. Microbiol. 1984, 34, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Wang, J.; Liu, Y.; Chen, S. Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J. Appl. Microbiol. 2005, 99, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Desnoues, N.; Lin, M.; Guo, X.; Ma, L.; Carreño-Lopez, R.; Elmerich, C. Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 2003, 149, 2251–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatayama, K.; Kawai, S.; Shoun, H.; Ueda, Y.; Nakamura, A. Pseudomonas azotifigens sp. nov., a novel nitrogen-fixing bacterium isolated from a compost pile. Int. J. Syst. Evol. Microbiol. 2005, 55, 1539–1544. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, J.; Dou, Y.; Chen, M.; Ping, S.; Peng, J.; Lu, W.; Zhang, W.; Yao, Z.; Li, H.; et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. USA 2008, 105, 7564–7569. [Google Scholar] [CrossRef] [Green Version]
- Iniguez, A.L.; Dong, Y.; Triplett, E.W. Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol. Plant. Microbe Interact. 2004, 17, 1078–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puente, M.; Li, C.; Bashan, Y. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings. Plant Biol. 2004, 6, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, B.R.; Tinoco-Ojanguren, C.; Bacilio, M.; Mendoza, A.; Bashan, Y. Endophytic bacteria of the rock-dwelling cactus Mammillaria fraileana affect plant growth and mobilization of elements from rocks. Environ. Exp. Bot. 2012, 81, 26–36. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Can naturally-occurring endophytic nitrogen-fixing bacteria of hybrid white spruce sustain boreal forest tree growth on extremely nutrient-poor soils? Soil Biol. Biochem. 2020, 140, 107642. [Google Scholar] [CrossRef]
- Gallon, J. The oxygen sensitivity of nitrogenase: A problem for biochemists and micro-organisms. Trends Biochem. Sci. 1981, 6, 19–23. [Google Scholar] [CrossRef]
- White, J.F.; Crawford, H.; Torres, M.S.; Mattera, R.; Irizarry, I.; Bergen, M. A proposed mechanism for nitrogen acquisition by grass seedlings through oxidation of symbiotic bacteria. Symbiosis 2012, 57, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamilton, C.E.; Gundel, P.E.; Helander, M.; Saikkonen, K. Endophytic mediation of reactive oxygen species and antioxidant activity in plants: A review. Fungal Divers. 2012, 54, 1–10. [Google Scholar] [CrossRef]
- Andreozzi, A.; Prieto, P.; Mercado-Blanco, J.; Monaco, S.; Zampieri, E.; Romano, S.; Valè, G.; Defez, R.; Bianco, C. Efficient colonization of the endophytes Herbaspirillum huttiense RCA24 and Enterobacter cloacae RCA25 influences the physiological parameters of Oryza sativa L. cv. Baldo rice. Environ. Microbiol. 2019, 21, 3489–3504. [Google Scholar] [CrossRef] [PubMed]
- Santos, K.; Moure, V.; Hauer, V.; Santos, A.S.; Donatti, L.; Galvão, C.; Pedrosa, F.; Souza, E.; Wassem, R.; Steffens, M. Wheat colonization by an Azospirillum brasilense ammonium-excreting strain reveals upregulation of nitrogenase and superior plant growth promotion. Plant Soil 2017, 415, 245–255. [Google Scholar] [CrossRef]
- Galambos, N.; Compant, S.; Moretto, M.; Sicher, C.; Puopolo, G.; Wäckers, F.; Sessitsch, A.; Pertot, I.; Perazzolli, M. Humic acid enhances the growth of tomato promoted by endophytic bacterial strains through the activation of hormone-, growth-, and transcription-related processes. Front. Plant Sci. 2020, 11, 1437. [Google Scholar] [CrossRef]
- Sevilla, M.; Burris, R.H.; Gunapala, N.; Kennedy, C. Comparison of benefit to sugarcane plant growth and 15N2 incorporation following inoculation of sterile plants with Acetobacter diazotrophicus wild-type and nif mutant strains. Mol. Plant. Microbe Interact. 2001, 14, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Paungfoo-Lonhienne, C.; Schmidt, S.; Webb, R.I.; Lonhienne, T.G. Rhizophagy—A new dimension of plant–microbe interactions. Mol. Microb. Ecol. Rhizosphere 2013, 1, 1199–1207. [Google Scholar]
