Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of C. ternatea Extract (CTE)
2.3. Participants
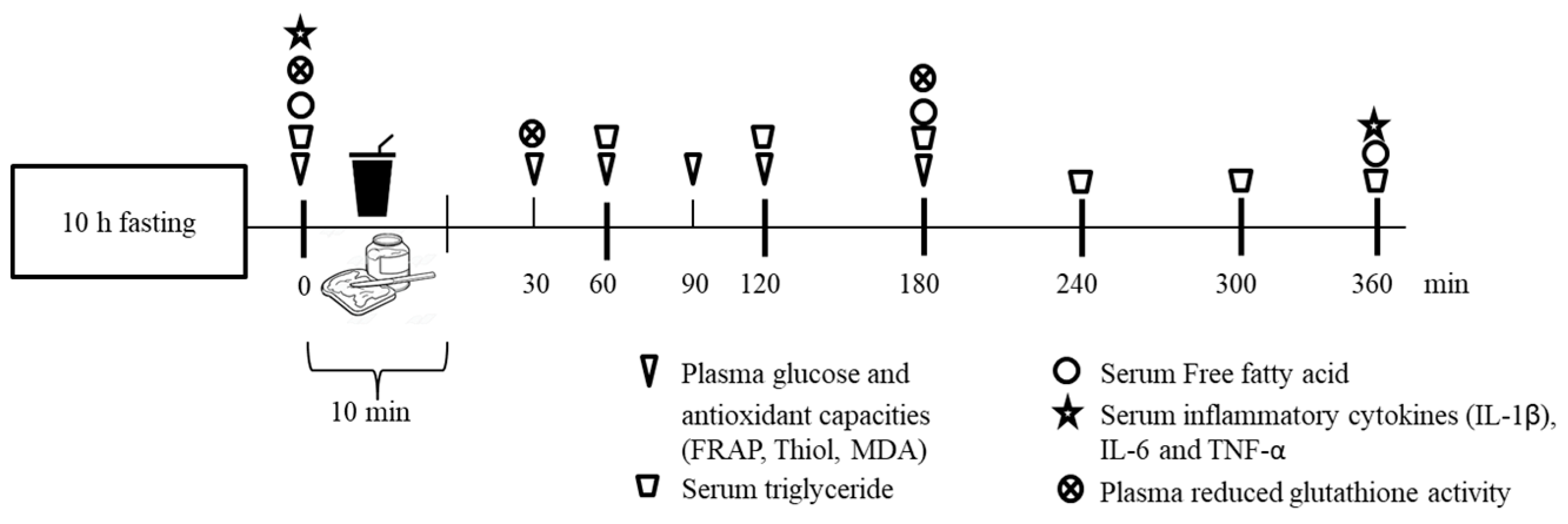
2.4. Study Design
2.5. Intervention
2.6. Plasma Glucose, Serum Triglyceride and Free Fatty Acid (FFA)
2.7. Plasma Ferric Reducing Antioxidant Power (FRAP)
2.8. Plasma Thiol
2.9. Plasma Lipid Peroxidation
2.10. Plasma Glutathione Peroxidase Activity
2.11. Plasma Inflammatory Cytokines
2.12. Statistical Analysis
3. Results
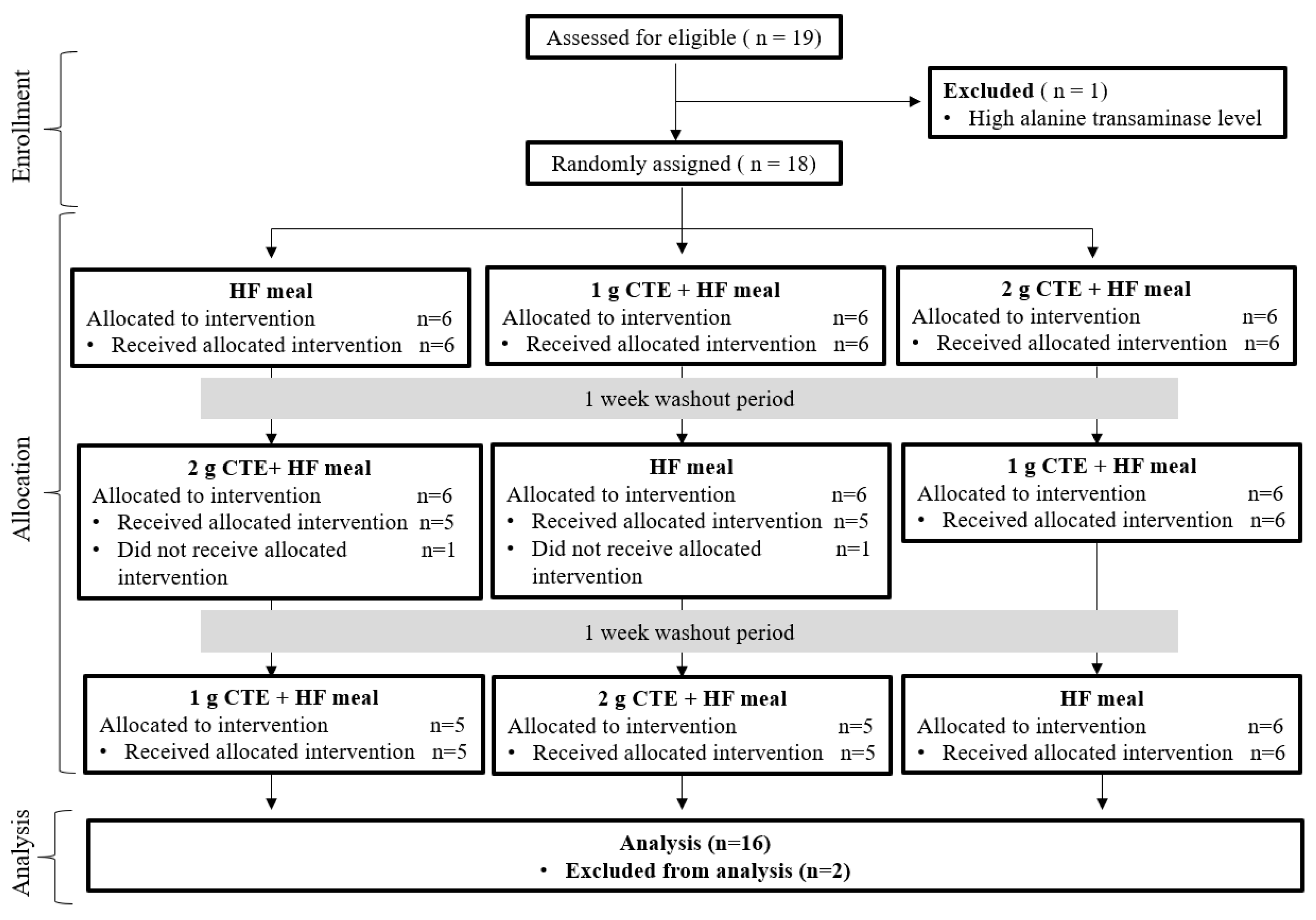
3.1. Participants
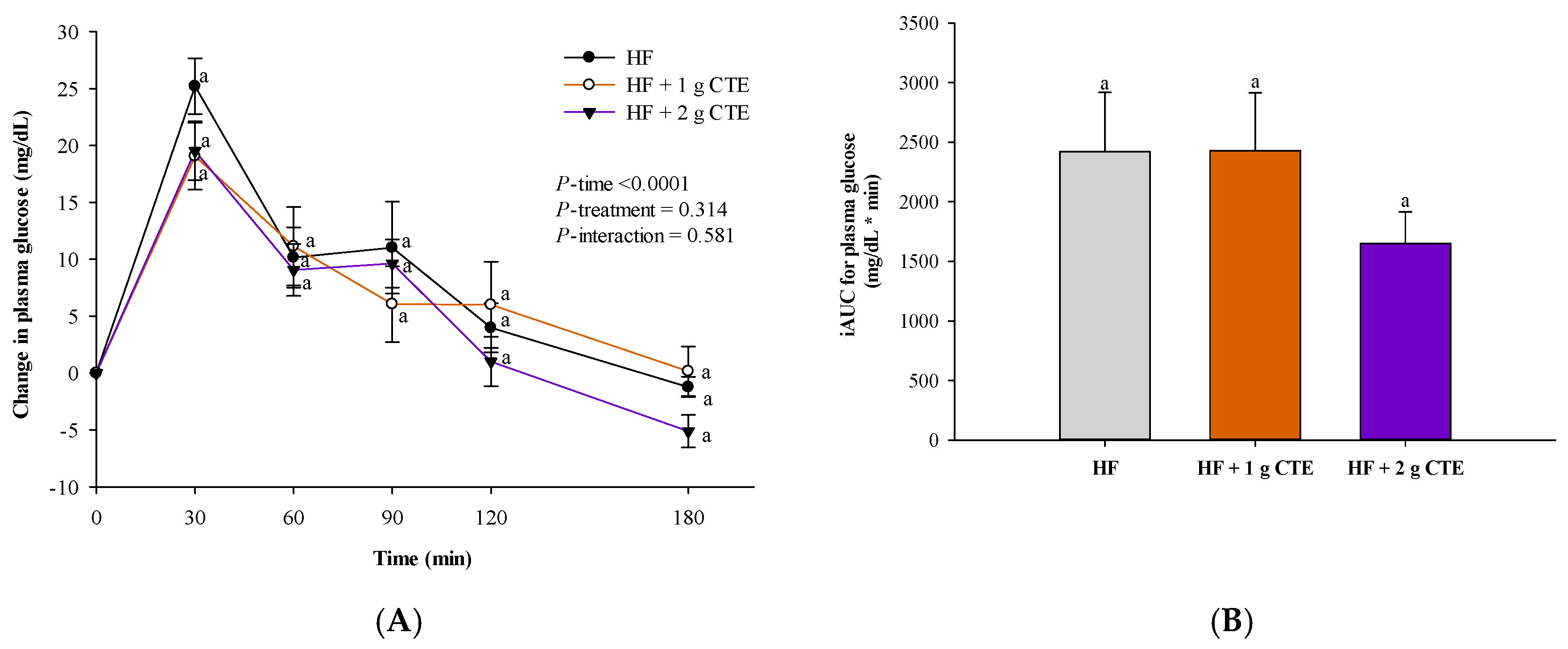
3.2. Postprandial Plasma Glucose Concentration
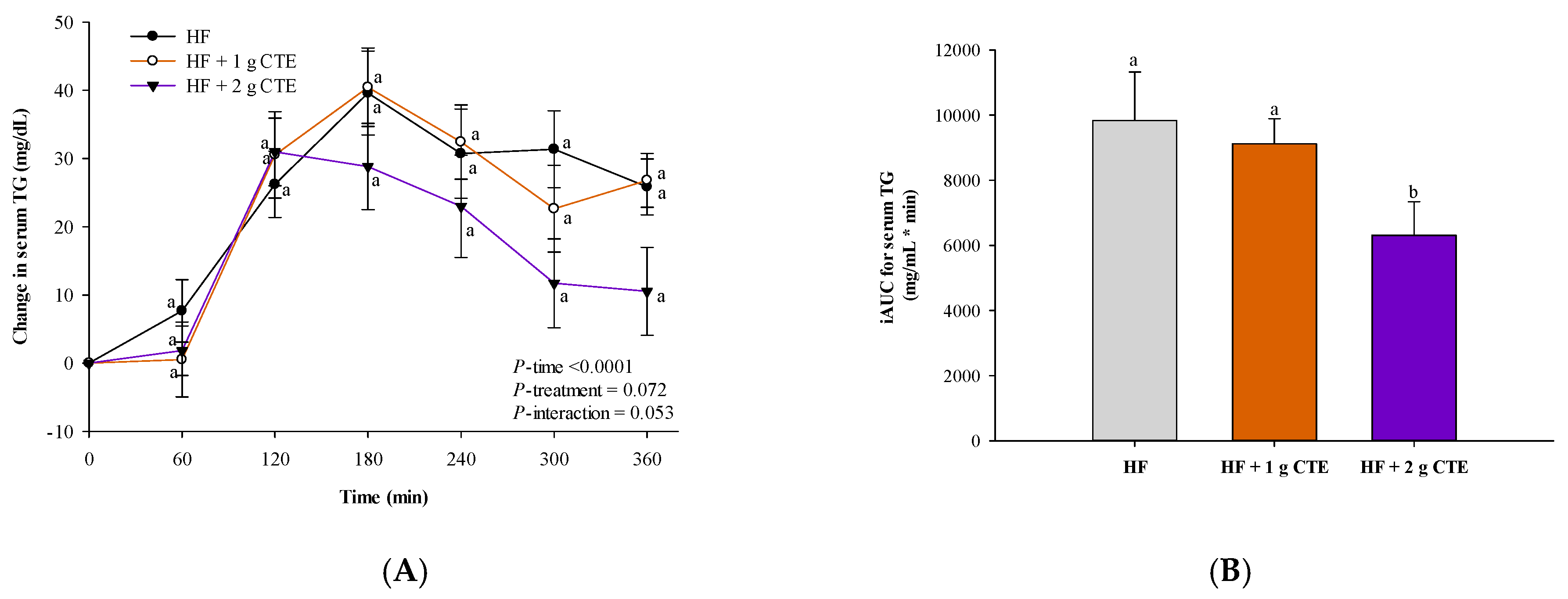
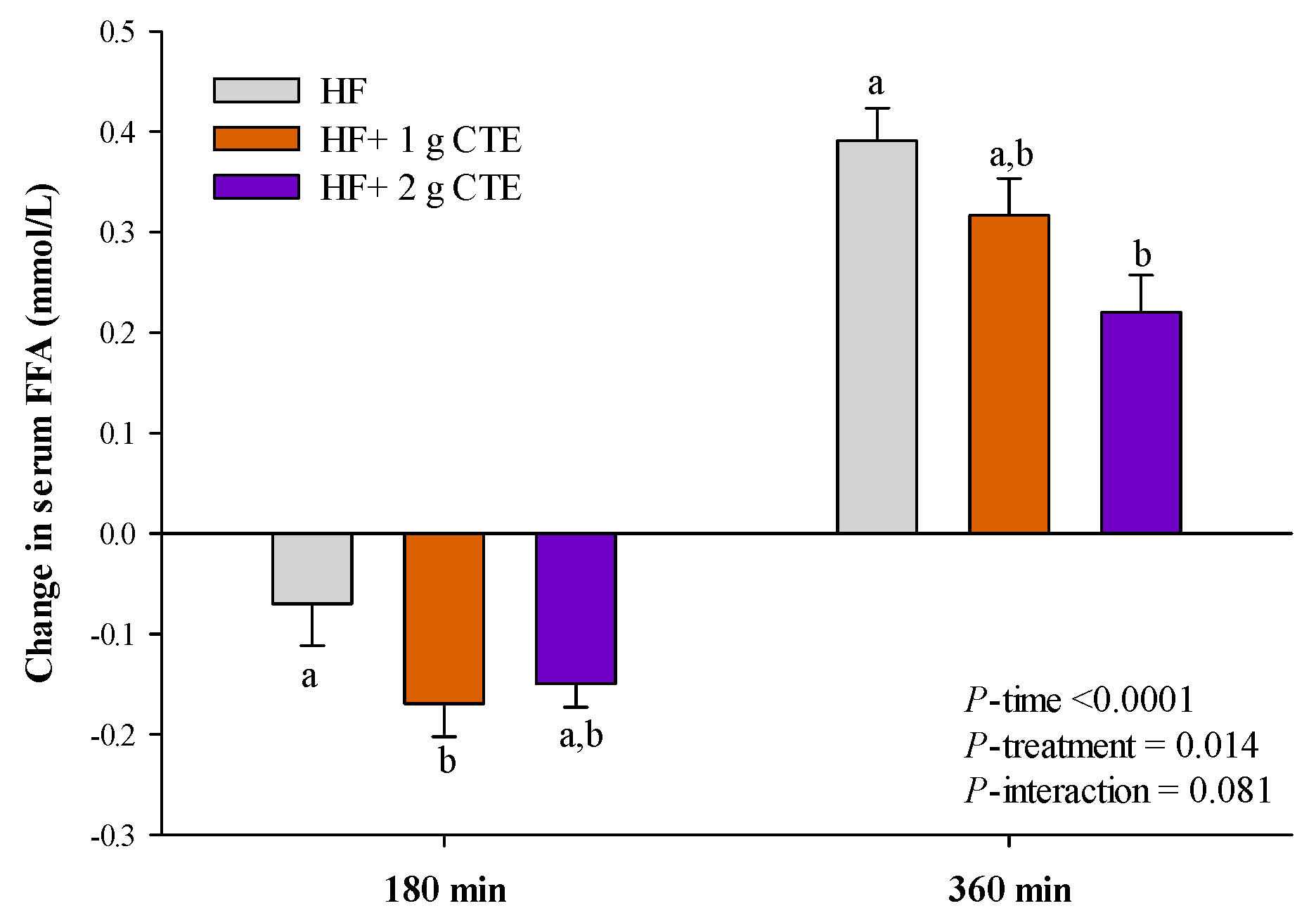
3.3. Postprandial Serum Triglyceride and FFA Concentration
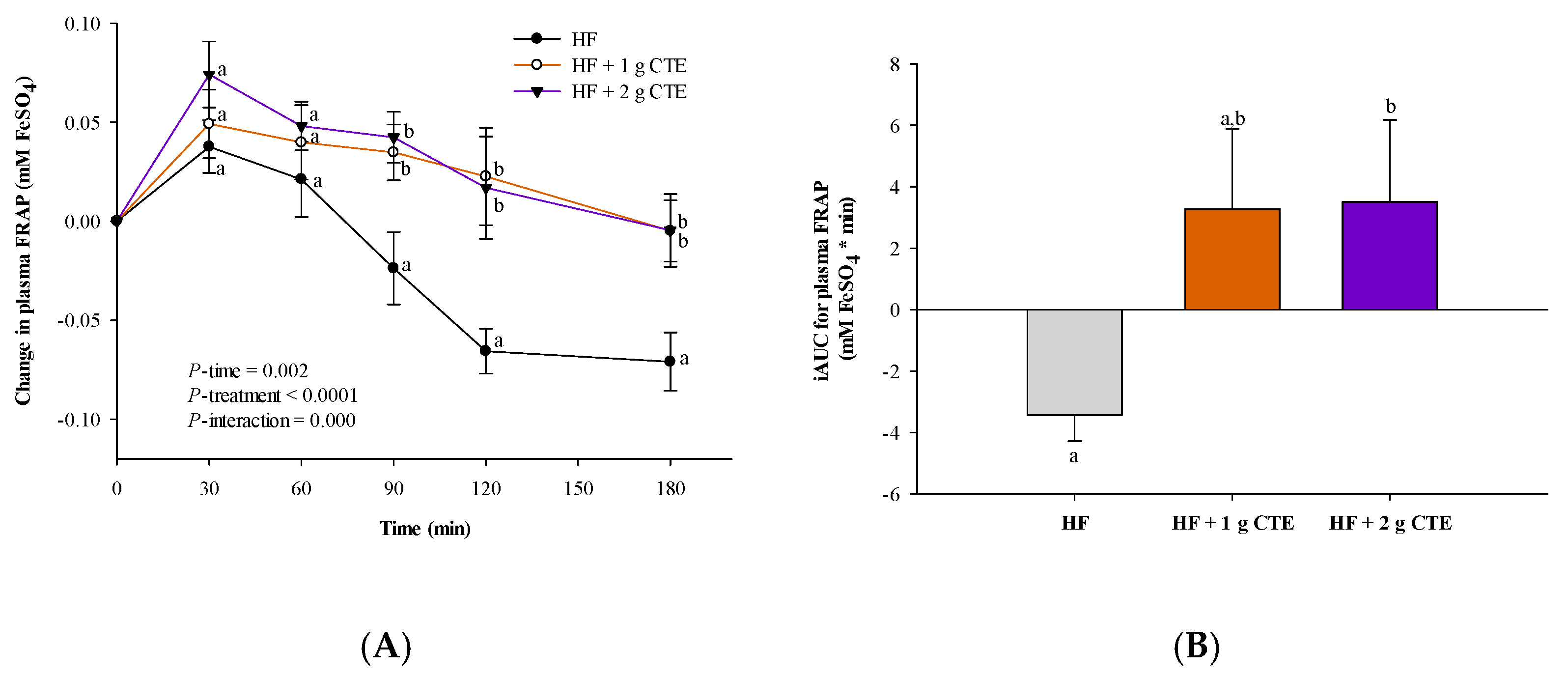
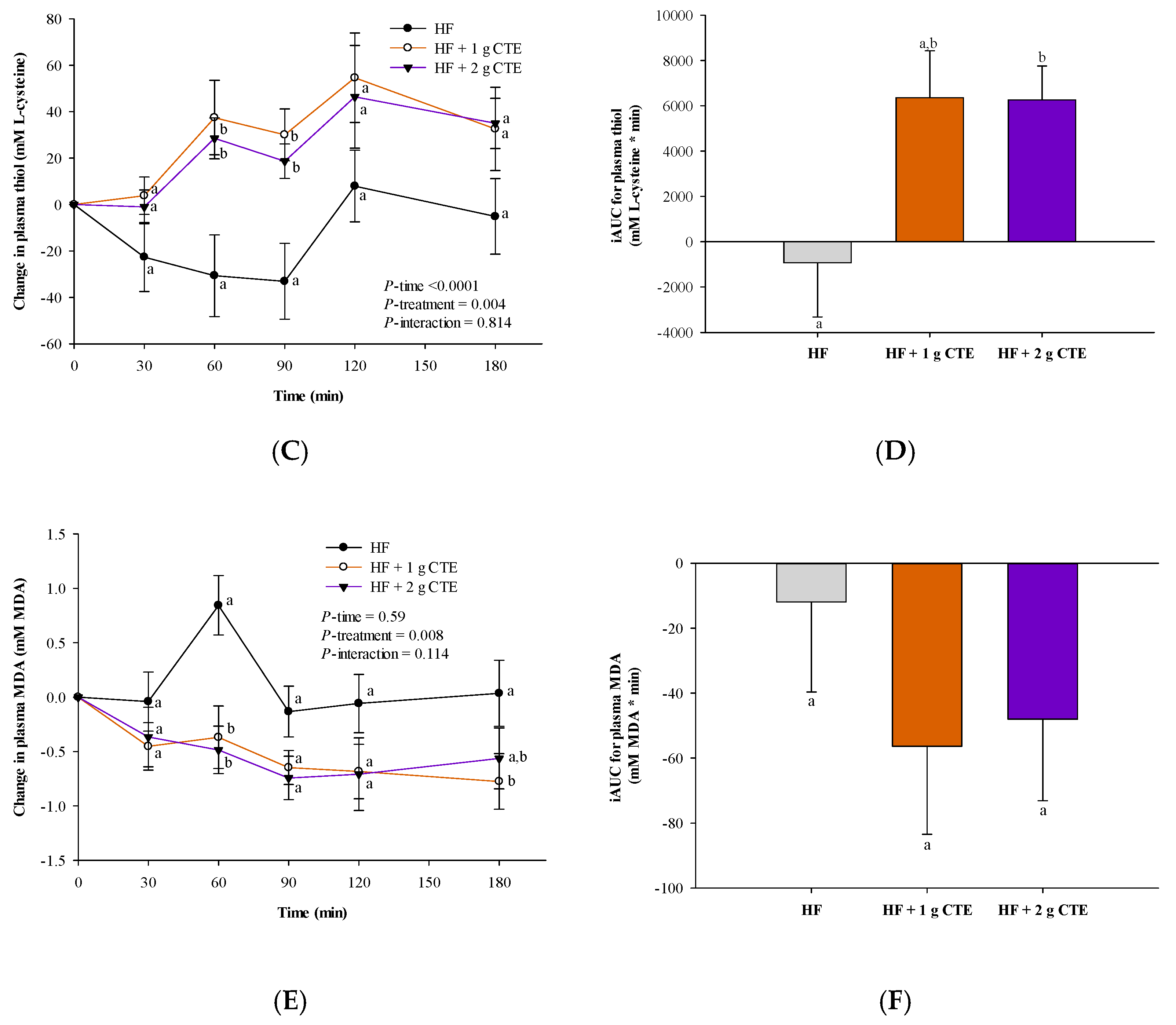
3.4. Postprandial Antioxidant Status
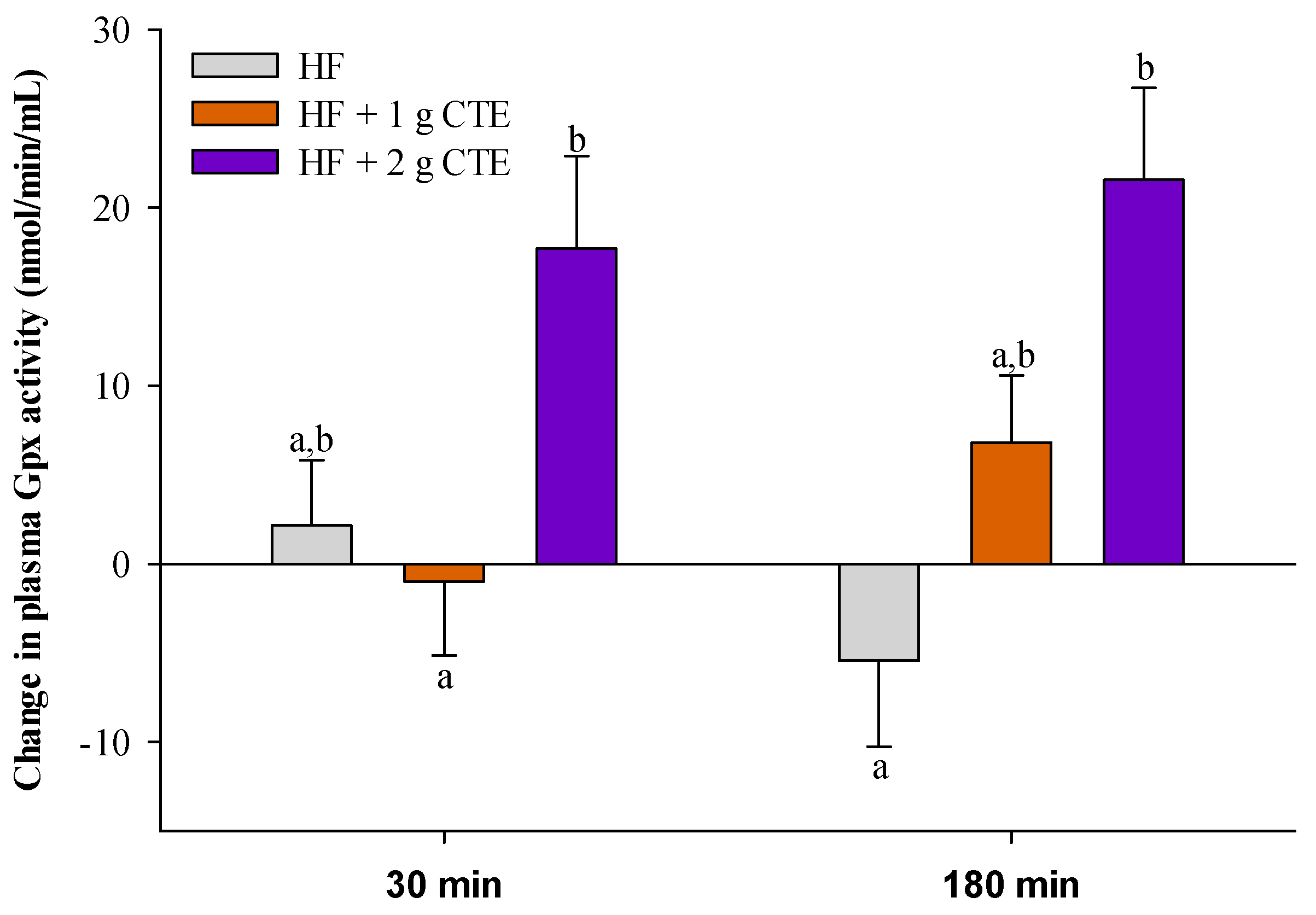
3.5. Postprandial Plasma Glutathione Peroxidase (Gpx) Activity
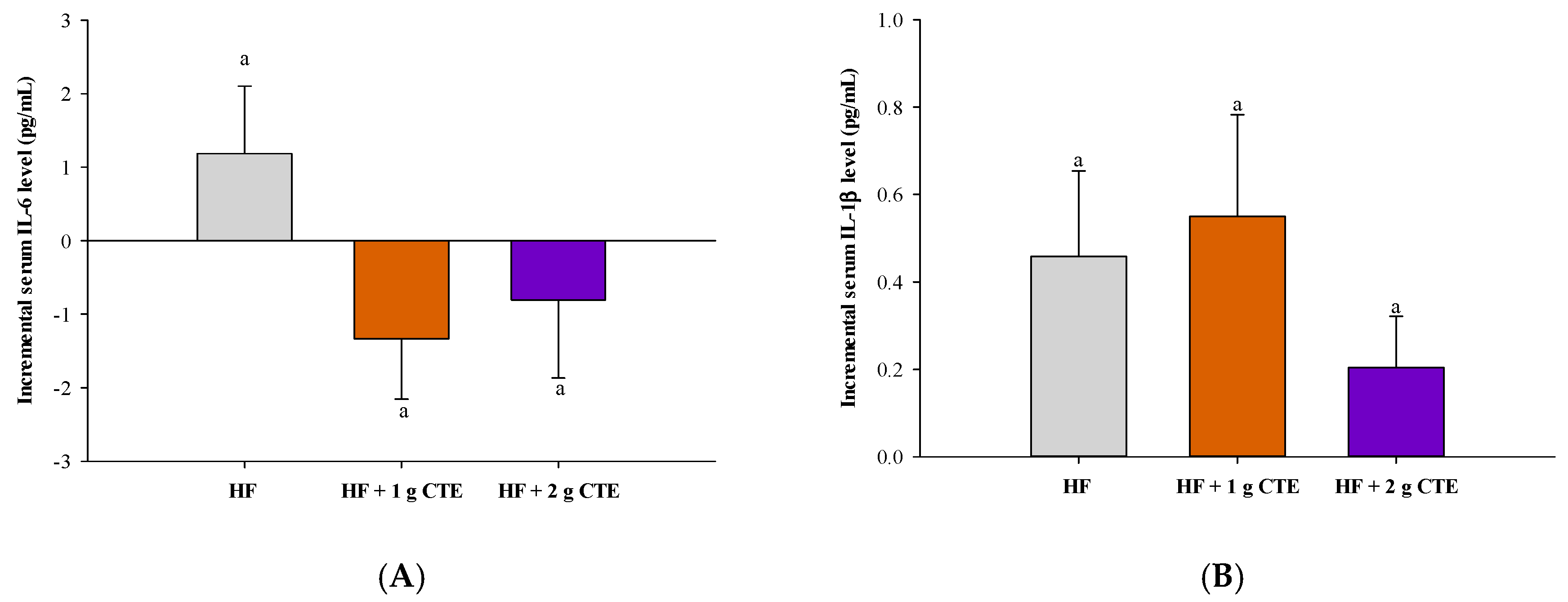
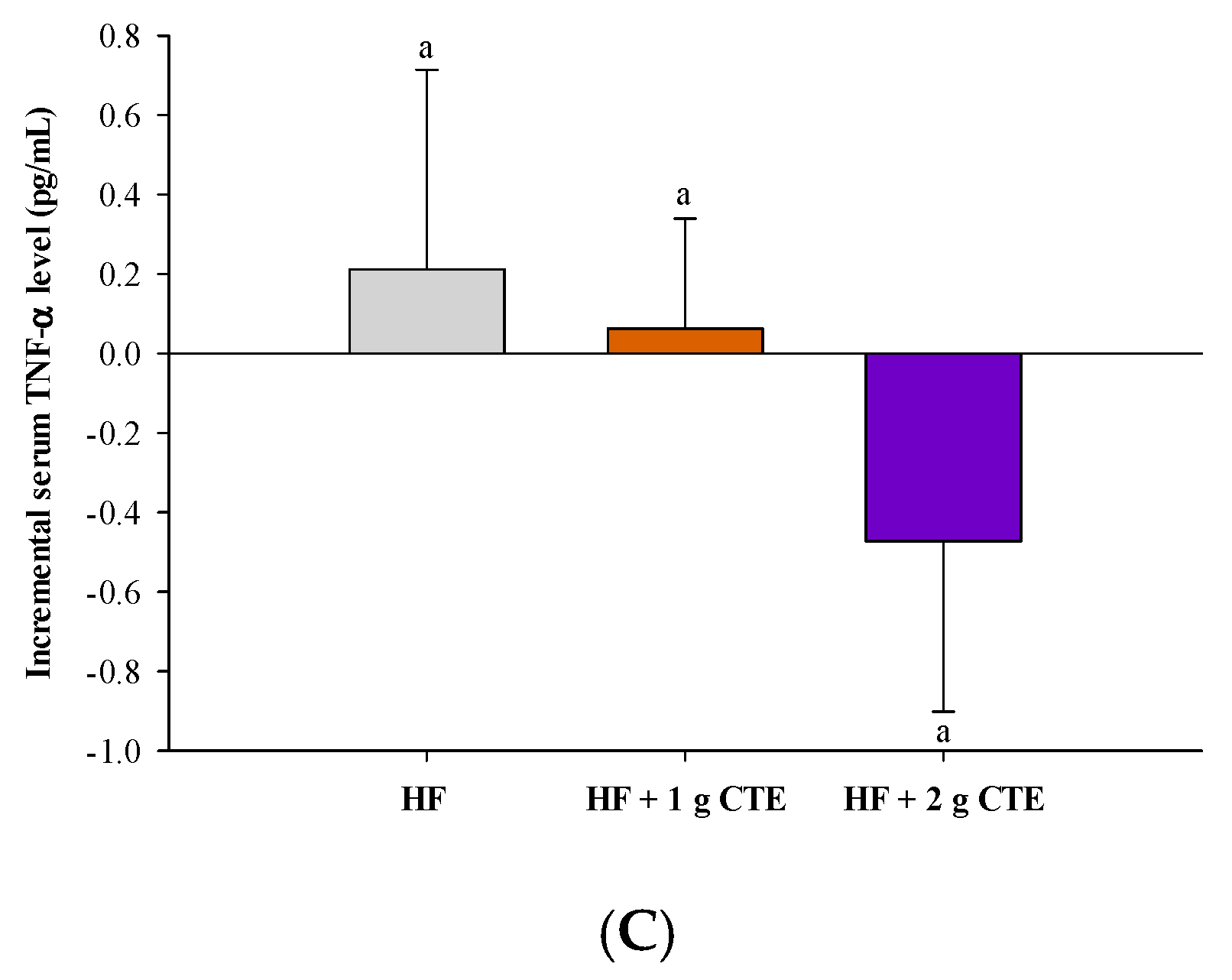
3.6. Postprandial Serum Pro-Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, Regional, and National Prevalence of Overweight and Obesity in Children and Adults during 1980–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Danaei, G.; Singh, G.M.; Paciorek, C.J.; Lin, J.K.; Cowan, M.J.; Finucane, M.M.; Farzadfar, F.; Stevens, G.A.; Riley, L.M.; Lu, Y.; et al. The Global Cardiovascular Risk Transition: Associations of Four Metabolic Risk Factors with National Income, Urbanization, and Western Diet in 1980 and 2008. Circulation 2013, 127, 1493–1502. [Google Scholar] [CrossRef]
- April-Sanders, A.K.; Rodriguez, C.J. Metabolically Healthy Obesity Redefined. JAMA Netw. Open 2021, 4, e218860. [Google Scholar] [CrossRef] [PubMed]
