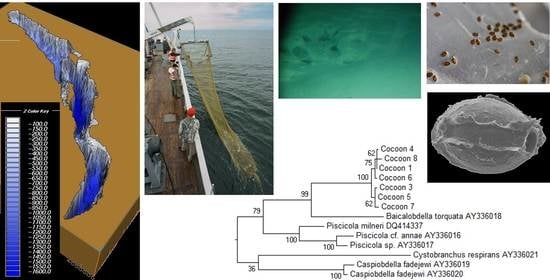
Amazing Discoveries of Benthic Fauna from the Abyssal Zone of Lake Baikal
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
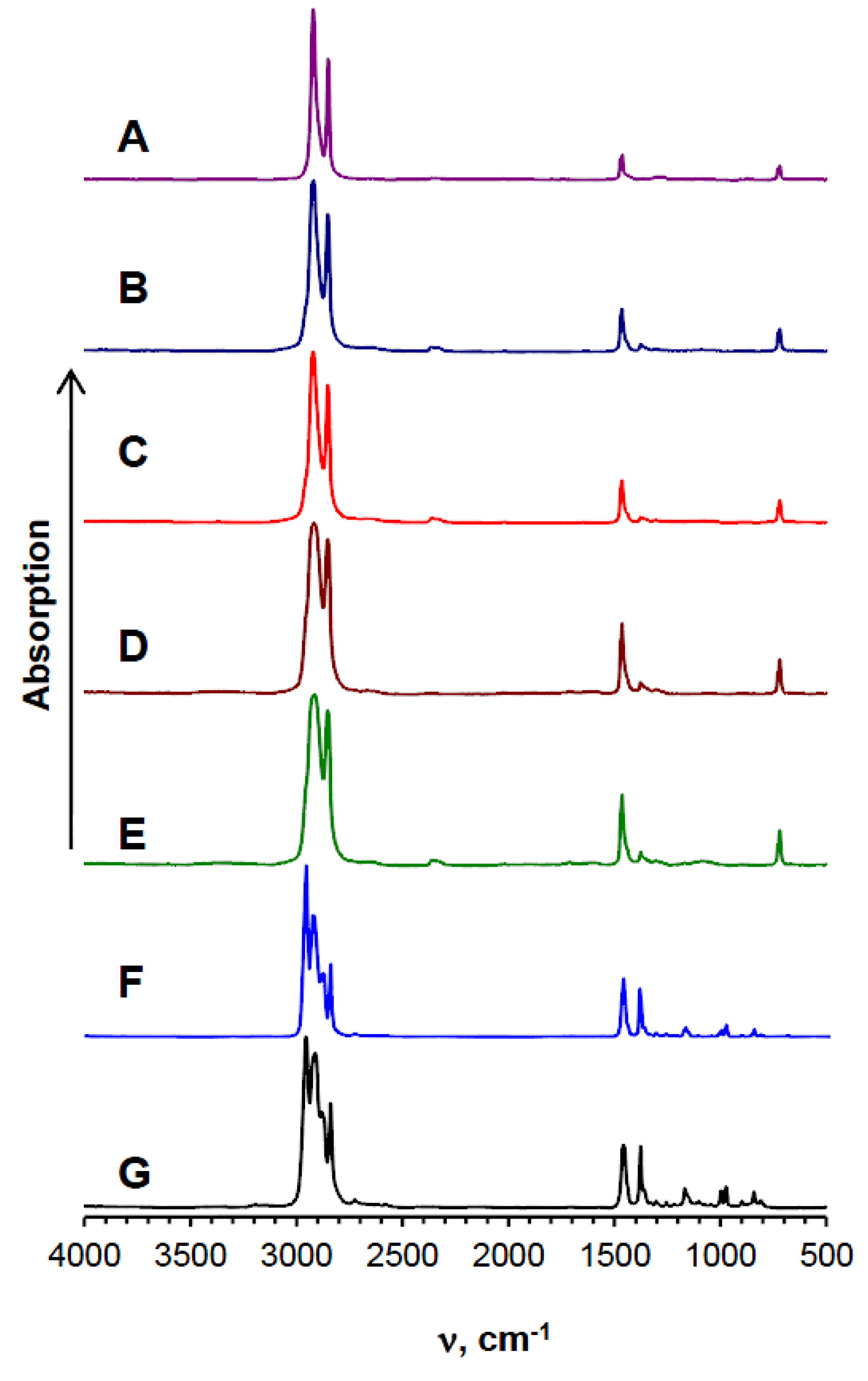
2.2. Plastic Composition Experimental Analysis
2.3. Morphological Analysis
2.4. DNA Analysis
3. Results
3.1. The Chemical Composition of Plastic Waste
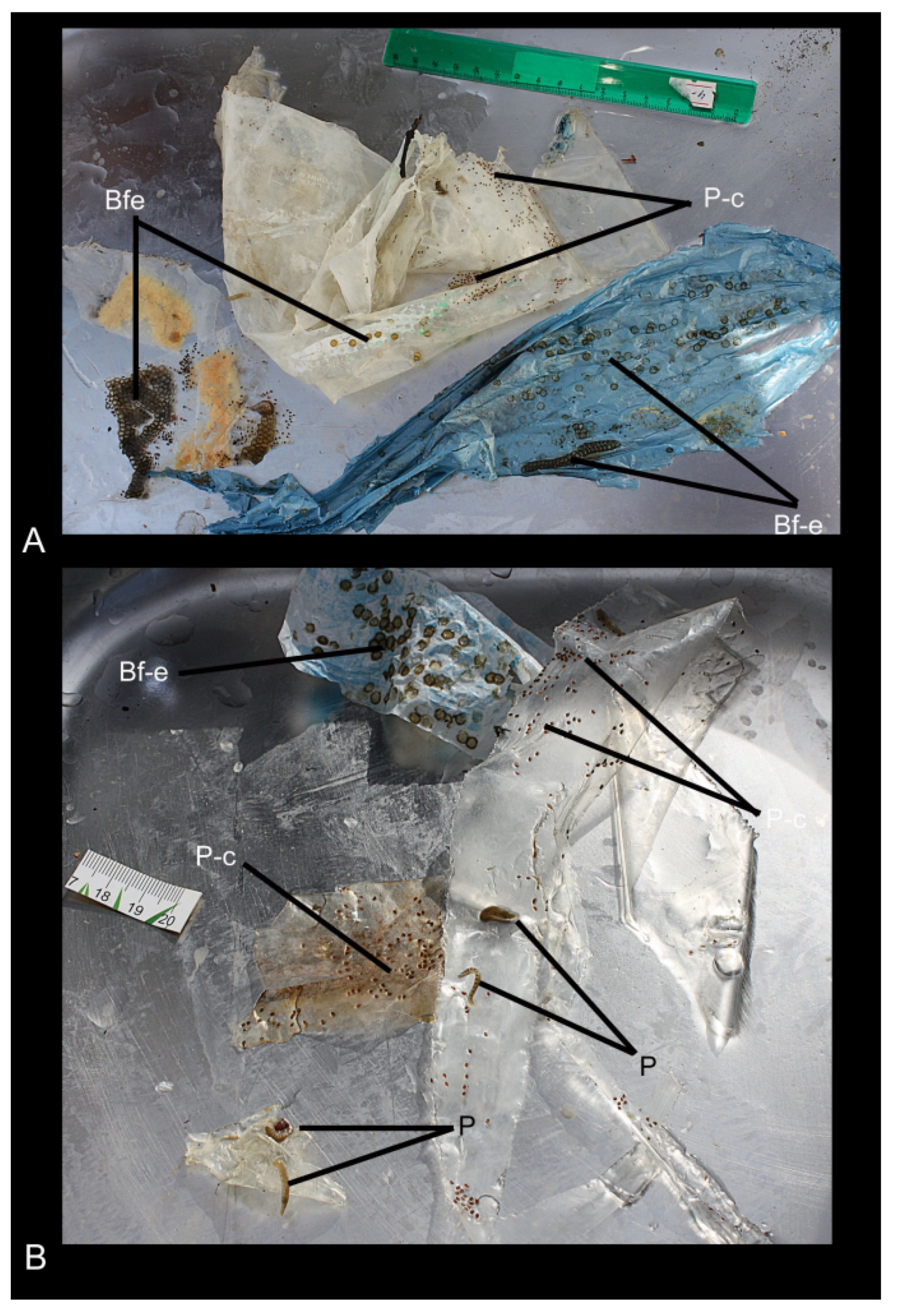
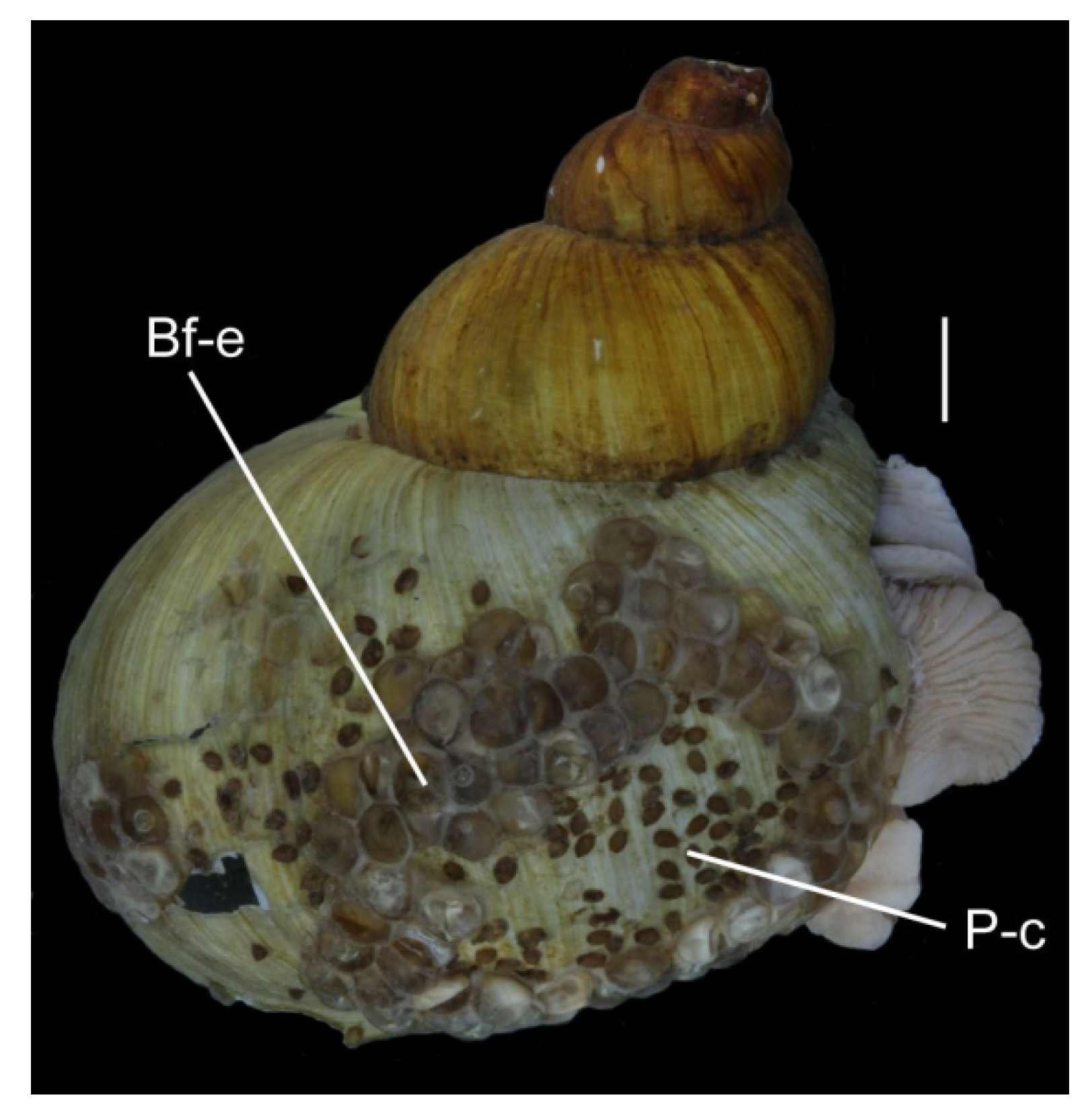
3.2. Waste-Associated Macroorganisms
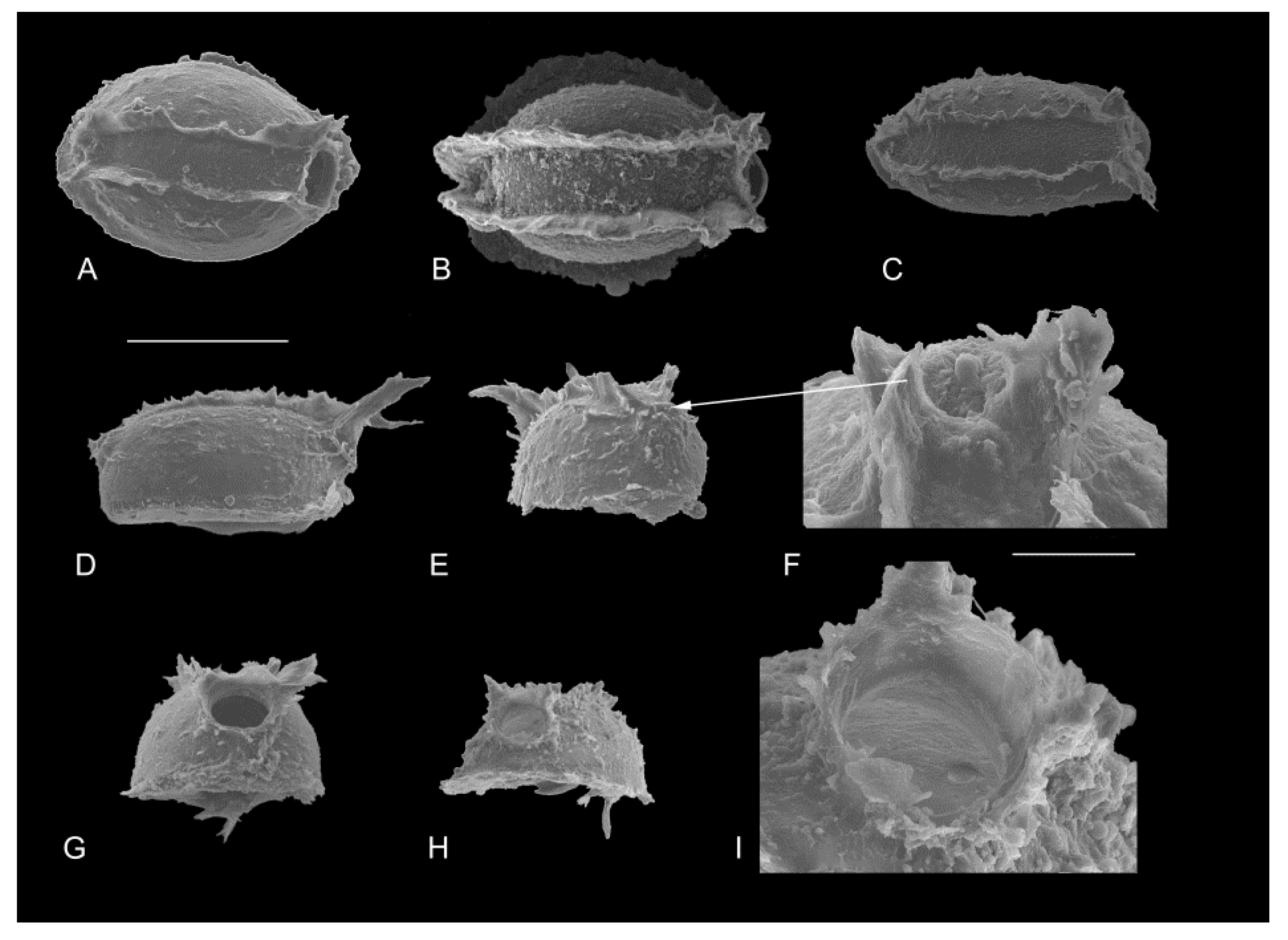
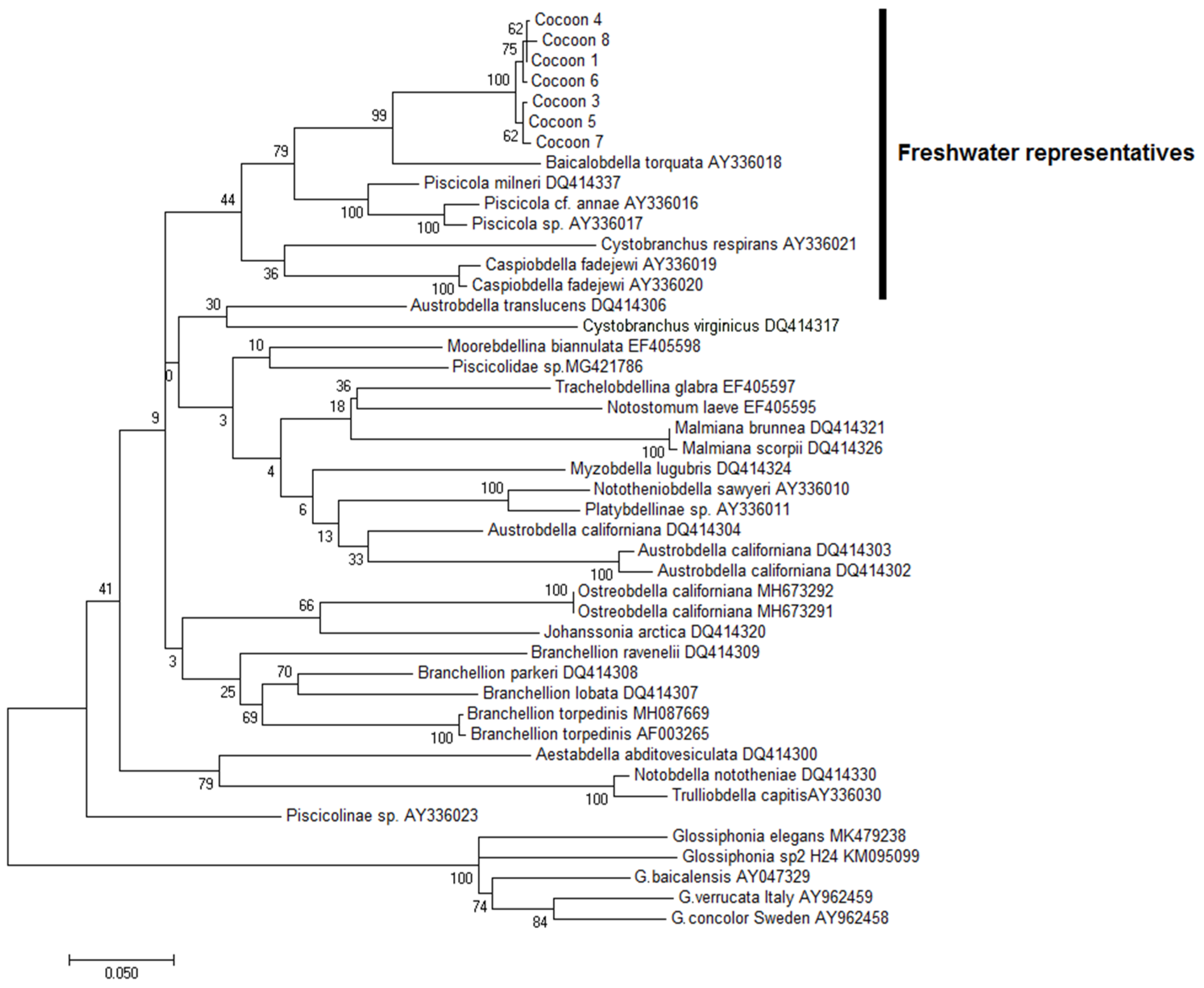
3.3. Identification of Cocoons
3.4. Detection of Stramenopiles
3.5. Identification of Bryozoans
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kozhova, O.M.; Izmest’eva, L.R. Lake Baikal. In Evolution and Biodiversity; Backhuys Publishers: Leiden, The Netherlands, 1998; p. 447. [Google Scholar]
- Taliev, D.N. Sculpins Fishes of Lake Baikal (Cottoidei); Publishing House of the Academy of Sciences of the USSR: Moscow, Russia, 1955; p. 602. [Google Scholar]
- UNESCO. Operational Guidelines for the Implementation of the World Heritage Convention; UNESCO: Paris, France, 1999. [Google Scholar]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Domysheva, V.M.; Kravchenko, O.S.; Grachev, M.A. Disturbances of the vertical zoning of green algae in the coastal part of the Listvennichnyi Gulf of Lake Baikal. Dokl. Biol. Sci. 2012, 448, 227–229. [Google Scholar] [CrossRef]
- Kravtsova, L.S.; Izhboldina, L.A.; Khanaev, I.V.; Pomazkina, G.V.; Rodionova, E.V.; Domysheva, V.M.; Sakirko, M.V.; Tomberg, I.V.; Kostornova, T.Y.; Kravchenko, O.S.; et al. Nearshore benthic blooms of filamentous green algae in Lake Baikal. J. Great Lakes Res. 2014, 40, 441–448. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Samsonov, D.P.; Yamamuro, M.; Moore, M.V.; Belykh, O.I.; Malnik, V.V.; Sakirko, M.V.; Shirokaya, A.A.; Bondarenko, N.A.; Domysheva, V.M.; et al. Rapid ecological change in the coastal zone of Lake Baikal (East Siberia): Is the site of the world’s greatest freshwater biodiversity in danger? J. Great Lakes Res. 2016, 42, 487–497. [Google Scholar] [CrossRef]
- Timoshkin, O.A.; Bondarenko, N.A.; Kulikova, N.N.; Lukhnev, A.G.; Maximova, N.V.; Malnik, V.V.; Moore, M.V.; Nepokrytykh, A.V.; Obolkina, L.A.; Rozhkova, N.A.; et al. Protection of Lake Baikal requires more stringent, not more lenient, environmental regulation. J. Great Lakes Res. 2019, 45, 401–402. [Google Scholar] [CrossRef]
- Volkova, E.A.; Bondarenko, N.A.; Timoshkin, O.A. Morphotaxonomy, distribution and abundance of Spirogyra (Zygnematophyceae, Charophyta) in Lake Baikal, East Siberia. Phycologia 2018, 57, 298–308. [Google Scholar] [CrossRef]
- Potapskaya, N.V.; Kulikova, N.N.; Timoshkin, O.A.; Zaitseva, E.P.; Nepokrytykh, A.V.; Mal’nik, V.V. Estimation of consumer waste accumulation in the coastal area of Baikal and Selenga delta. Geogr. Nat. Resour. 2016, 1, 62–69. [Google Scholar]