- White, J.F.; Kingsley, K.L.; Verma, S.K.; Kowalski, K.P. Rhizophagy cycle: An oxidative process in plants for nutrient extraction from symbiotic microbes. Microorganisms 2018, 6, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Kingsley, K.L.; White, J.F. Chemical interactions at the interface of plant root hair cells and intracellular bacteria. Microorganisms 2021, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Beltran-Garcia, M.J.; White, J.F., Jr.; Prado, F.M.; Prieto, K.R.; Yamaguchi, L.F.; Torres, M.S.; Kato, M.J.; Medeiros, M.H.; Di Mascio, P. Nitrogen acquisition in Agave tequilana from degradation of endophytic bacteria. Sci. Rep. 2014, 4, 6938. [Google Scholar] [CrossRef] [PubMed]
- Lonhienne, T.; Mason, M.G.; Ragan, M.A.; Hugenholtz, P.; Schmidt, S.; Paungfoo-Lonhienne, C. Yeast as a biofertilizer alters plant growth and morphology. Crop Sci. 2014, 54, 785–790. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef] [Green Version]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.; Urquiaga, S.; Schultz, N.; Balieiro, F.D.C.; Medeiros, P.S.; Santos, L.A.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi help tomato to acquire nutrients from ground plant material. Front. Microbiol. 2017, 8, 2437. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Pan, X.; Kubicek, C.; Druzhinina, I.; Chenthamara, K.; Labbé, J.L.; Yuan, Z. Diverse plant-associated pleosporalean fungi from saline areas: Ecological tolerance and nitrogen-status dependent effects on plant growth. Front. Microbiol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.E. Soil microorganisms and phosphorus availability. In Soil Biota: Management in Sustainable Farming Systems; Pankhurst, C.E., Doube, B.M., Gupta, V.S.S.R., Grace, P.R., Eds.; CSIRO: Mellbourne, VIC, Australia, 1994; pp. 50–62. [Google Scholar]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Sperber, J.I. The incidence of apatite-solubilizing organisms in the rhizosphere and soil. Aust. J. Agric. Res. 1958, 9, 778–781. [Google Scholar] [CrossRef]
- Raghu, K.; MacRae, I. Occurrence of Phosphate-dissolving Micro-organisms in the Rhizosphere of Rice Plants and in Submerged Soils. J. Appl. Bacteriol. 1966, 29, 582–586. [Google Scholar] [CrossRef]
- Katznelson, H.; Peterson, E.; Rouatt, J. Phosphate-dissolving microorganisms on seed and in the root zone of plants. Can. J. Bot. 1962, 40, 1181–1186. [Google Scholar] [CrossRef]
- Otieno, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745. [Google Scholar] [CrossRef] [Green Version]
- Varga, T.; Hixson, K.K.; Ahkami, A.H.; Sher, A.W.; Barnes, M.E.; Chu, R.K.; Battu, A.K.; Nicora, C.D.; Winkler, T.E.; Reno, L.R.; et al. Endophyte-Promoted Phosphorus Solubilization in Populus. Front. Plant Sci. 2020, 11, 1585. [Google Scholar] [CrossRef]
- Matos, A.D.; Gomes, I.C.; Nietsche, S.; Xavier, A.A.; Gomes, W.S.; Dos Santos Neto, J.A.; Pereira, M.C. Phosphate solubilization by endophytic bacteria isolated from banana trees. An. Acad. Bras. Cienc. 2017, 89, 2945–2954. [Google Scholar] [CrossRef] [Green Version]
- De Abreu, C.; Figueiredo, J.; Oliveira, C.; Dos Santos, V.; Gomes, E.; Ribeiro, V.; Barros, B.d.A.; LANA, U.d.P.; Marriel, I. Maize endophytic bacteria as mineral phosphate solubilizers. Embrapa Milho E Sorgo-Artigo Em Periód. Indexado ALICE 2017, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Pandey, A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere 2019, 9, 2–9. [Google Scholar] [CrossRef]
- Nath, R.; Sharma, G.; Barooah, M. Efficiency of tricalcium phosphate solubilization by two different endophytic Penicillium sp. isolated from tea (Camellia sinensis L.). Eur. J. Exp. Biol. 2012, 2, 1354–1358. [Google Scholar]
- Resende, M.P.; Jakoby, I.C.M.C.; dos Santos, L.C.R.; Soares, M.A.; Pereira, F.D.; Souchie, E.L.; Silva, F.G. Phosphate solubilization and phytohormone production by endophytic and rhizosphere Trichoderma isolates of guanandi (Calophyllum brasiliense Cambess). Afr. J. Microbiol. Res. 2014, 8, 2616–2623. [Google Scholar]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I.-J. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Ngwene, B.; Boukail, S.; Söllner, L.; Franken, P.; Andrade-Linares, D. Phosphate utilization by the fungal root endophyte Piriformospora indica. Plant Soil 2016, 405, 231–241. [Google Scholar] [CrossRef] [Green Version]