- Simons, P.J.; Pangaart, P.S.; Roomen, C.P.; Aerts, J.M.; Boon, L. Cytokine-Mediated Modulation of Leptin and Adiponectin Secretion during In Vitro Adipogenesis: Evidence that Tumor Necrosis Factor-α-and Interleukin-1β-Treated Human Preadipocytes Are Potent Leptin Producers. Cytokine 2005, 32, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Bondia-Pons, I.; Ryan, L.; Martinez, J.A. Oxidative Stress and Inflammation Interactions in Human Obesity. J. Physiol. Biochem. 2012, 68, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- De Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Hokanson, J.E.; Austin, M.A. Plasma Triglyceride Level Is a Risk Factor for Cardiovascular Disease Independent of High-Density Lipoprotein Cholesterol Level: A Metaanalysis of Population-Based Prospective Studies. J. Cardiovasc. Risk 1996, 3, 213–219. [Google Scholar] [CrossRef]
- Margioris, A.N. Fatty Acids and Postprandial Inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 129–137. [Google Scholar] [CrossRef]
- Peairs, A.D.; Rankin, J.W.; Lee, Y.W. Effects of Acute Ingestion of Different Fats on Oxidative Stress and Inflammation in Overweight and Obese Adults. Nutr. J. 2011, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceriello, A.; Taboga, C.; Tonutti, L.; Quagliaro, L.; Piconi, L.; Bais, B.; Ros, D.R.; Motz, E. Evidence for an Independent and Cumulative Effect of Postprandial Hypertriglyceridemia and Hyperglycemia on Endothelial Dysfunction and Oxidative Stress Generation: Effects of Short-And Long-Term Simvastatin Treatment. Circulation 2002, 106, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible Mutagens Derived from Lipids and Lipid Precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Randolph, J.; Cheema, M.; Tadapaneni, R.; Park, E.; Burton-Freeman, B.; Kappagoda, T. Effect of Grape Seed Extract on Postprandial Oxidative Status and Metabolic Responses in Men and Women with the Metabolic Syndrome-Randomized, Cross-Over, Placebo-Controlled Study. Funct. Food Health Dis. 2013, 2, 508–521. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Nguyen, T.H.; Kendall, C.W.; Faulkner, D.A.; Bashyam, B.; Kim, I.J.; Ireland, C.; Patel, D.; Vidgen, E.; Josse, A.R.; et al. The Effect of Strawberries in a Cholesterol-Lowering Dietary Portfolio. Metabolism 2008, 57, 1636–1644. [Google Scholar] [CrossRef]