- Chapman, M.G.; Clynick, B.G. Experiments testing the use of waste material in estuaries as habitat for subtidal organisms. J. Exp. Mar. Biol. Ecol. 2006, 338, 164–178. [Google Scholar] [CrossRef]
- Czarnecka, M.; Poznanska, M.; Kobak, J.; Wolnomiejski, N. The role of solid waste materials as habitats for macroinvertebrates in a lowland dam reservoir. Hydrobiologia 2009, 635, 125–135. [Google Scholar] [CrossRef]
- Kennedy, K.T.M.; El-Sabaawi, R.W. Decay patterns of invasive plants and plastic trash in urban streams. Urban Ecosyst. 2018, 21, 817–830. [Google Scholar] [CrossRef]
- Booth, A.J.; Kadye, W.T.; Vu, T.; Wright, M. Rapid colonisation of artificial substrates by macroinvertebrates in a South African lentic environment. Afr. J. Aquat. Sci. 2013, 38, 175–183. [Google Scholar] [CrossRef]
- Hebert, P.; Cywinska, A.; Ball, S.; Dewaard, J. Biological identifications through DNA barcodes. Proc. R. Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.; Hollingsworth, P.; Hajibabaei, M. From writing to reading the encyclopedia of life. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150321. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.K.; Biswas, A.; Goswami, U. A simple method for scanning electron microscopy (SEM) study of Cladocera: Bosmina (Bosmina) tripurae. WJFMS 2011, 3, 71–78. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Darriba, D.; Posada, D.A.; Kozlov, M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2019, 37, 291–294. [Google Scholar] [CrossRef]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Kaygorodova, I.; Bolbat, N.; Bolbat, A. Species delimitation through DNA barcoding of freshwater leeches of the Glossiphonia genus (Hirudinea: Glossiphoniidae) from Eastern Siberia, Russia. J. Zoolog. Syst. Evol. Res. 2020, 58, 1437–1446. [Google Scholar] [CrossRef]
- Saglam, N.; Saunders, R.; Lang, S.A.; Shain, D.H. Phylogeny and cocoon production in the parasitic leech Myzobdella lugubris Leidy, 1851 (Hirudinidae, Piscicolidae). Acta Parasitol. 2018, 63, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Karabanov, E.B.; Sideleva, V.G.; Izhboldina, L.A.; Melnik, N.G.; Zubin, A.A.; Zubina, L.V.; Smirnov, N.V.; Parfyonova, V.V.; Fedorova, O.A.; Gorbunova, L.A.; et al. Underwater Landscapes of Baikal; Nauka: Novosibirsk, Russia, 1990; p. 183. [Google Scholar]
- Sitnikova, T.Y.; Shimaraev, M.N. Deep-water “dwarfs” and “giants” among endemic Baikal gastropods. Zh. Obs. Biol. 2001, 62, 226–238. [Google Scholar]
- Brönmark, C. Leech predation on juvenile freshwater snails: Effects of size, species and substrate. Oecologia 1992, 91, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, R.T. Leech Biology and Behavior: Feeding Biology, Ecology, and Systematics, 1st ed.; Oxford University Press: Oxford, UK, 1986; Volume II. [Google Scholar]
- Young, J.O. Intra- and interspecific predation of the cocoon of Erpobdella octoculata (L.) (Annelida: Hirudinea). Hydrobiologia 1988, 169, 85–89. [Google Scholar] [CrossRef]