- Puente, M.E.; Li, C.Y.; Bashan, Y. Endophytic bacteria in cacti seeds can improve the development of cactus seedlings. Environ. Exp. Bot. 2009, 66, 402–408. [Google Scholar] [CrossRef]
- Kucey, R. Phosphate-solubilizing bacteria and fungi in various cultivated and virgin Alberta soils. Can. J. Soil Sci. 1983, 63, 671–678. [Google Scholar] [CrossRef]
- Barrow, J.; Osuna, P. Phosphorus solubilization and uptake by dark septate fungi in fourwing saltbush, Atriplex canescens (Pursh) Nutt. J. Arid Environ. 2002, 51, 449–459. [Google Scholar] [CrossRef]
- Spagnoletti, F.N.; Tobar, N.; Di Pardo, A.F.; Chiocchio, V.M.; Lavado, R.S. Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl. Soil Ecol. 2017, 111, 25–32. [Google Scholar] [CrossRef]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Caldwell, B.A.; Jumpponen, A.; Trappe, J.M. Utilization of major detrital substrates by dark-septate, root endophytes. Mycologia 2000, 92, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Jumpponen, A.; Mattson, K.G.; Trappe, J.M. Mycorrhizal functioning of Phialocephala fortinii with Pinus contorta on glacier forefront soil: Interactions with soil nitrogen and organic matter. Mycorrhiza 1998, 7, 261–265. [Google Scholar] [CrossRef]
- Upson, R.; Read, D.J.; Newsham, K.K. Nitrogen form influences the response of Deschampsia antarctica to dark septate root endophytes. Mycorrhiza 2009, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.S.; Cordery, I.; Iacovides, I. Coping with Water Scarcity: Addressing the Challenges; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- El Kharraz, J.; El-Sadek, A.; Ghaffour, N.; Mino, E. Water scarcity and drought in WANA countries. Procedia Eng. 2012, 33, 14–29. [Google Scholar] [CrossRef] [Green Version]
- Falkenmark, M. Growing water scarcity in agriculture: Future challenge to global water security. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2013, 371, 20120410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoekstra, A.Y.; Mekonnen, M.M. The water footprint of humanity. Proc. Natl. Acad. Sci. USA 2012, 109, 3232–3237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global water resources: Vulnerability from climate change and population growth. Science 2000, 289, 284–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexandratos, N.; Bruinsma, J. World agriculture towards 2030/2050: The 2012 revision. Agric. Food Policy 2012. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, e14823. [Google Scholar] [CrossRef]
- Waller, F.; Achatz, B.; Baltruschat, H.; Fodor, J.; Becker, K.; Fischer, M.; Heier, T.; Hückelhoven, R.; Neumann, C.; Von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar] [CrossRef] [Green Version]
- Lyon, B. Southern Africa summer drought and heat waves: Observations and coupled model behavior. J. Clim. 2009, 22, 6033–6046. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S. Plant drought stress: Effects, mechanisms and management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Endophytic fungus Aspergillus japonicus mediates host plant growth under normal and heat stress conditions. BioMed Res. Int. 2018, 2018, 7696831. [Google Scholar]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Zhang, Q.; Gong, M.; Yuan, J.; Hou, Y.; Zhang, H.; Wang, Y.; Hou, X. Dark septate endophyte improves drought tolerance in Sorghum. Int. J. Agric. Biol. 2017, 19, 53–60. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces fungal endophyte isolated from extreme hot desert-adapted plant on heat stress tolerance of cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Moghaddam, M.S.H.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant. Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef]