- Polley, K.R.; Oswell, N.J.; Pegg, R.B.; Cooper, J.A. Tart Cherry Consumption with or without Prior Exercise Increases Antioxidant Capacity and Decreases Triglyceride Levels Following a High-Fat Meal. Appl. Physiol. Nutr. Metab. 2019, 44, 1209–1218. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Banaszewski, K.; Cappozzo, J.; Sandhya, K.; Ellis, C.L.; Tadapaneni, R.; Kappagoda, C.T.; Burton-Freeman, B.M. Strawberry Anthocyanin and Its Association with Postprandial Inflammation and Insulin. Br. J. Nutr. 2011, 106, 913–922. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic Medicine Clitoria ternatea-From Traditional Use to Scientific Assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef]
- Terahara, N.; Oda, M.; Matsui, T.; Osajima, Y.; Saito, N.; Toki, K.; Honda, T. Five New Anthocyanins, Ternatins A3, B4, B3, B2, and D2, from Clitoria ternatea Flowers. J. Nat. Prod. 1996, 59, 139–144. [Google Scholar] [CrossRef]
- Chayaratanasin, P.; Adisakwattana, S.; Thilavech, T. Protective Role of Clitoria ternatea L. Flower Extract on Methylglyoxal-Induced Protein Glycation and Oxidative Damage to DNA. BMC Complement. Med. Ther. 2021, 21, 1–11. [Google Scholar] [CrossRef]
- Chayaratanasin, P.; Barbieri, M.A.; Suanpairintr, N.; Adisakwattana, S. Inhibitory Effect of Clitoria ternatea Flower Petal Extract on Fructose-Induced Protein Glycation and Oxidation-Dependent Damages to Albumin In Vitro. BMC Complement. Altern. Med. 2015, 15, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chayaratanasin, P.; Caobi, A.; Suparpprom, C.; Saenset, S.; Pasukamonset, P.; Suanpairintr, N.; Barbieri, M.A.; Adisakwattana, S. Clitoria ternatea Flower Petal Extract Inhibits Adipogenesis and Lipid Accumulation in 3T3-L1 Preadipocytes by Downregulating Adipogenic Gene Expression. Molecules 2019, 24, 1894. [Google Scholar] [CrossRef] [Green Version]
