Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Chemicals
2.3. Experiments (Exposure to Anaesthetics)
2.3.1. Reflexes and Stages of Anaesthesia
2.3.2. Experiment 1—Compound Screening
2.3.3. Experiment 2—Mass Testing
2.3.4. Experiment 3—Monitoring Discomfort and Aversive Behavior
2.3.5. Experiment 4—Buffering of Lidocaine HCl
2.4. Data Presentation and Statistics
3. Results
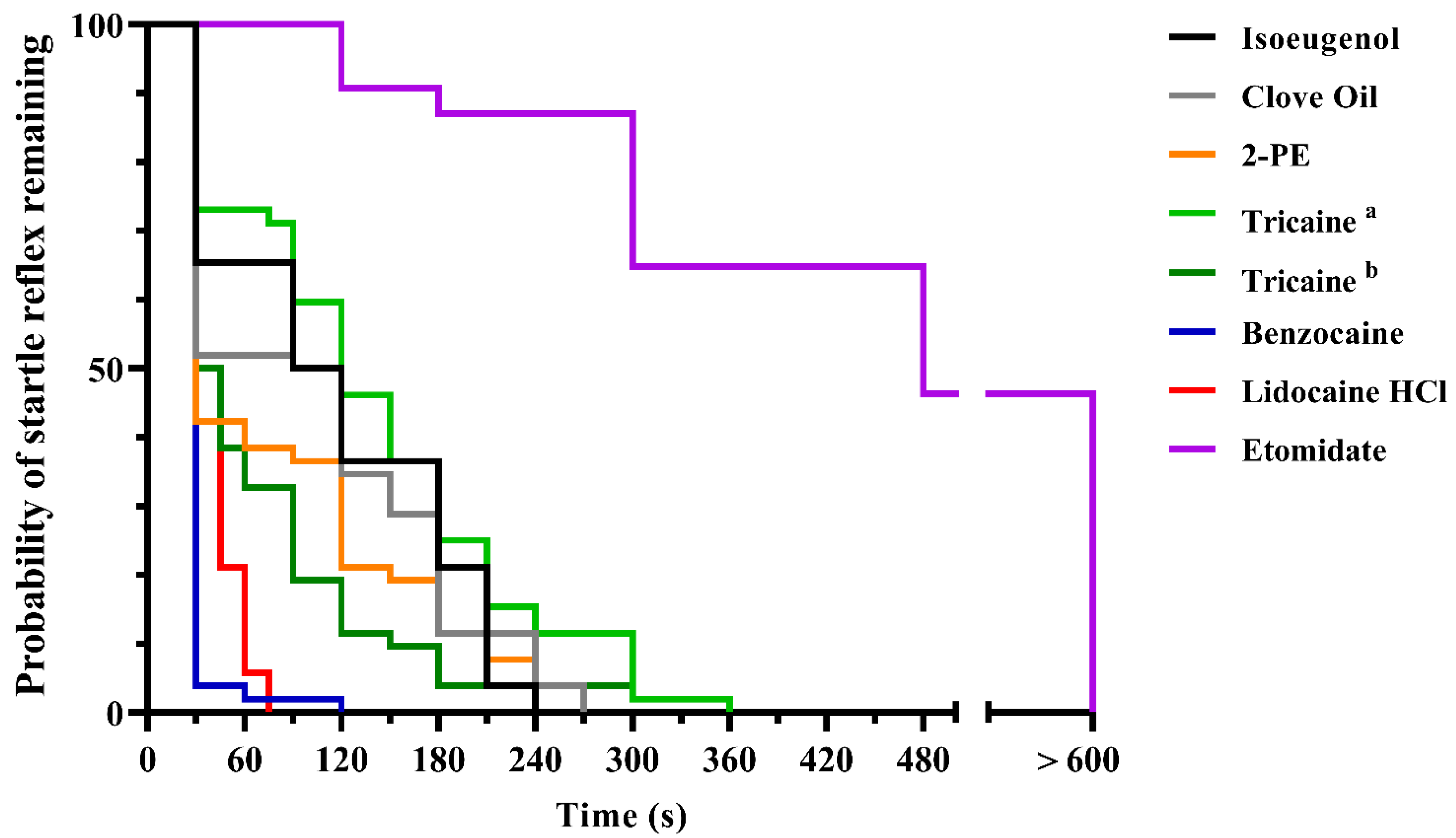
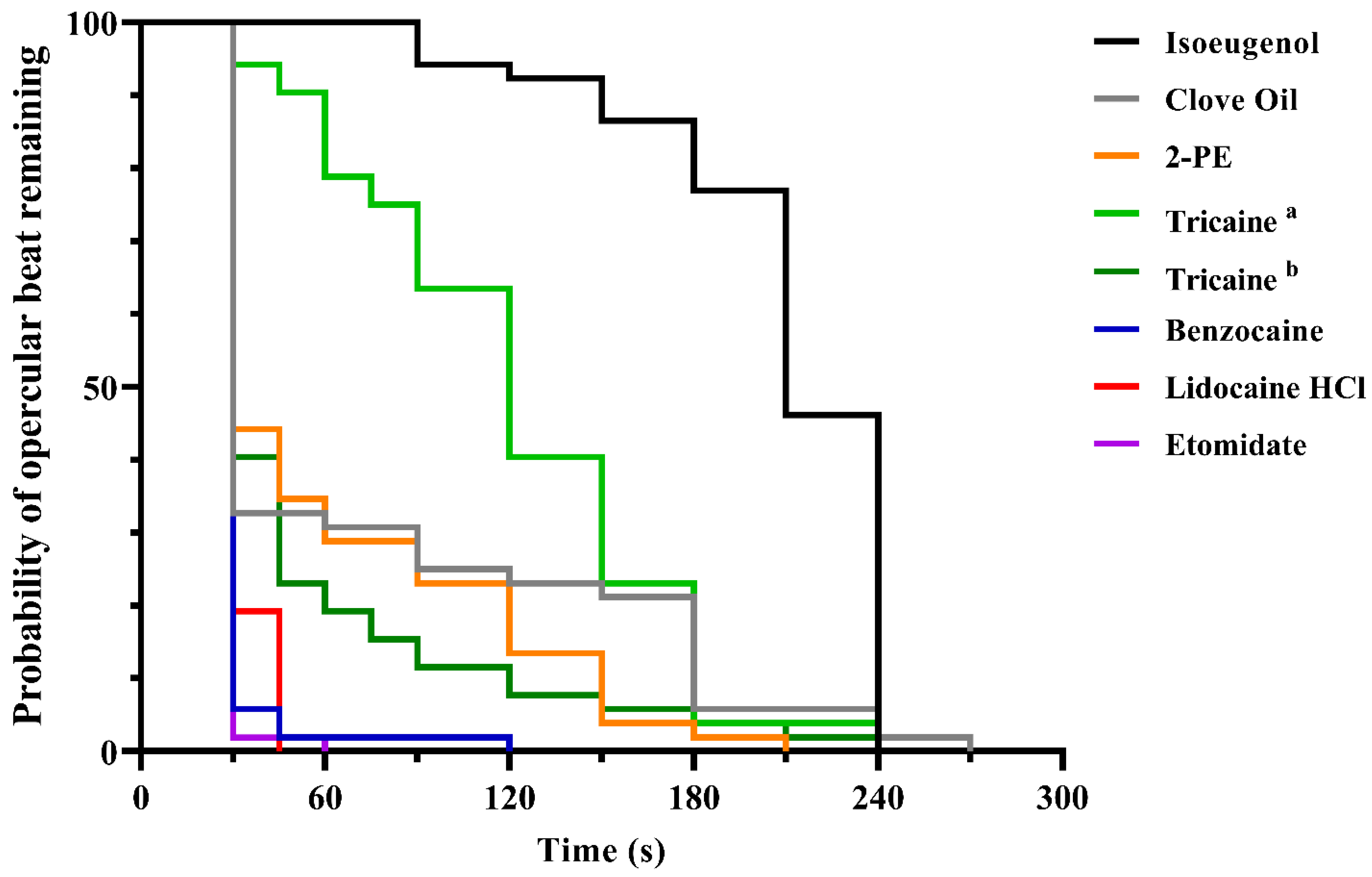
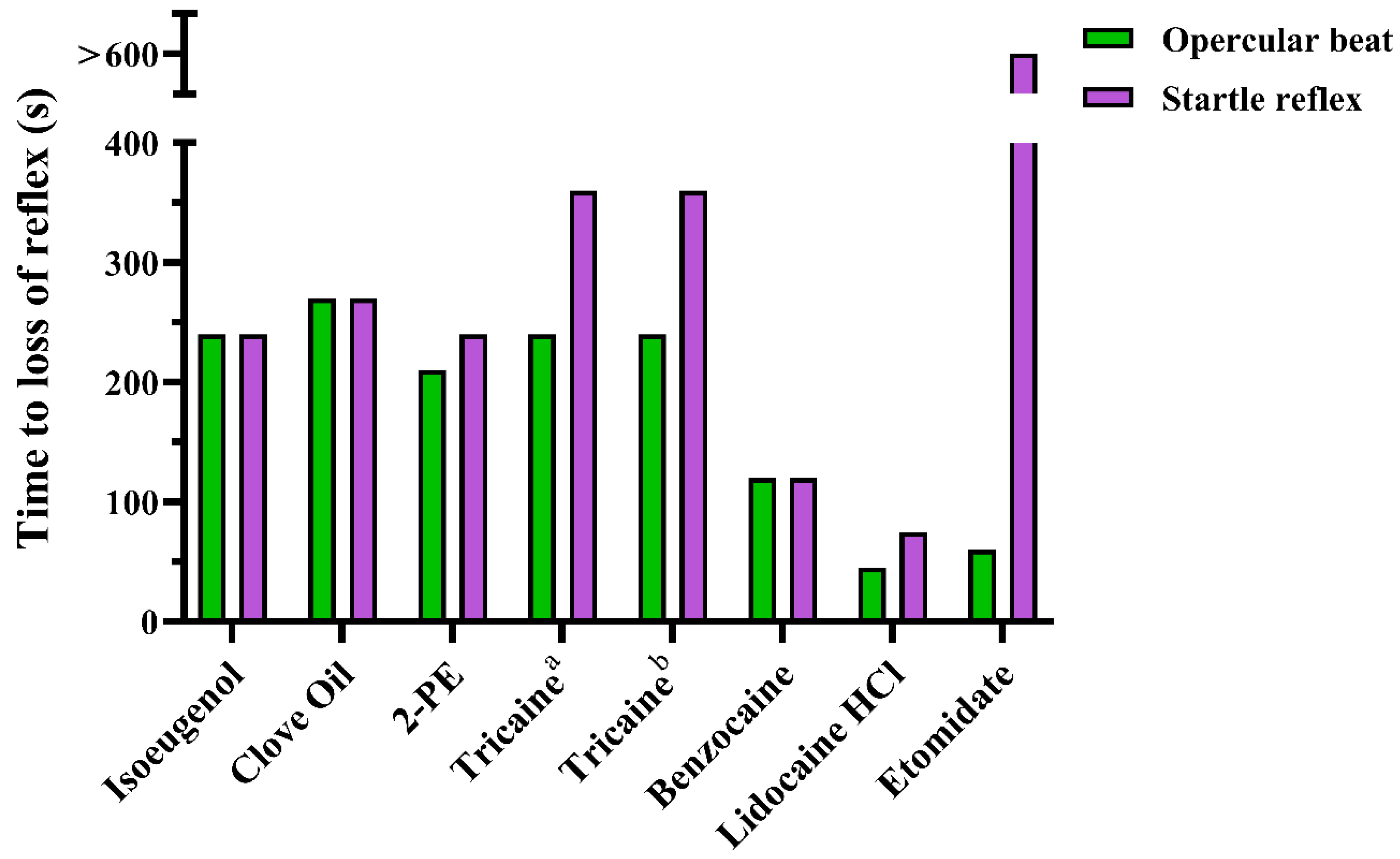
3.1. Experiment 1
3.2. Experiment 2
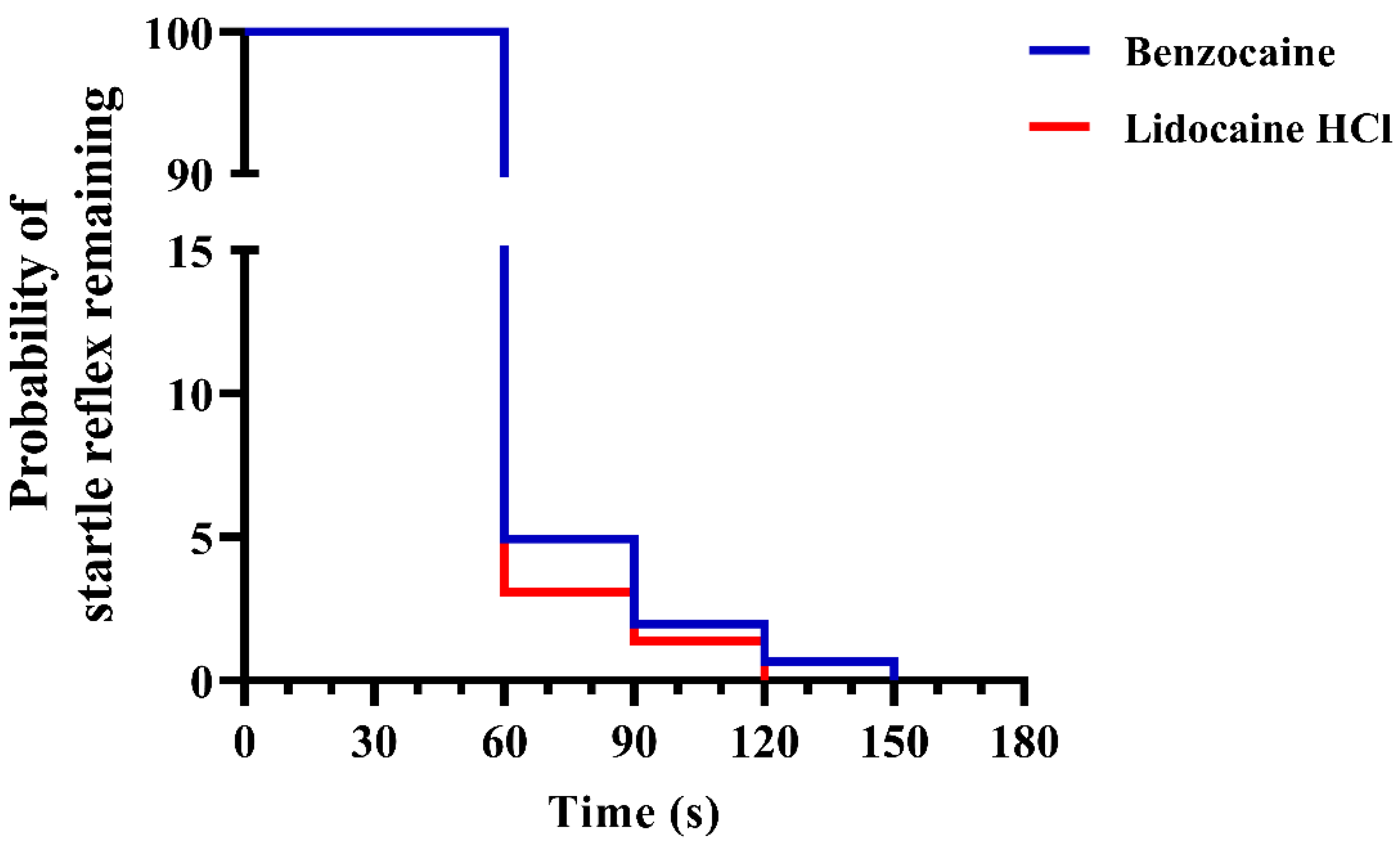
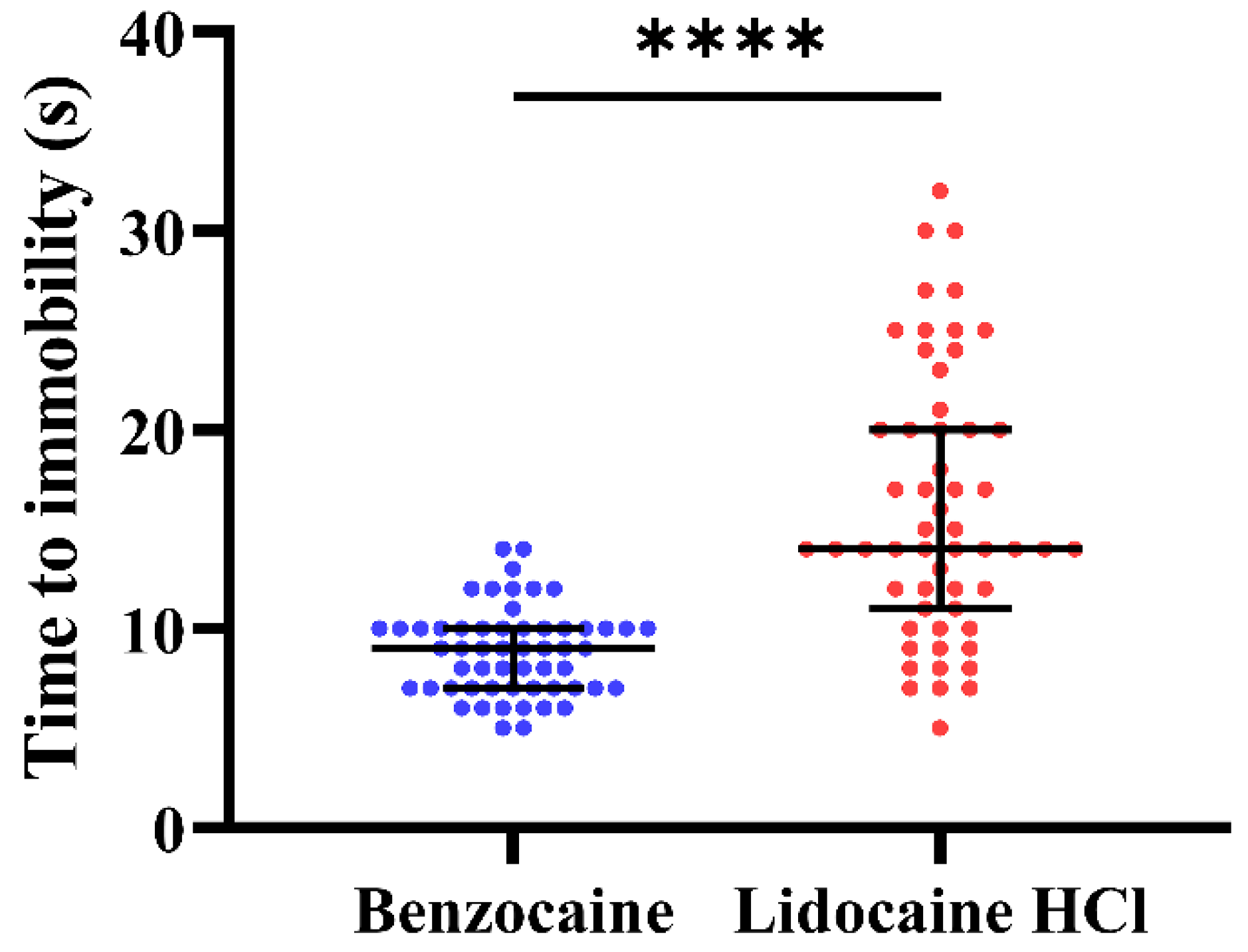
3.3. Experiment 3
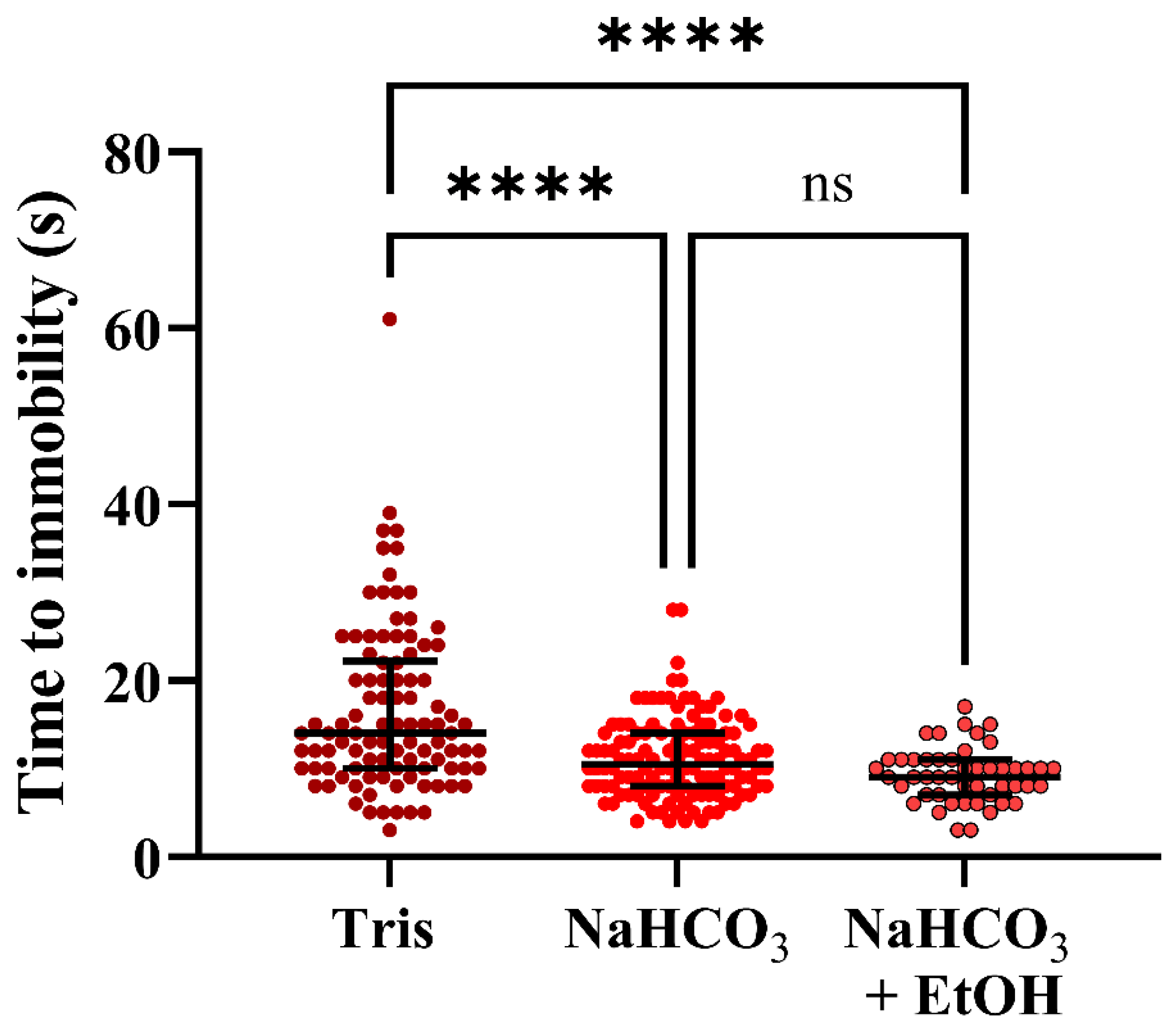
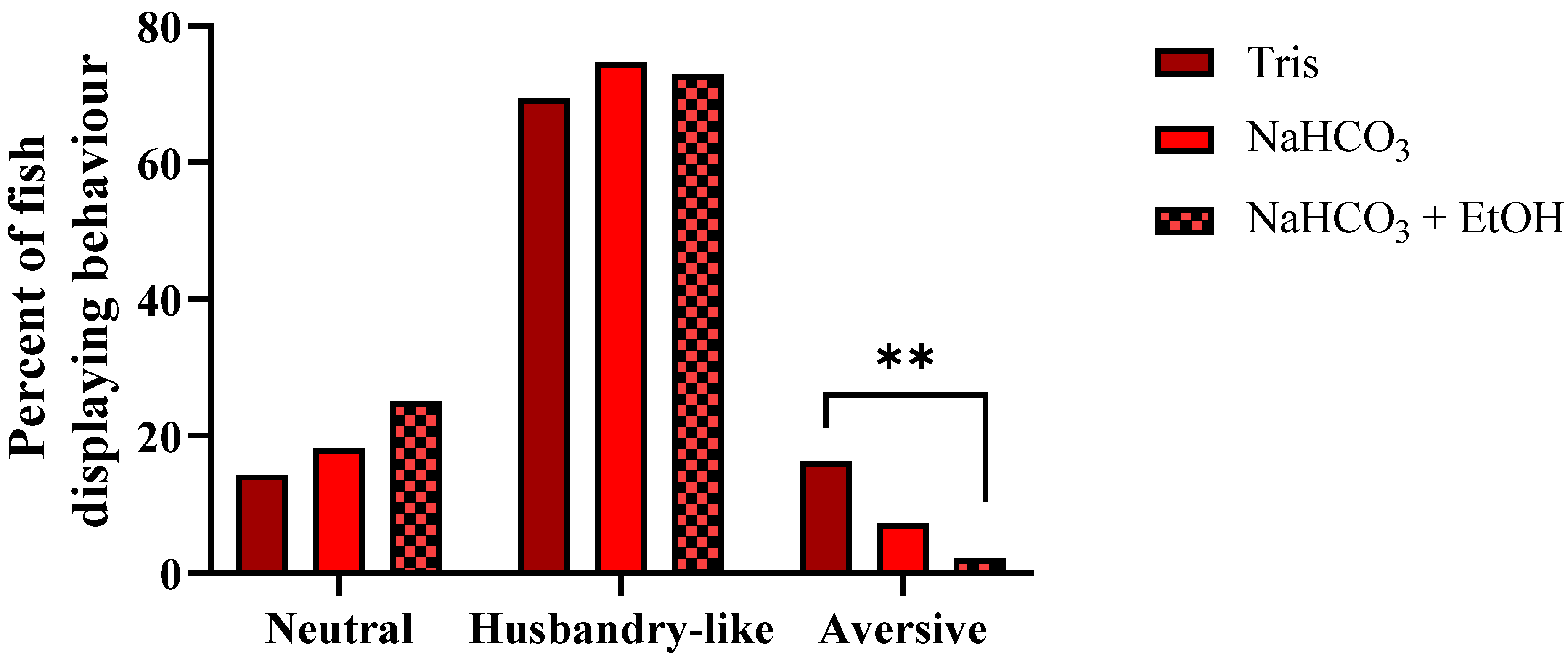
3.4. Experiment 4
4. Discussion
4.1. Experiments 1 and 2; Screening and Mass Testing