- Nagasawa, K.; Fujiwara, K. Two Piscicolid leeches (Hirudinida) and their cocoons on Snow Crabs Chionoecetes opilio in Japan, with the first record of Johanssonia arctica from the Sea of Japan. Biogeography 2008, 10, 65–72. [Google Scholar]
- Adamiak-Brud, Z.; Jabłonska-Barna, I.; Bielecki, A.; Terlecki, J. Settlement preferences of leeches (Clitellata: Hirudinida) for different artificial substrates. Hydrobiologia 2015, 758, 275–286. [Google Scholar] [CrossRef][Green Version]
- Koperski, P. Stone-dwelling leeches (Hirudinea, Clitellata) of Lake Hancza (Poland): Different sampling methods determine different taxonomic structures. Pol. J. Ecol. 2003, 51, 353–361. [Google Scholar]
- Kaygorodova, I.A. Annotated checklist of the leech species diversity in the Maloe More Strait of Lake Baikal, Russia. ZooKeys 2015, 545, 37–52. [Google Scholar] [CrossRef]
- Matveenko, E.Y.; Kaygorodova, I.A. Ecological zonation of the Baikal endemic piscine leeches of the genus Baicalobdella (Hirudinea, Piscicolidae). Limnol. Freshw. Biol. 2020, 4, 801–802. [Google Scholar] [CrossRef]
- Snimschikova, L.N. The Lists of species of animals and plants inhabiting in Baikal. Hirudinea. In Lake Baikal: Evolution and Biodiversity; Kozhova, O.M., Izmest’eva, L.R., Eds.; Backuys Publishers: Leiden, The Netherlands, 1998; pp. 369–370. [Google Scholar]
- Vinogradov, A.V. Eurystomata and Phylactolaemata fauna at the main relict continental water-bodies of Euroasia. Izv. RAS SamSC 2008, 10, 531–546. [Google Scholar]
- Pinochet, J.; Urbina, M.A.; Lagos, M.E. Marine invertebrate larvae love plastics: Habitat selection and settlement on artificial substrates. Environ. Pollut. 2020, 257, 113571. [Google Scholar] [CrossRef] [PubMed]
- Pelseneer, P. The origin of fresh water animals [L’origine des animaux d’eau douce]. Acad. Analecta 1906, 12, 699–741. [Google Scholar]
- Fehlauer-Ale, K.H.; Vieira, L.M.; Winston, J.E. Molecular and morphological characterization of Amathia distans Busk and Amathia brasiliensis Busk (Bryozoa: Ctenostomata) from the tropical and subtropical Western Atlantic. Zootaxa 2011, 2962, 49–62. [Google Scholar] [CrossRef]
- Waeschenbach, A.; Vieira, L.M.; Reverter-Gil, O.; Souto-Derungs, J.; Nascimento, K.B.; Fehlauer-Ale, K.H. A phylogeny of Vesiculariidae (Bryozoa, Ctenostomata) supports synonymization of three genera and reveals possible cryptic diversity. Zool. Scr. 2015, 44, 667–683. [Google Scholar] [CrossRef]
- Mason, T.A.; McIlroy, J.P.; Shain, D.H. A cysteine-rich protein in the Theromyzon (Annelida: Hirudinea) cocoon membrane. FEBS J. 2004, 561, 167–172. [Google Scholar] [CrossRef]
- Coleman, J.; Shain, D.H. Clitellate cocoons and their secretion. In Annelids in Modern Biology; Shain, D.H., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 18, pp. 328–346. [Google Scholar]