- Adisakwattana, S.; Ruengsamran, T.; Kampa, P.; Sompong, W. In Vitro Inhibitory Effects of Plant-Based Foods and Their Combinations on Intestinal Alpha-Glucosidase and Pancreatic Alpha-Amylase. BMC Complement. Altern. Med. 2012, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Escher, G.B.; Marques, M.B.; do Carmo, M.A.V.; Azevedo, L.; Furtado, M.M.; Sant’Ana, A.S.; Silva, M.C.; Genovese, M.I.; Wen, M.; Zhang, L.; et al. Clitoria ternatea L. Petal Bioactive Compounds Display Antioxidant, Antihemolytic and Antihypertensive Effects, Inhibit α-Amylase and α-Glucosidase Activities and Reduce Human LDL Cholesterol and DNA Induced Oxidation. Food Res. Int. 2020, 128, 108763. [Google Scholar] [CrossRef]
- Pasukamonset, P.; Pumalee, T.; Sanguansuk, N.; Chumyen, C.; Wongvasu, P.; Adisakwattana, S.; Ngamukote, S. Physicochemical, Antioxidant and Sensory Characteristics of Sponge Cakes Fortified with Clitoria ternatea Extract. J. Food Sci. Technol. 2018, 55, 2881–2889. [Google Scholar] [CrossRef]
- Chusak, C.; Henry, C.J.; Chantarasinlapin, P.; Techasukthavorn, V.; Adisakwattana, S. Influence of Clitoria ternatea Flower Extract on the In Vitro Enzymatic Digestibility of Starch and Its Application in Bread. Foods 2018, 7, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chusak, C.; Thilavech, T.; Henry, C.J.; Adisakwattana, S. Acute Effect of Clitoria ternatea Flower Beverage on Glycemic Response and Antioxidant Capacity in Healthy Subjects: A Randomized Crossover Trial. BMC Complement. Altern. Med. 2018, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- WHO Expert Consultation. Appropriate Body-Mass Index for Asian Populations and Its Implications for Policy and Intervention Strategies. Lancet 2004, 363, 157–163. [Google Scholar] [CrossRef]