- Zahra, T.; Hamedi, J.; Mahdigholi, K. Endophytic actinobacteria of a halophytic desert plant Pteropyrum olivieri: Promising growth enhancers of sunflower. 3 Biotech. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Sasan, R.K.; Bidochka, M.J. The insect-pathogenic fungus Metarhizium robertsii (Clavicipitaceae) is also an endophyte that stimulates plant root development. Am. J. Bot. 2012, 99, 101–107. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Jung, H.-Y.; Lee, J.-H.; Lee, I.-J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.; Khan, A.L.; Shahzad, R.; Ullah, I.; Khan, A.R.; Lee, I.-J. Mutualistic fungal endophytes produce phytohormones and organic acids that promote japonica rice plant growth under prolonged heat stress. J. Zhejiang Univ.-Sci. B 2015, 16, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Khalvati, M.; Hu, Y.; Mozafar, A.; Schmidhalter, U. Quantification of water uptake by arbuscular mycorrhizal hyphae and its significance for leaf growth, water relations, and gas exchange of barley subjected to drought stress. Plant Biol. 2005, 7, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Lozano, J.; Azcón, R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995, 95, 472–478. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Ettoumi, B.; Vigani, G.; Mapelli, F.; Borin, S.; Abou-Hadid, A.F.; El-Behairy, U.A.; Sorlini, C.; Cherif, A.; et al. A drought resistance-promoting microbiome is selected by root system under desert farming. PLoS ONE 2012, 7, e48479. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.L.; Torres, I.F.; García, C.; López-Mondéjar, R.; Bastida, F. Land use shapes the resistance of the soil microbial community and the C cycling response to drought in a semi-arid area. Sci. Total Environ. 2019, 648, 1018–1030. [Google Scholar] [CrossRef]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [Green Version]
- Munns, R.; Termaat, A. Whole-plant responses to salinity. Funct. Plant Biol. 1986, 13, 143–160. [Google Scholar] [CrossRef]
- Soldan, R.; Mapelli, F.; Crotti, E.; Schnell, S.; Daffonchio, D.; Marasco, R.; Fusi, M.; Borin, S.; Cardinale, M. Bacterial endophytes of mangrove propagules elicit early establishment of the natural host and promote growth of cereal crops under salt stress. Microbiol. Res. 2019, 223, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Piernik, A.; Hrynkiewicz, K.; Wojciechowska, A.; Szymańska, S.; Lis, M.I.; Muscolo, A. Effect of halotolerant endophytic bacteria isolated from Salicornia europaea L. on the growth of fodder beet (Beta vulgaris L.) under salt stress. Arch. Agron. Soil Sci. 2017, 63, 1404–1418. [Google Scholar] [CrossRef]
- De Zélicourt, A.; Synek, L.; Saad, M.M.; Alzubaidy, H.; Jalal, R.; Xie, Y.; Andrés-Barrao, C.; Rolli, E.; Guerard, F.; Mariappan, K.G.; et al. Ethylene induced plant stress tolerance by Enterobacter sp. SA187 is mediated by 2-keto-4-methylthiobutyric acid production. PLoS Genet. 2018, 14, e1007273. [Google Scholar] [CrossRef] [Green Version]
- Abdelshafy Mohamad, O.A.; Ma, J.-B.; Liu, Y.-H.; Zhang, D.; Hua, S.; Bhute, S.; Hedlund, B.P.; Li, W.-J.; Li, L. Beneficial Endophytic Bacterial Populations Associated With Medicinal Plant Thymus vulgaris Alleviate Salt Stress and Confer Resistance to Fusarium oxysporum. Front. Plant. Sci. 2020, 11, 47. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.-H.; Schäfer, P.; Schwarczinger, I.; et al. Salt tolerance of barley induced by the root endophyte Piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Farias, G.C.; Nunes, K.G.; Soares, M.A.; de Siqueira, K.A.; Lima, W.C.; Neves, A.L.R.; de Lacerda, C.F.; Gomes Filho, E. Dark septate endophytic fungi mitigate the effects of salt stress on cowpea plants. Braz. J. Microbiol. 2020, 51, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D. Alleviation of salt stress by plant growth regulators and IAA producing bacteria in wheat. Acta Physiol. Plant. 2009, 31, 861–864. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Hashem, A. Pseudomonas induces salinity tolerance in cotton (Gossypium hirsutum) and resistance to Fusarium root rot through the modulation of indole-3-acetic acid. Saudi J. Biol. Sci. 2015, 22, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, F. Alleviation of salt stress by halotolerant and halophilic plant growth-promoting bacteria in wheat (Triticum aestivum). Braz. J. Microbiol. 2016, 47, 621–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. SpringerPlus 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria Capable of Producing ACC-deaminase May Mitigate Salt Stress in Wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Bebber, D.P. Range-expanding pests and pathogens in a warming world. Annu. Rev. Phytopathol. 2015, 53, 335–356. [Google Scholar] [CrossRef]