- Rossi, A.M.; Saidel, W.M.; Gravante, C.J.; Sayers, C.W.; Shain, D.H. Mechanics of cocoon secretion in a segmented worm (Annelida: Hirudinidae). Micron 2016, 86, 30–35. [Google Scholar] [CrossRef]
- Langenhoven, S.D.; Halleen, F.; Spies, C.; Stempien, E.; Mostert, L. Detection and quantification of black foot and crown and root rot pathogens in grapevine nursery soils in the Western Cape of South Africa. Phytopathol. Mediterr. 2018, 57, 519–537. [Google Scholar]
- Tkaczyk, M. Phytopythium: Origin, differences and meaning in modern plant pathology. Folia For. Pol. A 2020, 62, 227–232. [Google Scholar] [CrossRef]
- Grossmann, L.; Bock, C.; Schweikert, M.; Boenigk, J. Small but manifold-hidden diversity in “Spumella-like Flagellates”. J. Eukaryot. Microbiol. 2016, 63, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Annenkova, N.V.; Giner, C.R.; Logares, R. Tracing the origin of planktonic protists in an Ancient Lake. Microorganisms 2020, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Berney, C.; Hartikainen, H.; Mahamdallie, S.; Gardner, M.; Boenigk, J.; Cavalier-Smith, T.; Bass, D. High-throughput sequencing of microbial eukaryotes in Lake Baikal reveals ecologically differentiated communities and novel evolutionary radiations. FEMS Microbiol. Ecol. 2017, 93, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rech, S.; Borrell Pichs, Y.J.; García-Vazquez, E. Anthropogenic marine litter composition in coastal areas may be a predictor of potentially invasive rafting fauna. PLoS ONE 2018, 13, e0191859. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
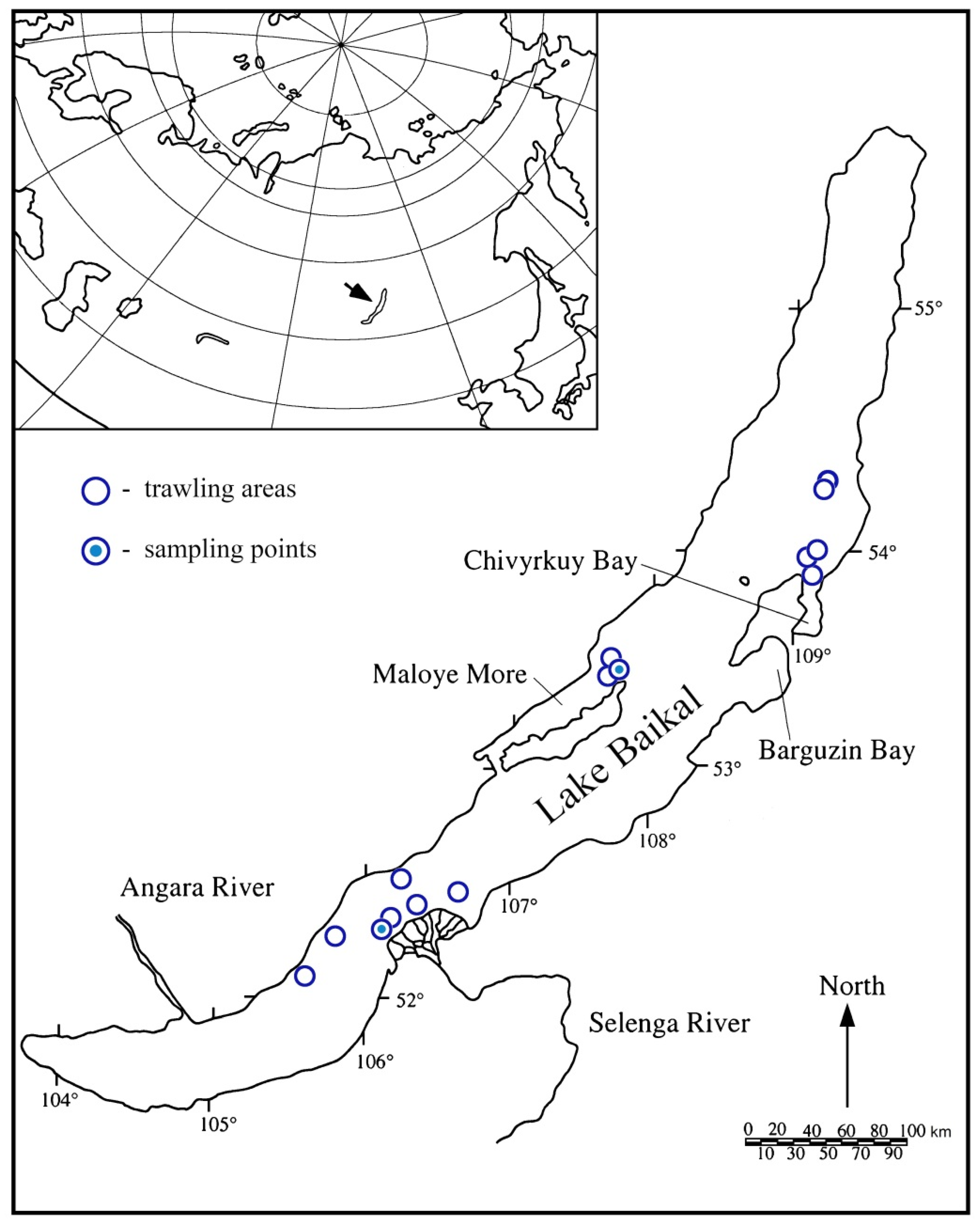


| Trawl | Place Name | Coordinates down (N/E) | Coordinates up (N/E) | Depths (m) |
|---|---|---|---|---|
| 1 | Selenga River delta | 52°17.270′/106°03.910′ | 52°18.600′/106°06.095′ | 111 to 133 |
| 2 | Selenga River delta | 52°19.910′/106°07.441′ | 52°22.280′/106°10.015′ | 142 to 181 |
| 3 | Selenga River delta | 52°24.933′/106°13.027′ | 52°26.217′/106°16.940′ | 271 to 276 |
| 4 | Proval Bay | 52°30.502′/106°36.704′ | 52°31.196′/106°40.088′ | 553 to 478 |
| 5 | Chivyrkuy Bay | 53°55.900′/109°08.473′ | 53°58.341′/109°05.869′ | 790 to 760 |
| 6 | Chivyrkuy Bay | 53°56.260′/109°06.425′ | 53°57.765′/109°07.358′ | 785 to 810 |
| 7 | Chivyrkuy Bay | 53°49.813′/109°08.584′ | 53°50.787′/109°03.886′ | 125 to 180 |
| 8 | Davsha | 54°23.662′/109°14.127′ | 54°23.091′/109°15.727′ | 700 to 715 |
| 9 | Davsha | 54°21.745′/109°14.749′ | 54°21.006′/109°12.765′ | 715 to 715 |
| 10 | Northern section of the Maloye More strait | 53°26.746′/107°44.691′ | 53°26.496′/107°43.012′ | 313 to 300 |
| 11 | Northern section of the Maloye More strait | 53°25.280′/107°45.085′ | 53°24.821′/107°44.079′ | 175 to 188 |
| 12 | Northern section of the Maloye More strait | 53°24.979′/107°44.783′ | 53°24.585′/107°43.074′ | 163 to 186 |
| 13 | Buguldeyka | 52°30.870′/106°07.467′ | 52°29.179′/106°07.226′ | 360 to 395 |
| 14 | Peschanaya | 52°15.864′/105°50.365′ | 52°14.974′/105°49.767′ | 1005 to 1015 |