- Mazza, G.; Kay, C.D.; Cottrell, T.; Holub, B.J. Absorption of Anthocyanins from Blueberries and Serum Antioxidant Status in Human Subjects. J. Agric. Food Chem. 2002, 50, 7731–7737. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapenna, D.; Ciofani, G.; Pierdomenico, S.D.; Giamberardino, M.A.; Cuccurullo, F. Reaction Conditions Affecting the Relationship between Thiobarbituric Acid Reactivity and Lipid Peroxidesin Human Plasma. Free Radic. Biol. Med. 2001, 31, 331–335. [Google Scholar] [CrossRef]
- Emerson, S.R.; Kurti, S.P.; Harms, C.A.; Haub, M.D.; Melgarejo, T.; Logan, C.; Rosenkranz, S.K. Magnitude and Timing of the Postprandial Inflammatory Response to a High-Fat Meal in Healthy Adults: A Systematic Review. Adv. Nutr. 2017, 8, 213–225. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Tunez, I.; Tasset, I.; Murri, M.; Tinahones, F. Similar Increase in Oxidative Stress after Fat Overload in Persons with Baseline Hypertriglyceridemia with or without the Metabolic Syndrome. Clin. Biochem. 2008, 41, 701–705. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting Triglycerides and Risk of Myocardial Infarction, Ischemic Heart Disease, and Death in Men and Women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemente-Postigo, M.; Queipo-Ortuno, M.I.; Murri, M.; Boto-Ordonez, M.; Perez-Martinez, P.; Andres-Lacueva, C.; Cardona, F.; Tinahones, F.J. Endotoxin Increase after Fat Overload Is Related to Postprandial Hypertriglyceridemia in Morbidly Obese Patients. J. Lipid Res. 2012, 53, 973–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry Modulates LDL Oxidation and Postprandial Lipemia in Response to Highfat Meal in Overweight Hyperlipidemic Men and Women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Romeo, F.V.; Rapisarda, P. Screening of the Anthocyanin Profile and In Vitro Pancreatic Lipase Inhibition by Anthocyanin-Containing Extracts of Fruits, Vegetables, Legumes and Cereals. J. Sci. Food Agric. 2016, 96, 4713–4723. [Google Scholar] [CrossRef] [PubMed]