4.2. Experiments 3 and 4; Behavior and Buffers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Union. Directive 2010/63/EU of the European Parliament and of the Council; On the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Staib-Lasarzik, I.; Kriege, O.; Timaru-Kast, R.; Pieter, D.; Werner, C.; Engelhard, K.; Thal, S.C. Anesthesia for Euthanasia Influences mRNA Expression in Healthy Mice and after Traumatic Brain Injury. J. Neurotrauma 2014, 31, 1664–1671. [Google Scholar] [CrossRef] [Green Version]
- Gause, B.R.; Trushenski, J.T.; Bowzer, J.C.; Bowker, J.D. Efficacy and Physiological Responses of Grass Carp to Different Sedation Techniques: I. Effects of Various Chemicals on Sedation and Blood Chemistry. N. Am. J. Aquac. 2012, 74, 560–566. [Google Scholar] [CrossRef]
- Matsche, M.A. Efficacy and Physiological Response to Chemical Anesthesia in Wild Hickory Shad during Spawning Season. Mar. Coast. Fish. 2017, 9, 296–304. [Google Scholar] [CrossRef]
- Kinth, P.; Mahesh, G.; Panwar, Y. Mapping of Zebrafish Research: A Global Outlook. Zebrafish 2013, 10, 510–517. [Google Scholar] [CrossRef] [Green Version]