| 15 | Verkhniye Khomuty | 52°06.481′/105°37.545′ | 52°05.767′/105°39.826′ | 1136 to 1200 |
| Description | Max Score | Query Cover | Per. Ident | Accession |
|---|---|---|---|---|
| Baicalobdella torquata | 833 | 95% | 90.51% | AY336018 |
| Piscicola milneri | 813 | 99% | 88.82% | DQ414337 |
| Piscicola cf. annae | 771 | 94% | 88.71% | AY336016 |
| Moorebdellina biannulata | 763 | 99% | 87.46% | EF405598 |
| Piscicola sp. | 745 | 93% | 88.12% | AY336017 |
| Branchellion parkeri | 725 | 99% | 86.40% | DQ414308 |
| Austrobdella translucens | 725 | 99% | 86.40% | DQ414306 |
| Nototheniobdella sawyeri | 708 | 99% | 86.04% | AY336010 |
| Branchellion torpedinis | 699 | 98% | 85.84% | MH087669 |
| Caspiobdella fadejewi | 689 | 94% | 86.46% | AY336019 |
| Piscicolidae sp. | 688 | 98% | 85.58% | MG421786 |
| Branchellion torpedinis | 686 | 97% | 85.69% | AF003265 |
| Caspiobdella fadejewi | 682 | 94% | 86.26% | AY336020 |
| Cystobranchus respirans | 669 | 94% | 85.90% | AY336021 |
| Trachelobdellina glabra | 647 | 99% | 84.31% | EF405597 |
| Branchellion lobata | 636 | 99% | 83.99% | DQ414307 |
| Aestabdella abditovesiculata | 636 | 99% | 84.08% | DQ414300 |
| Piscicolinae sp. | 634 | 97% | 84.36% | AY336023 |
| Myzobdella lugubris | 630 | 99% | 83.91% | DQ414324 |
| Austrobdella californiana | 630 | 99% | 83.86% | DQ414304 |
| Johanssonia arctica | 625 | 99% | 83.71% | DQ414320 |
| Cystobranchus virginicus | 625 | 99% | 83.69% | DQ414317 |
| Branchellion ravenelii | 623 | 98% | 83.76% | DQ414309 |
| Notostomum laeve | 619 | 99% | 83.56% | EF405595 |
| Ostreobdella californiana | 616 | 97% | 83.72% | MH673292 |
| Ostreobdella californiana | 616 | 97% | 83.72% | MH673291 |
| Malmiana brunnea | 597 | 99% | 82.98% | DQ414321 |
| Austrobdella californiana | 597 | 99% | 82.96% | DQ414303 |
| Platybdellinae sp. | 597 | 84% | 85.66% | AY336011 |
| Malmiana scorpii | 586 | 99% | 82.68% | DQ414326 |
| Notobdella nototheniae | 579 | 98% | 82.60% | DQ414330 |
| Austrobdella californiana | 564 | 94% | 82.93% | DQ414302 |
| Trulliobdella capitis | 556 | 98% | 82.07% | AY336030 |
| Glossiphonia elegans | 555 | 98% | 82.00% | MK479238 |
| Glossiphonia complanata | 527 | 98% | 81.27% | JQ821635 |
| Description | Max Score | Query Cover | Per. Ident | Accession |
|---|---|---|---|---|
| Amathia evelinae | 601 | 99% | 83.41% | KM373436 |
| Amathia vidovici | 601 | 99% | 83.36% | KM373394 |
| Amathia cf. vidovici | 601 | 99% | 83.36% | JF490059 |
| Amathia distans | 601 | 99% | 83.28% | JF490058 |