- Bebber, D.P.; Ramotowski, M.A.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Appelberg, G.; Harding, S.; Bärring, L. Spatio-temporal impact of climate change on the activity and voltinism of the spruce bark beetle, Ips typographus. Glob. Chang. Biol. 2009, 15, 486–499. [Google Scholar] [CrossRef]
- Olsen, M. Cotton (Texas) Root Rot; College of Agriculture, University of Arizona: Tucson, AZ, USA, 2015. [Google Scholar]
- Nassar, A.H.; El-Tarabily, K.A.; Sivasithamparam, K. Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol. Fertil. Soils 2005, 42, 97–108. [Google Scholar] [CrossRef]
- Forchetti, G.; Masciarelli, O.; Izaguirre, M.J.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria improve seedling growth of sunflower under water stress, produce salicylic acid, and inhibit growth of pathogenic fungi. Curr. Microbiol. 2010, 61, 485–493. [Google Scholar] [CrossRef]
- Khan, A.L.; Waqas, M.; Kang, S.-M.; Al-Harrasi, A.; Hussain, J.; Al-Rawahi, A.; Al-Khiziri, S.; Ullah, I.; Ali, L.; Jung, H.-Y.; et al. Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J. Microbiol. 2014, 52, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Delauré, S.L.; De Bolle, M.F.; Cammue, B.P. The role of ethylene in host-pathogen interactions. Annu. Rev. Phytopathol. 2006, 44, 393–416. [Google Scholar] [CrossRef]
- Loaces, I.; Ferrando, L.; Scavino, A.F. Dynamics, diversity and function of endophytic siderophore-producing bacteria in rice. Microb. Ecol. 2011, 61, 606–618. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Strobel, G.A.; Miller, R.V.; Martinez-Miller, C.; Condron, M.M.; Teplow, D.B.; Hess, W. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 1999, 145, 1919–1926. [Google Scholar] [CrossRef] [Green Version]
- Zachow, C.; Jahanshah, G.; de Bruijn, I.; Song, C.; Ianni, F.; Pataj, Z.; Gerhardt, H.; Pianet, I.; Lämmerhofer, M.; Berg, G.; et al. The novel lipopeptide poaeamide of the endophyte Pseudomonas poae RE* 1-1-14 is involved in pathogen suppression and root colonization. Mol. Plant-Microbe Interact. 2015, 28, 800–810. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Li, F.; Lu, Q.; Wu, G.; Jiang, Z.; Liu, S.; Habden, X.; Razumova, E.A.; Osterman, I.A.; Sergiev, P.V.; et al. Studies on diversity, novelty, antimicrobial activity, and new antibiotics of cultivable endophytic actinobacteria isolated from psammophytes collected in Taklamakan Desert. J. Pharm. Anal. 2020. [Google Scholar]
- Gashgari, R.; Gherbawy, Y.; Ameen, F.; Alsharari, S. Molecular characterization and analysis of antimicrobial activity of endophytic fungi from medicinal plants in Saudi Arabia. Jundishapur J. Microbiol. 2016, 9, e26157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, M. Medicinal Plants of the Desert and Canyon West; UNM Press: Albuquerque, NM, USA, 1989. [Google Scholar]
- Upadhyay, B.; Roy, S.; Kumar, A. Traditional uses of medicinal plants among the rural communities of Churu district in the Thar Desert, India. J. Ethnopharmacol. 2007, 113, 387–399. [Google Scholar]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial activity of medicinal plants correlates with the proportion of antagonistic endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willoughby, L. A new kind of antagonistic association, between bacteria and aquatic fungi. Trans. Br. Mycol. Soc. 1983, 80, 91–97. [Google Scholar] [CrossRef]
- De Boer, W.; Klein Gunnewiek, P.J.; Kowalchuk, G.A.; Van Veen, J.A. Growth of chitinolytic dune soil β-subclass Proteobacteria in response to invading fungal hyphae. Appl. Environ. Microbiol. 2001, 67, 3358–3362. [Google Scholar] [CrossRef] [Green Version]
- Godfrey, S.; Harrow, S.; Marshall, J.; Klena, J. Characterization by 16S rRNA sequence analysis of pseudomonads causing blotch disease of cultivated Agaricus bisporus. Appl. Environ. Microbiol. 2001, 67, 4316–4323. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-H.; Menge, J.A.; Cooksey, D.A. Mutations affecting hyphal colonization and pyoverdine production in pseudomonads antagonistic toward Phytophthora parasitica. Appl. Environ. Microbiol. 1994, 60, 473–481. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, D.Y.; Crouch, J.A. Bacterial/fungal interactions: From pathogens to mutualistic endosymbionts. Annu. Rev. Phytopathol. 2009, 47, 63–82. [Google Scholar] [CrossRef] [Green Version]