- European Commission. 2019 Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union in 2015–2017; Report from the commission to the European parliament and the council; Report no. COM(2020) 16 final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Lidster, K.; Readman, G.D.; Prescott, M.J.; Owen, S. International survey on the use and welfare of zebrafish Danio rerio in research. J. Fish Biol. 2017, 90, 1891–1905. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Bunte, R.M.; Carty, A.J. Evaluation of Rapid Cooling and Tricaine Methanesulfonate (MS222) as Methods of Euthanasia in Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 785–789. [Google Scholar]
- Collymore, C.; Banks, E.K.; Turner, P.V. Lidocaine Hydrochloride Compared with MS222 for the Euthanasia of Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2016, 55, 816–820. [Google Scholar] [PubMed]
- Sánchez-Vázquez, F.J.; Terry, M.I.; Felizardo, V.O.; Vera, L.M. Daily Rhythms of Toxicity and Effectiveness of Anesthetics (MS222 and Eugenol) in Zebrafish (Danio Rerio). Chronobiol. Int. 2011, 28, 109–117. [Google Scholar] [CrossRef]
- Wong, D.; Von Keyserlingk, M.A.G.; Richards, J.G.; Weary, D.M. Conditioned Place Avoidance of Zebrafish (Danio rerio) to Three Chemicals Used for Euthanasia and Anaesthesia. PLoS ONE 2014, 9, e88030. [Google Scholar] [CrossRef] [Green Version]
- Readman, G.D.; Owen, S.; Murrell, J.C.; Knowles, T. Do Fish Perceive Anaesthetics as Aversive? PLoS ONE 2013, 8, e73773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, P.; Lloyd, R.; McKimm, R.; Metselaar, M.; Navarro, J.; O’Farrell, M.; Readman, G.D.; Speilberg, L.; Mocho, J.-P. Anaesthesia of laboratory, aquaculture and ornamental fish: Proceedings of the first LASA-FVS Symposium. Lab. Anim. 2021, 55, 317–328. [Google