- Garza, A.L.; Milagro, F.I.; Boque, N.; Campión, J.; Martínez, J.A. Natural Inhibitors of Pancreatic Lipase as New Players in Obesity Treatment. Planta Med. 2011, 77, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.M.; Yoon, Y.; Yoon, H.; Park, H.M.; Song, S.; Yeum, K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Hara, C.; Ojo, B.; Emerson, S.R.; Simenson, A.J.; Peterson, S.; Perkins-Veazie, P.; Payton, M.E.; Hermann, J.; Smith, B.J.; Lucas, E.A. Acute Freeze-Dried Mango Consumption with a High-Fat Meal Has Minimal Effects on Postprandial Metabolism, Inflammation and Antioxidant Enzymes. Nutr. Metab. Insights 2019, 12, 1178638819869946. [Google Scholar] [CrossRef]
- Giera, M.; Lingeman, H.; Niessen, W.M. Recent Advancements in the LC-And GC-Based Analysis of Malondialdehyde (MDA): A Brief Overview. Chromatographia 2012, 75, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Frei, B.; Stocker, R.; Ames, B.N. Antioxidant Defenses and Lipid Peroxidation in Human Blood Plasma. Proc. Natl. Acad. Sci. USA 1988, 85, 9748–9752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matés, J.M.; Pérez-Gómez, C.; Castro, I.N. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Alturfan, A.; Emekli-Alturfan, E.; Uslu, E. Consumption of Pistachio Nuts Beneficially Affected Blood Lipids and Total Antioxidant Activity in Rats Fed a High-Cholesterol Diet. Folia Biol. 2009, 55, 132–136. [Google Scholar]
- De Souza, R.G.M.; Gomes, A.C.; Navarro, A.M.; Cunha, L.C.; Silva, M.A.C.; Junior, F.B.; Mota, J.F. Baru Almonds Increase the Activity of Glutathione Peroxidase in Overweight and Obese Women: A Randomized, Placebo-Controlled Trial. Nutrients 2019, 11, 1750. [Google Scholar] [CrossRef] [Green Version]