- Endara, M.-J.; Coley, P.D. The resource availability hypothesis revisited: A meta-analysis. Funct. Ecol. 2011, 25, 389–398. [Google Scholar] [CrossRef]
- Li, H.; Soares, M.A.; Torres, M.S.; Bergen, M.; White, J.F., Jr. Endophytic bacterium, Bacillus amyloliquefaciens, enhances ornamental hosta resistance to diseases and insect pests. J. Plant Interact. 2015, 10, 224–229. [Google Scholar] [CrossRef]
- Breen, J. Acremonium endophyte interactions with enhanced plant resistance to insects. Annu. Rev. Entomol. 1994, 39, 401–423. [Google Scholar] [CrossRef]
- Qin, J.; Wu, M.; Liu, H.; Gao, Y.; Ren, A. Endophyte infection and methyl jasmonate treatment increased the resistance of Achnatherum sibiricum to insect herbivores independently. Toxins 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cosme, M.; Lu, J.; Erb, M.; Stout, M.J.; Franken, P.; Wurst, S. A fungal endophyte helps plants to tolerate root herbivory through changes in gibberellin and jasmonate signaling. New Phytol. 2016, 211, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Knoch, T.R.; Faeth, S.H.; Arnott, D.L. Endophytic fungi alter foraging and dispersal by desert seed-harvesting ants. Oecologia 1993, 95, 470–473. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Tran, H.; Ficke, A.; Asiimwe, T.; Höfte, M.; Raaijmakers, J.M. Role of the cyclic lipopeptide massetolide A in biological control of Phytophthora infestans and in colonization of tomato plants by Pseudomonas fluorescens. New Phytol. 2007, 175, 731–742. [Google Scholar] [CrossRef] [Green Version]
- Yun, D.C.; Yang, S.Y.; Kim, Y.C.; Kim, I.S.; Kim, Y.H. Identification of surfactin as an aphicidal metabolite produced by Bacillus amyloliquefaciens G1. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 751–753. [Google Scholar] [CrossRef]
- Bazely, D.R.; Vicari, M.; Emmerich, S.; Filip, L.; Lin, D.; Inman, A. Interactions between herbivores and endophyte-infected Festuca rubra from the Scottish islands of St. Kilda, Benbecula and Rum. J. Appl. Ecol. 1997, 34, 847–860. [Google Scholar]
- Caradus, J.R.; Johnson, L.J. Epichloë Fungal Endophytes—From a Biological Curiosity in Wild Grasses to an Essential Component of Resilient High Performing Ryegrass and Fescue Pastures. J. Fungi 2020, 6, 322. [Google Scholar] [CrossRef]
- Guerre, P. Ergot alkaloids produced by endophytic fungi of the genus Epichloë. Toxins 2015, 7, 773–790. [Google Scholar] [CrossRef] [Green Version]
- Bush, L.P.; Wilkinson, H.H.; Schardl, C.L. Bioprotective alkaloids of grass-fungal endophyte symbioses. Plant Physiol. 1997, 114, 1. [Google Scholar] [CrossRef] [Green Version]
- Popay, A.J.; Jensen, J.G.; Mace, W.J. Root herbivory: Grass species, Epichloë endophytes and moisture status make a difference. Microorganisms 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol Sci 2009, 1, 48–58. [Google Scholar]
- Yousefi, A.A.; Khavazi, K.; Moezi, A.A.; Rejali, F.; Nadian, H.A. Phosphate solubilizing bacteria and arbuscular mycorrhizal fungi impacts on inorganic phosphorus fractions and wheat growth. World Appl. Sci. J. 2011, 15, 1310–1318. [Google Scholar]
- Bandara, W.; Seneviratne, G.; Kulasooriya, S.A. Interactions among endophytic bacteria and fungi: Effects and potentials. J. Biosci. 2006, 31, 645–650. [Google Scholar] [CrossRef]
- Araújo, W.L.; Maccheroni, W., Jr.; Aguilar-Vildoso, C.I.; Barroso, P.A.; Saridakis, H.O.; Azevedo, J.L. Variability and interactions between endophytic bacteria and fungi isolated from leaf tissues of citrus rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Malo, S. IN BRIEF: Widely banned pesticide allowed on Florida citrus crops sparks lawsuit. Westlaw Today, 3 March 2021. [Google Scholar]
- Thompson, D. Pesticide caused kids’ brain damage, California lawsuits say. Associated Press News, 12 July 2021. [Google Scholar]