Scholar] [CrossRef]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Readman, G.D.; Owen, S.; Knowles, T.; Murrell, J.C. Species specific anaesthetics for fish anaesthesia and euthanasia. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, I.-S.; Gil, H.W.; Lee, T.H.; Nam, Y.K.; Lim, S.G.; Kim, D.S. Effects of Clove Oil and Lidocaine-HCl Anesthesia on Water Parameter during Simulated Transportation in the Marine Medaka, Oryzias dancena. Dev. Reprod. 2017, 21, 19–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strykowski, J.L.; Schech, J.M. Effectiveness of Recommended Euthanasia Methods in Larval Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 76–79. [Google Scholar]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR J. 2012, 53, 192–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, C.K.; Bright, L.A.; Marx, J.O.; Andersen, R.P.; Mullins, M.C.; Carty, A.J. Effectiveness of Rapid Cooling as a Method of Euthanasia for Young Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2018, 57, 58–63. [Google Scholar]
- Mocho, J.-P.; Martin, D.J.; Millington, M.E.; Torres, Y.S. Environmental Screening of Aeromonas hydrophila, Mycobacterium spp., and Pseudocapillaria tomentosa in Zebrafish Systems. J. Vis. Exp. 2017, 130, e55306. [Google Scholar] [CrossRef] [Green Version]
- Collymore, C.; Tolwani, A.; Lieggi, C.; Rasmussen, S. Efficacy and Safety of 5 Anesthetics in Adult Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 198–203. [Google Scholar]
- Grush, J.; Noakes, D.L.G.; Moccia, R.D. The Efficacy of Clove Oil As An Anesthetic for the Zebrafish, Danio rerio (Hamilton). Zebrafish 2004, 1, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olt, J.; Allen, C.E.; Marcotti, W. In vivo physiological recording from the lateral line of juvenile zebrafish. J. Physiol. 2016, 594, 5427–5438. [Google Scholar] [CrossRef] [Green Version]