- Amri, Z.; Ghorbel, A.; Turki, M.; Akrout, F.M.; Ayadi, F.; Elfeki, A.; Hammami, M. Effect of Pomegranate Extracts on Brain Antioxidant Markers and Cholinesterase Activity in High Fat-High Fructose Diet Induced Obesity in Rat Model. BMC Complement. Altern. Med. 2017, 17, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hassimotto, N.M.; Lajolo, F.M. Antioxidant Status in Rats after Long-Term Intake of Anthocyanins and Ellagitannins from Blackberries. J. Sci. Food Agric. 2011, 91, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Z.H.; Johnson, A.P.; Rando, T.A. NF-κB and AP-1 Mediate Transcriptional Responses to Oxidative Stress in Skeletal Muscle Cells. Free Radic. Biol. Med. 2001, 31, 1405–1416. [Google Scholar] [CrossRef]
- Couillard, C.; Bergeron, N.; Prud’homme, D.; Bergeron, J.; Tremblay, A.; Bouchard, C.; Mauriège, P.; Després, J.P. Postprandial Triglyceride Response in Visceral Obesity in Men. Diabetes 1998, 47, 953–960. [Google Scholar] [CrossRef]
- De Vries, M.A.; Klop, B.; Janssen, H.W.; Njo, T.L.; Westerman, E.M.; Cabezas, M.C. Postprandial Inflammation: Targeting Glucose and Lipids. Adv. Exp. Med. Biol. 2014, 824, 161–170. [Google Scholar]
- Esposito, K.; Ciotola, M.; Sasso, F.C.; Cozzolino, D.; Saccomanno, F.; Assaloni, R. Effect of a Single High-Fat Meal on Endothelial Function in Patients with the Metabolic Syndrome: Role of Tumor Necrosis Factor-α. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Herieka, M.; Erridge, C. High-Fat Meal Induced Postprandial Inflammation. Mol. Nutr. Food Res. 2014, 58, 136–146. [Google Scholar] [CrossRef]
- Peluso, I.; Villano, D.; Roberts, S.; Cesqui, E.; Raguzzini, A.; Borges, G.; Crozier, A.; Catasta, G.; Toti, E.; Serafini, M. Consumption of Mixed Fruit-Juice Drink and Vitamin C Reduces Postprandial Stress Induced by a High Fat Meal in Healthy Overweight Subjects. Curr. Pharm. Des. 2014, 20, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.W.; Tallent, R.; Navalta, J.W.; Salazar, A.; Lyons, T.J.; Basu, A. Effects of Acute Cocoa Supplementation on Postprandial Apolipoproteins, Lipoprotein Subclasses, and Inflammatory Biomarkers in Adults with Type 2 Diabetes after a High-Fat Meal. Nutrients 2020, 12, 1902. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SEM |