| Amathia imbricata | 599 | 99% | 83.16% | KM373430 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373419 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373415 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373414 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373413 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373412 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373411 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373410 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373406 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373404 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373401 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373399 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373395 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373379 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373368 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373367 |
| Amathia vidovici | 595 | 99% | 83.21% | KM373366 |
| Amathia vidovici | 593 | 98% | 83.26% | KM373370 |
| Amathia vidovici | 588 | 98% | 83.05% | KM373421 |
| Amathia vidovici | 588 | 96% | 83.41% | KM373418 |
| Amathia vidovici | 588 | 97% | 83.26% | KM373396 |
| Amathia vidovici | 588 | 98% | 83.05% | KM373380 |
| Amathia vidovici | 588 | 97% | 83.26% | KM373374 |
| Amathia vidovici | 588 | 97% | 83.26% | KM373373 |
| Amathia vidovici | 588 | 97% | 83.26% | KM373372 |
| Amathia vidovici | 588 | 97% | 83.26% | KM373369 |
| Amathia vidovici | 588 | 98% | 83.05% | KM373365 |
| Amathia vidovici | 588 | 98% | 83.05% | KM373364 |
| Amathia vidovici | 584 | 97% | 83.20% | KM373417 |
| Amathia vidovici | 584 | 97% | 83.10% | KM373400 |
| Amathia imbricata | 580 | 99% | 82.55% | KM373431 |
| Amathia vidovici | 580 | 96% | 83.23% | KM373397 |
| Amathia vidovici | 579 | 96% | 83.20% | KM373362 |
| Amathia vidovici | 579 | 96% | 83.20% | KM373361 |
| Amathia vidovici | 577 | 95% | 83.25% | KM373420 |
| Amathia vidovici | 577 | 96% | 83.12% | KM373382 |
| Amathia vidovici | 577 | 96% | 83.12% | KM373381 |
| Amathia vidovici | 577 | 96% | 83.12% | KM373376 |
| Amathia vidovici | 577 | 96% | 83.12% | KM373375 |
| Amathia vidovici | 577 | 95% | 83.25% | KM373371 |
| Amathia maxima | 571 | 98% | 82.72% | KM373432 |
| Amathia vidovici | 571 | 96% | 82.92% | KM373402 |
| Amathia vidovici | 571 | 96% | 82.97% | KM373384 |
| Amathia vidovici | 571 | 96% | 82.94% | KM373359 |
| Amathia vidovici | 566 | 95% | 82.97% | KM373378 |
| Amathia vidovici | 538 | 95% | 81.86% | KM373377 |
| Bowerbankia gracilis | 518 | 98% | 81.25% | FJ196083 |
| Amathia vidovici | 510 | 71% | 86.20% | KM373407 |
| Amathia vidovici | 510 | 71% | 86.20% | KM373405 |
| Anguinella palmata | 501 | 98% | 80.79% | JN680973 |
| Amathia vidovici | 499 | 71% | 85.77% | KM373393 |
| Amathia vidovici | 499 | 71% | 85.77% | KM373391 |
| Amathia vidovici | 499 | 71% | 85.77% | KM373360 |
| Amathia vidovici | 492 | 95% | 80.57% | KM373383 |
| Anguinella palmata | 464 | 98% | 79.69% | KM373422 |
| Cocoons | 0.014 | 0.014 | 0.013 | 0.014 | |
| Piscicola cf. annae AY336016 | 0.11641 | 0.006 | 0.015 | 0.010 | |
| Piscicola sp. AY336017 | 0.12425 | 0.023 | 0.015 | 0.009 | |
| Baicalobdella torquata AY336018 | 0.09859 | 0.123 | 0.129 | 0.014 | |
| Piscicola milneri DQ414337 | 0.11676 | 0.059 | 0.052 | 0.115 |