- Cohen, P. Roundup maker to pay $10 billion to settle cancer suits. New York Times, 24 June 2020. [Google Scholar]
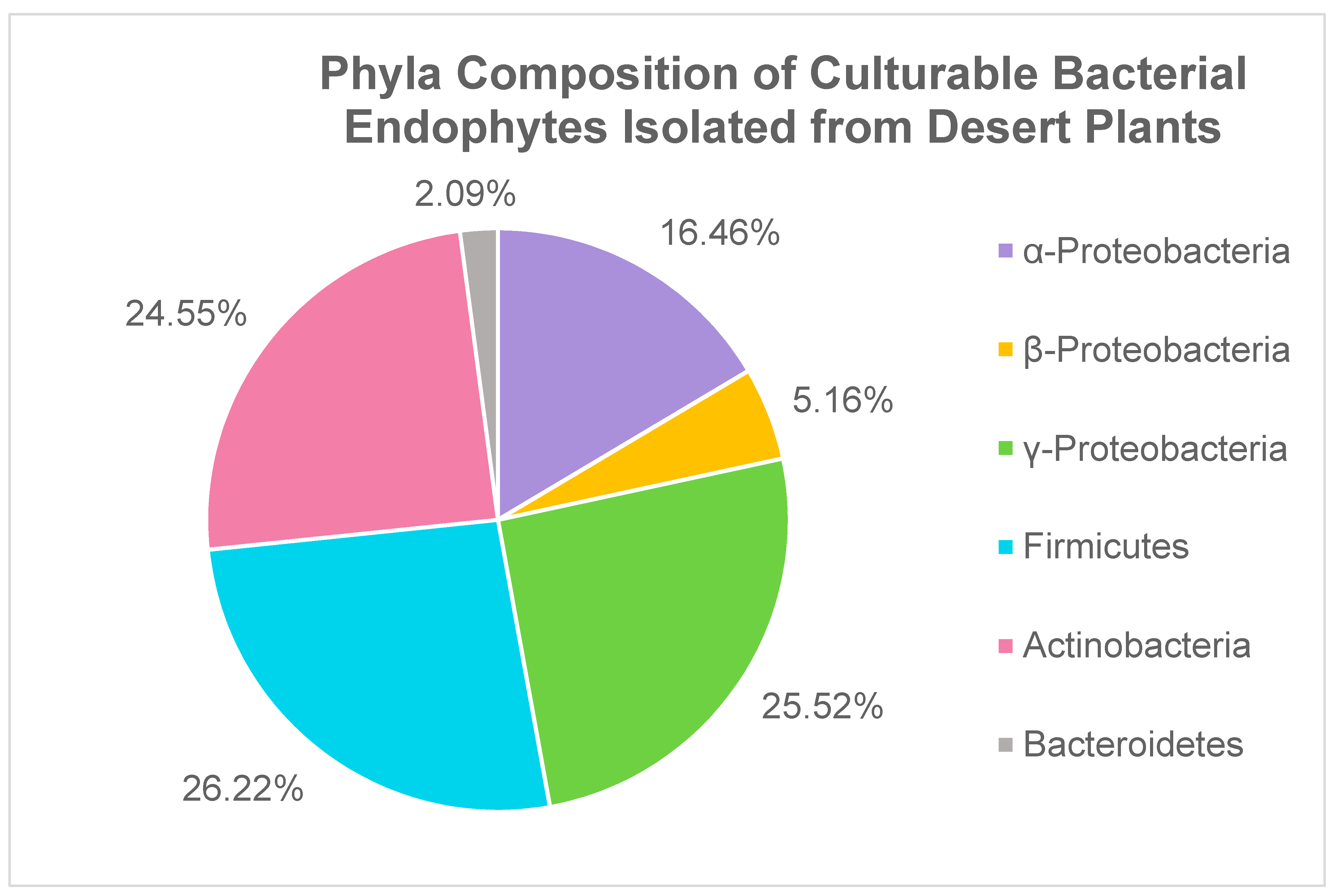
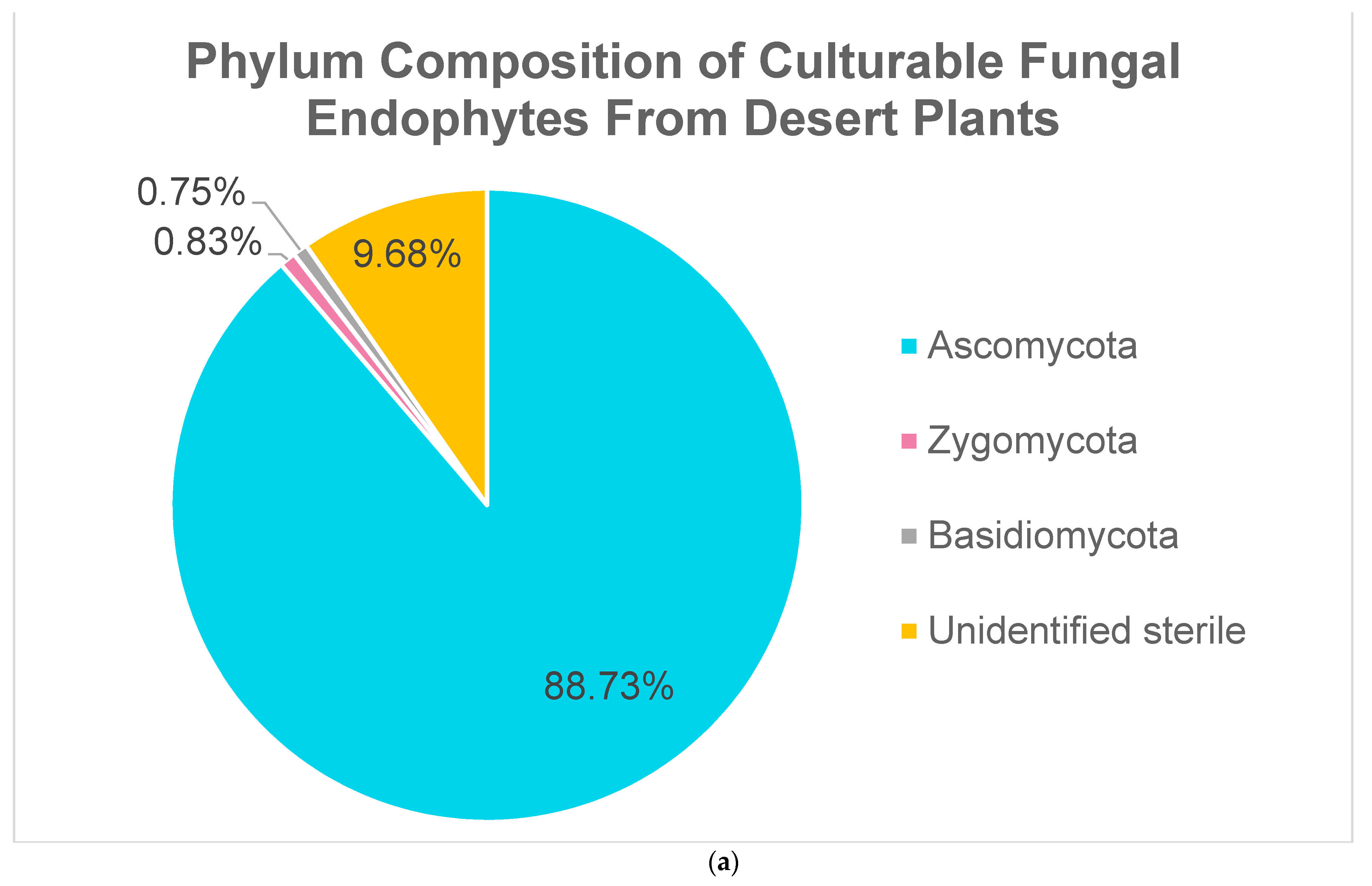
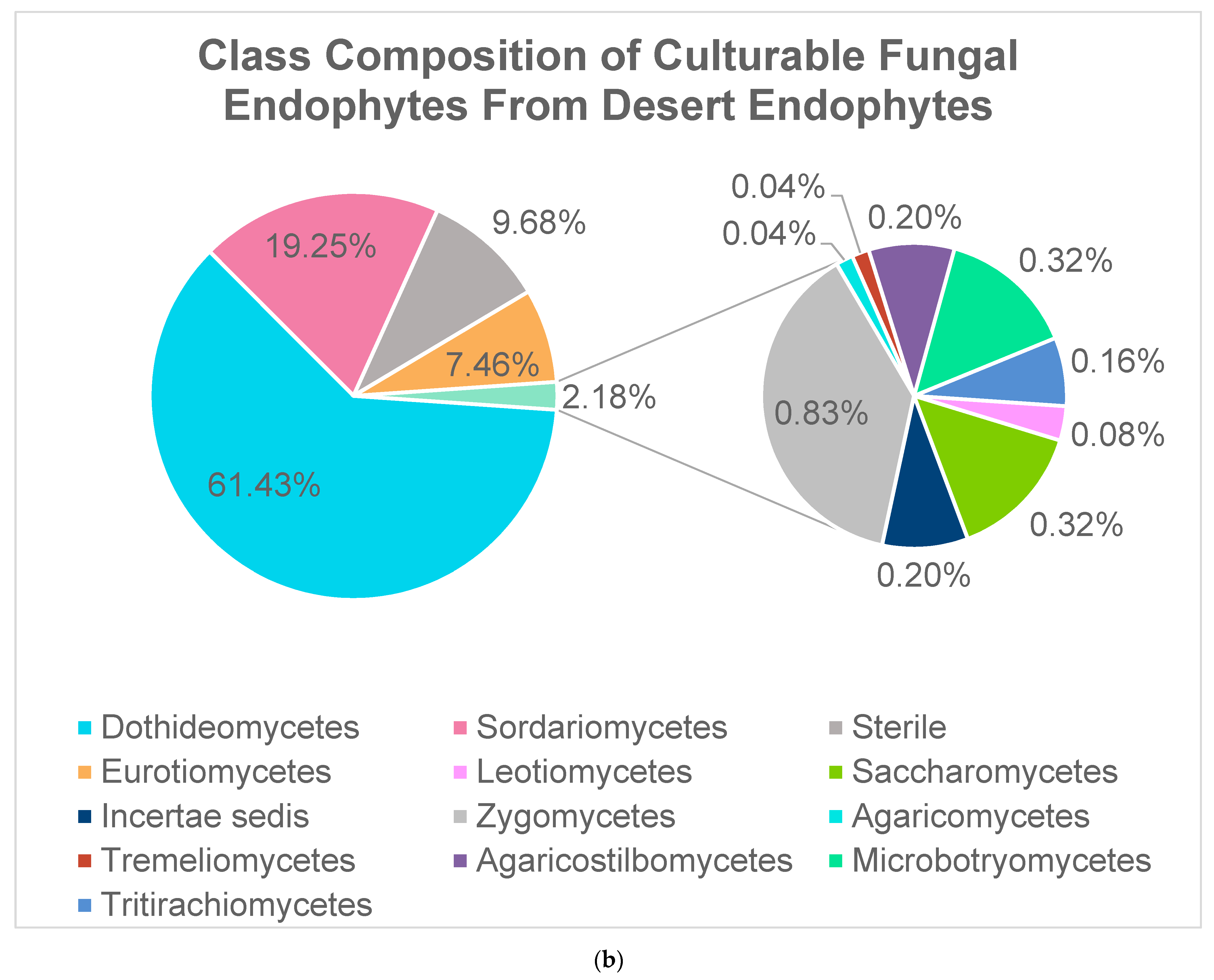
| Phylum | Genus | # of Isolates | % of Phylum | % of All Bacterial Endophytes |
|---|---|---|---|---|
| Actinobacteria | Microbacterium | 91 | 51.70% | 12.69% |
| Micrococcus | 6 | 3.41% | 0.84% | |
| Arthrobacter | 6 | 3.41% | 0.84% | |
| Streptomyces | 20 | 11.36% | 2.79% | |
| Cellulomonas | 31 | 17.61% | 4.32% | |
| Citrococcus | 1 | 0.57% | 0.14% | |
| Curtobacterium | 3 | 1.70% | 0.42% | |
| Kocuria | 1 | 0.57% | 0.14% | |
| Promicromonospora | 1 | 0.57% | 0.14% | |
| Aeromicrobium | 2 | 1.14% | 0.28% | |
| Amycolatopsis | 3 | 1.70% | 0.42% | |
| Cellulosimicrobium | 1 | 0.57% | 0.14% | |
| Corynebacterium | 1 | 0.57% | 0.14% | |
| Gordonia | 3 | 1.70% | 0.42% | |
| Herbiconiux | 1 | 0.57% | 0.14% | |
| Leucobacter | 1 | 0.57% | 0.14% | |
| Kineococcus | 1 | 0.57% | 0.14% | |
| Mycobacterium | 1 | 0.57% | 0.14% | |
| Nonomuraea | 1 | 0.57% | 0.14% | |
| Spirillospora | 1 | 0.57% | 0.14% | |
| α-Proteobacteria | Gluconobacter | 33 | 27.97% | 4.60% |
| Agrobacterium | 14 | 11.86% | 1.95% | |
| Rhizobium | 13 | 11.02% | 1.81% | |
| Mesorhizobium | 11 | 9.32% | 1.53% | |
| Inquilinus | 8 | 6.78% | 1.12% | |
| Sinorhizobium/Ensifer | 8 | 6.78% | 1.12% | |
| Azospirillum | 7 | 5.93% | 0.98% | |
| Paracoccus | 4 | 3.39% | 0.56% | |
| Sphingomonas | 4 | 3.39% | 0.56% | |
| Unknown genus | 4 | 3.39% | 0.56% | |
| Devosia | 3 | 2.54% | 0.42% | |
| Brevundimonas | 2 | 1.69% | 0.28% | |
| Methylobacterium | 2 | 1.69% | 0.28% | |
| Altererythrobacter | 1 | 0.85% | 0.14% | |
| Miroviga | 1 | 0.85% | 0.14% | |
| Ochrobactrum | 1 | 0.85% | 0.14% | |
| Rhodobacter | 1 | 0.85% | 0.14% | |
| Roseomonas | 1 | 0.85% | 0.14% | |
| β-Proteobacteria | Cupriavidus | 20 | 54.05% | 2.79% |
| Variovorax | 7 | 18.92% | 0.98% | |
| Ralstonia | 3 | 8.11% | 0.42% | |
| Massilia | 2 | 5.41% | 0.28% | |
| Achromobacter | 1 | 2.70% | 0.14% | |
| Bordetella | 1 | 2.70% | 0.14% | |
| Burkholderia | 1 | 2.70% | 0.14% | |
| Thibacillus | 1 | 2.70% | 0.14% | |
| Tetrathiobacter | 1 | 2.70% | 0.14% | |
| γ-Proteobacteria | Pseudomonas | 53 | 28.96% | 7.39% |
| Acinetobacter | 37 | 20.22% | 5.16% | |
| Enterobacter | 24 | 13.11% | 3.35% | |
| Pantoea | 14 | 7.65% | 1.95% | |
| Stenotrophomonas | 11 | 6.01% | 1.53% | |
| Cronobacter | 11 | 6.01% | 1.53% | |
| Erwinia | 8 | 4.37% | 1.12% | |
| Klebsiella | 7 | 3.83% | 0.98% | |
| Serratia | 6 | 3.28% | 0.84% | |
| Buttiauxella | 2 | 1.09% | 0.28% | |
| Citrobacter | 2 | 1.09% | 0.28% | |
| Halomonas | 2 | 1.09% | 0.28% | |
| Rahnella | 2 | 1.09% | 0.28% | |
| Aeromonas | 1 | 0.55% | 0.14% | |
| Azotobacter | 1 | 0.55% | 0.14% | |
| Lelliottia | 1 | 0.55% | 0.14% | |
| Proteus | 1 | 0.55% | 0.14% | |
| Firmicutes | Bacillus | 148 | 78.72% | 20.64% |
| Paenibacillus | 17 | 9.04% | 2.37% | |
| Brevibacillus | 5 | 2.66% | 0.70% | |
| Staphylococcus | 5 | 2.66% | 0.70% | |
| Rumeliibacillus | 3 | 1.60% | 0.42% | |
| Planomicrobium | 2 | 1.06% | 0.28% | |
| Enterococcus | 1 | 0.53% | 0.14% | |
| Exiguobacterium | 1 | 0.53% | 0.14% | |
| Leuconostoc | 1 | 0.53% | 0.14% | |
| Lysinibacillus | 1 | 0.53% | 0.14% | |
| Planococcus | 2 | 1.06% | 0.28% | |
| Saccharibacillus | 1 | 0.53% | 0.14% | |
| Streptococcus | 1 | 0.53% | 0.14% | |
| Bacteroidetes | Sphingobacterium | 7 | 46.67% | 0.98% |
| Chryseobacterium | 4 | 26.67% | 0.56% | |
| Olivibacter | 3 | 20.00% | 0.42% | |
| Algoriphagus | 1 | 6.67% | 0.14% | |
| Totals | 717 | 100% |
| Phylum | Subphylum | Class | Genus | # of Isolates | % of Class | % of Phylum | % of Total |
|---|---|---|---|---|---|---|---|
| Ascomycota | Pezizomycotina | Dothideomycetes | Alternaria | 476 | 30.75% | 21.29% | 18.89% |
| Phoma | 444 | 28.68% | 19.86% | 17.62% | |||
| Aureobasidium | 242 | 15.63% | 10.82% | 9.60% | |||
| Cladosporium | 150 | 9.69% | 6.71% | 5.95% | |||
| Ascochyta | 95 | 6.14% | 4.25% | 3.77% | |||
| Coniothyrium | 37 | 2.39% | 1.66% | 1.47% | |||
| Epicoccum | 36 | 2.33% | 1.61% | 1.43% | |||
| Leptosphaeria | 16 | 1.03% | 0.72% | 0.63% | |||