|---|---|
| Age (years) | 23.5 ± 0.6 |
| Weight (kg) | 75.7 ± 1.9 |
| Height (cm) | 171.2 ± 1.7 |
| Body mass index (BMI; kg/m2) | 25.7 ± 0.7 |
| Fasting plasma glucose (mg/dL) | 88.7 ± 1.7 |
| Total cholesterol (mg/dL) | 190.1 ± 8.7 |
| Serum triglyceride (mg/dL) | 97.9 ± 12.6 |
| Creatinine (mg/dL) | 1.06 ± 0.04 |
| Blood urea nitrogen (mg/dL) | 14.7 ± 0.8 |
| Aspartate transaminase (U/L) | 23.9 ± 2.0 |
| Alanine transaminase (U/L) | 33.7 ± 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thilavech, T.; Adisakwattana, S.; Channuwong, P.; Radarit, K.; Jantarapat, K.; Ngewlai, K.; Sonprasan, N.; Chusak, C. Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants. Biology 2021, 10, 975. https://doi.org/10.3390/biology10100975
Thilavech T, Adisakwattana S, Channuwong P, Radarit K, Jantarapat K, Ngewlai K, Sonprasan N, Chusak C. Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants. Biology. 2021; 10(10):975. https://doi.org/10.3390/biology10100975
Chicago/Turabian StyleThilavech, Thavaree, Sirichai Adisakwattana, Pilailak Channuwong, Korntima Radarit, Kanthida Jantarapat, Kittisak Ngewlai, Nantarat Sonprasan, and Charoonsri Chusak. 2021. "Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants" Biology 10, no. 10: 975. https://doi.org/10.3390/biology10100975
APA StyleThilavech, T., Adisakwattana, S., Channuwong, P., Radarit, K., Jantarapat, K., Ngewlai, K., Sonprasan, N., & Chusak, C. (2021). Clitoria ternatea Flower Extract Attenuates Postprandial Lipemia and Increases Plasma Antioxidant Status Responses to a High-Fat Meal Challenge in Overweight and Obese Participants. Biology, 10(10), 975. https://doi.org/10.3390/biology10100975