| Hislopia placoides | 0.019 | 0.022 | 0.020 | 0.022 | 0.019 | 0.023 | 0.021 | 0.020 | 0.030 | 0.023 | 0.023 | 0.022 | 0.024 | 0.022 | 0.025 | 0.027 | 0.025 | 0.032 | 0.030 | 0.022 | 0.022 | 0.039 | |
| Amathia vidovici 2 group | 0.208 | 0.013 | 0.012 | 0.017 | 0.017 | 0.014 | 0.014 | 0.017 | 0.030 | 0.015 | 0.016 | 0.014 | 0.024 | 0.014 | 0.015 | 0.020 | 0.025 | 0.033 | 0.030 | 0.022 | 0.021 | 0.038 | |
| Amathia vidovici 1 group | 0.236 | 0.115 | 0.016 | 0.020 | 0.017 | 0.016 | 0.015 | 0.020 | 0.032 | 0.016 | 0.019 | 0.017 | 0.025 | 0.015 | 0.017 | 0.022 | 0.028 | 0.038 | 0.036 | 0.025 | 0.023 | 0.039 | |
| Amathia distans | 0.208 | 0.111 | 0.136 | 0.019 | 0.017 | 0.017 | 0.016 | 0.017 | 0.033 | 0.015 | 0.016 | 0.014 | 0.025 | 0.015 | 0.017 | 0.021 | 0.024 | 0.038 | 0.033 | 0.023 | 0.022 | 0.040 | |
| Amathia tertia | 0.237 | 0.175 | 0.203 | 0.179 | 0.020 | 0.019 | 0.020 | 0.015 | 0.029 | 0.016 | 0.019 | 0.016 | 0.027 | 0.018 | 0.020 | 0.023 | 0.025 | 0.032 | 0.032 | 0.024 | 0.020 | 0.043 | |
| Amathia evelinae | 0.217 | 0.192 | 0.187 | 0.190 | 0.220 | 0.020 | 0.019 | 0.019 | 0.030 | 0.019 | 0.021 | 0.019 | 0.023 | 0.018 | 0.022 | 0.022 | 0.022 | 0.035 | 0.032 | 0.023 | 0.020 | 0.036 | |
| Amathia pustulosa | 0.244 | 0.127 | 0.144 | 0.161 | 0.187 | 0.234 | 0.017 | 0.019 | 0.029 | 0.015 | 0.017 | 0.017 | 0.026 | 0.017 | 0.019 | 0.020 | 0.027 | 0.031 | 0.032 | 0.025 | 0.024 | 0.037 | |
| Amathia maxima | 0.224 | 0.127 | 0.121 | 0.140 | 0.193 | 0.205 | 0.153 | 0.020 | 0.030 | 0.016 | 0.019 | 0.015 | 0.026 | 0.016 | 0.019 | 0.021 | 0.026 | 0.037 | 0.032 | 0.023 | 0.021 | 0.043 | |
| Amathia imbricata | 0.208 | 0.169 | 0.198 | 0.166 | 0.125 | 0.211 | 0.194 | 0.196 | 0.031 | 0.017 | 0.018 | 0.018 | 0.025 | 0.016 | 0.020 | 0.022 | 0.024 | 0.034 | 0.034 | 0.024 | 0.024 | 0.040 | |
| Amathia gracilis | 0.368 | 0.393 | 0.395 | 0.408 | 0.357 | 0.389 | 0.377 | 0.380 | 0.390 | 0.030 | 0.031 | 0.030 | 0.027 | 0.032 | 0.031 | 0.026 | 0.030 | 0.027 | 0.027 | 0.028 | 0.030 | 0.030 | |
| Amathia citrina | 0.243 | 0.144 | 0.150 | 0.135 | 0.165 | 0.215 | 0.132 | 0.140 | 0.177 | 0.389 | 0.016 | 0.014 | 0.026 | 0.014 | 0.016 | 0.020 | 0.026 | 0.035 | 0.032 | 0.024 | 0.022 | 0.043 | |
| Amathia crispa | 0.238 | 0.154 | 0.181 | 0.153 | 0.185 | 0.229 | 0.157 | 0.178 | 0.180 | 0.392 | 0.143 | 0.017 | 0.027 | 0.017 | 0.020 | 0.021 | 0.026 | 0.037 | 0.034 | 0.024 | 0.023 | 0.040 | |
| Vesicularia spinosa | 0.235 | 0.127 | 0.143 | 0.128 | 0.154 | 0.206 | 0.164 | 0.120 | 0.162 | 0.368 | 0.123 | 0.156 | 0.026 | 0.013 | 0.017 | 0.019 | 0.024 | 0.034 | 0.032 | 0.022 | 0.022 | 0.041 | |
| Anguinella palmata | 0.259 | 0.271 | 0.272 | 0.268 | 0.299 | 0.272 | 0.284 | 0.272 | 0.259 | 0.345 | 0.288 | 0.305 | 0.263 | 0.026 | 0.027 | 0.028 | 0.025 | 0.029 | 0.026 | 0.024 | 0.026 | 0.035 | |
| Amathia semiconvoluta | 0.224 | 0.125 | 0.118 | 0.123 | 0.175 | 0.190 | 0.154 | 0.130 | 0.153 | 0.398 | 0.119 | 0.153 | 0.109 | 0.280 | 0.017 | 0.020 | 0.024 | 0.036 | 0.034 | 0.025 | 0.021 | 0.042 | |