| Boeremia | 1 | 0.07% | 0.05% | 0.04% | |||
| Cochliobolus | 7 | 0.45% | 0.31% | 0.28% | |||
| Stemphylium | 7 | 0.45% | 0.31% | 0.28% | |||
| Curvularia | 6 | 0.39% | 0.26% | 0.24% | |||
| Preussia | 6 | 0.39% | 0.266% | 0.24% | |||
| Drechslera | 5 | 0.32% | 0.22% | 0.20% | |||
| Embellisia | 4 | 0.26% | 0.18% | 0.16% | |||
| Macrophomina | 3 | 0.19% | 0.13% | 0.12% | |||
| Guignardia | 2 | 0.13% | 0.09% | 0.08% | |||
| Aerobasidium | 1 | 0.07% | 0.05% | 0.04% | |||
| Paraconiothyrium | 2 | 0.13% | 0.09% | 0.08% | |||
| Paraphoma | 2 | 0.13% | 0.09% | 0.08% | |||
| Torula | 2 | 0.13% | 0.09% | 0.08% | |||
| Unocladium | 2 | 0.13% | 0.09% | 0.08% | |||
| Pseudocochliobolus | 1 | 0.07% | 0.09% | 0.04% | |||
| Unknown Pleosporales | 1 | 0.07% | 0.09% | 0.04% | |||
| Sordariomycetes | Fusarium | 131 | 27.01% | 5.86% | 5.20% | ||
| Nigrospora | 57 | 11.75% | 2.55% | 2.26% | |||
| Acremonium | 54 | 11.13% | 2.42% | 2.14% | |||
| Chaetomium | 41 | 8.45% | 1.83% | 1.63% | |||
| Coniella | 40 | 8.25% | 1.79% | 1.59% | |||
| Trichoderma | 27 | 5.57% | 1.21% | 1.07% | |||
| Monosporascus | 23 | 4.74% | 1.03% | 0.91% | |||
| Sordaria | 17 | 3.51% | 0.76% | 0.67% | |||
| Chrysonilia | 15 | 3.09% | 0.67% | 0.60% | |||
| Diaporthe | 15 | 3.09% | 0.67% | 0.60% | |||
| Cytospora | 14 | 2.89% | 0.63% | 0.56% | |||
| Pestalotiopsis | 9 | 1.86% | 0.40% | 0.36% | |||
| Geniculosporium | 8 | 1.65% | 0.36% | 0.32% | |||
| Nodulisporium | 8 | 1.65% | 0.36% | 0.32% | |||
| Gibberella | 7 | 1.44% | 0.31% | 0.28% | |||
| Phomopsis | 7 | 1.44% | 0.31% | 0.28% | |||
| Sarocladium | 3 | 0.62% | 0.13% | 0.12% | |||
| Bartalinia | 1 | 0.21% | 0.05% | 0.04% | |||
| Biscogniauxia | 1 | 0.21% | 0.05% | 0.04% | |||
| Coniochaeta | 1 | 0.21% | 0.05% | 0.04% | |||
| Myrothecium | 1 | 0.21% | 0.05% | 0.04% | |||
| Nectria | 1 | 0.21% | 0.05% | 0.04% | |||
| Neonectria | 1 | 0.21% | 0.05% | 0.04% | |||
| Plectosphaerella | 1 | 0.21% | 0.05% | 0.04% | |||
| Purpureocillium | 1 | 0.21% | 0.05% | 0.04% | |||
| Unk. Coniochaetales | 1 | 0.21% | 0.05% | 0.04% | |||
| Eurotiomycetes | Penicillium | 134 | 71.28% | 5.99% | 5.32% | ||
| Aspergillus | 51 | 27.13% | 2.28% | 2.02% | |||
| Phinocladiella | 3 | 1.60% | 0.13% | 0.12% | |||
| Leotiomycetes | Cadophora | 1 | 50.00% | 0.05% | 0.04% | ||
| Phialocephala | 1 | 50.00% | 0.05% | 0.04% | |||
| Saccharomycotina | Saccharomycetes | Debaryomyces | 6 | 75.00% | 0.27% | 0.24% | |
| Candida | 2 | 25.00% | 0.09% | 0.08% | |||
| Incertae sedis | Incertae sedis | Rhizopycnis | 4 | 80.00% | 0.18% | 0.16% | |
| Aporospora | 1 | 20.00% | 0.05% | 0.04% | |||
| Zygomycota | n/a | Zygomycetes | Mucor | 9 | 42.86% | 42.86% | 0.36% |
| Rhizopus | 8 | 38.10% | 38.10% | 0.32% | |||
| Cunninghamella | 3 | 14.29% | 14.29% | 0.12% | |||
| Syncephalastrum | 1 | 4.76% | 4.76% | 0.04% | |||
| Basidiomycota | Agaricomycotina | Agaricomycetes | Rhizoctonia | 1 | 100.00% | 5.26% | 0.04% |
| Tremeliomycetes | Cryptococcus | 1 | 100.00% | 5.26% | 0.04% | ||
| Pucciniomycotina | Agaricostilbomycetes | Sterigmatomyces | 5 | 100.00% | 26.32% | 0.20% | |
| Microbotryomycetes | Rhodotorula | 6 | 75.00% | 31.58% | 0.24% | ||
| Sporobolomyces | 2 | 25.00% | 10.53% | 0.08% | |||
| Tritirachiomycetes | Tritirachium | 4 | 100.00% | 21.05% | 0.16% | ||
| Sterile | Unk. | Unknown | Unknown | 244 | 100.00% | 100.00% | 9.68% |
| Total | 2520 | 100% | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; White, J.F. Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future. Biology 2021, 10, 961. https://doi.org/10.3390/biology10100961
Zhang Q, White JF. Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future. Biology. 2021; 10(10):961. https://doi.org/10.3390/biology10100961
Chicago/Turabian StyleZhang, Qiuwei, and James F. White. 2021. "Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future" Biology 10, no. 10: 961. https://doi.org/10.3390/biology10100961
APA StyleZhang, Q., & White, J. F. (2021). Bioprospecting Desert Plants for Endophytic and Biostimulant Microbes: A Strategy for Enhancing Agricultural Production in a Hotter, Drier Future. Biology, 10(10), 961. https://doi.org/10.3390/biology10100961