| Amathia lendigera | 0.261 | 0.143 | 0.164 | 0.157 | 0.206 | 0.230 | 0.182 | 0.177 | 0.197 | 0.381 | 0.148 | 0.190 | 0.163 | 0.280 | 0.156 | 0.022 | 0.025 | 0.037 | 0.034 | 0.027 | 0.024 | 0.041 | |
| Amathia convoluta | 0.310 | 0.210 | 0.232 | 0.213 | 0.253 | 0.274 | 0.214 | 0.226 | 0.244 | 0.342 | 0.209 | 0.231 | 0.189 | 0.313 | 0.210 | 0.230 | 0.026 | 0.027 | 0.029 | 0.026 | 0.024 | 0.036 | |
| Alcyonidium verrilli | 0.265 | 0.276 | 0.297 | 0.262 | 0.281 | 0.255 | 0.285 | 0.264 | 0.269 | 0.379 | 0.281 | 0.281 | 0.244 | 0.275 | 0.252 | 0.265 | 0.312 | 0.034 | 0.031 | 0.026 | 0.025 | 0.036 | |
| Alcyonidium mamillatum | 0.365 | 0.394 | 0.419 | 0.420 | 0.375 | 0.412 | 0.350 | 0.409 | 0.402 | 0.336 | 0.388 | 0.416 | 0.380 | 0.320 | 0.406 | 0.400 | 0.318 | 0.399 | 0.029 | 0.034 | 0.030 | 0.036 | |
| Triticella flava | 0.350 | 0.357 | 0.398 | 0.375 | 0.362 | 0.366 | 0.372 | 0.353 | 0.373 | 0.323 | 0.360 | 0.388 | 0.360 | 0.266 | 0.379 | 0.386 | 0.335 | 0.349 | 0.331 | 0.026 | 0.031 | 0.033 | |
| Triticella pedicellata | 0.245 | 0.242 | 0.270 | 0.245 | 0.244 | 0.257 | 0.266 | 0.240 | 0.263 | 0.355 | 0.259 | 0.268 | 0.233 | 0.256 | 0.266 | 0.284 | 0.293 | 0.274 | 0.377 | 0.266 | 0.022 | 0.037 | |
| Bowerbankia gracilis | 0.239 | 0.210 | 0.234 | 0.215 | 0.209 | 0.220 | 0.239 | 0.207 | 0.235 | 0.375 | 0.225 | 0.251 | 0.223 | 0.291 | 0.215 | 0.243 | 0.252 | 0.256 | 0.355 | 0.336 | 0.233 | 0.035 | |
| Flustrellidra hispida | 0.481 | 0.463 | 0.454 | 0.464 | 0.490 | 0.446 | 0.435 | 0.479 | 0.493 | 0.392 | 0.511 | 0.471 | 0.481 | 0.429 | 0.478 | 0.469 | 0.448 | 0.416 | 0.419 | 0.390 | 0.447 | 0.412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondratov, I.G.; Sitnikova, T.Y.; Kaygorodova, I.A.; Denikina, N.N.; Annenkov, V.V.; Khanaev, I.V.; Kirilchik, S.V.; Nebesnykh, I.A.; Dzyuba, E.V. Amazing Discoveries of Benthic Fauna from the Abyssal Zone of Lake Baikal. Biology 2021, 10, 972. https://doi.org/10.3390/biology10100972
Kondratov IG, Sitnikova TY, Kaygorodova IA, Denikina NN, Annenkov VV, Khanaev IV, Kirilchik SV, Nebesnykh IA, Dzyuba EV. Amazing Discoveries of Benthic Fauna from the Abyssal Zone of Lake Baikal. Biology. 2021; 10(10):972. https://doi.org/10.3390/biology10100972
Chicago/Turabian StyleKondratov, Ilya G., Tatiana Ya. Sitnikova, Irina A. Kaygorodova, Natalia N. Denikina, Vadim V. Annenkov, Igor V. Khanaev, Sergei V. Kirilchik, Ivan A. Nebesnykh, and Elena V. Dzyuba. 2021. "Amazing Discoveries of Benthic Fauna from the Abyssal Zone of Lake Baikal" Biology 10, no. 10: 972. https://doi.org/10.3390/biology10100972
APA StyleKondratov, I. G., Sitnikova, T. Y., Kaygorodova, I. A., Denikina, N. N., Annenkov, V. V., Khanaev, I. V., Kirilchik, S. V., Nebesnykh, I. A., & Dzyuba, E. V. (2021). Amazing Discoveries of Benthic Fauna from the Abyssal Zone of Lake Baikal. Biology, 10(10), 972. https://doi.org/10.3390/biology10100972