- McFarland, W.N.; Klontz, G.W. Anesthesia in fishes. Fed. Proc. 1969, 28, 1535–1540. [Google Scholar] [PubMed]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.-M.; Bromage, N.; Bunyan, J.; Erhardt, W.; Flecknell, P.; Gregory, N.; Hackbarth, H.; et al. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Machnik, P.; Schirmer, E.; Glück, L.; Schuster, S. Recordings in an integrating central neuron provide a quick way for identifying appropriate anaesthetic use in fish. Sci. Rep. 2018, 8, 17541. [Google Scholar] [CrossRef]
- Carrasco, S.; Sumano, H.; Navarro-Fierro, R. The use of lidocaine-sodium bicarbonate as anaesthetic in fish. Aquaculture 1984, 41, 395–398. [Google Scholar] [CrossRef]
- Sneddon, L.U. Evolution of nociception and pain: Evidence from fish models. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190290. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Rich, J.T.; Neely, J.G.; Paniello, R.C.; Voelker, C.C.J.; Nussenbaum, B.; Wang, E. A practical guide to understanding Kaplan-Meier curves. Otolaryngol. Neck Surg. 2010, 143, 331–336. [Google Scholar] [CrossRef] [Green Version]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Peto, R.; Peto, J. Asymptotically Efficient Rank Invariant Test Procedures. J. R. Stat. Soc. Ser. A 1972, 135, 185. [Google Scholar] [CrossRef]
- Harrington, D. Linear Rank Tests in Survival Analysis. In Encyclopedia of Biostatistics; Armitage, P., Colton, T., Eds.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- McHugh, M.L. The Chi-square test of independence. Biochem. Med. 2013, 23, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Agresti, A. A Survey of Exact Inference for Contingency Tables: Rejoinder. Stat. Sci. 1992, 7, 131. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons among Means. J. Am. Stat. Assoc. 1961, 56, 52–64. [Google Scholar] [CrossRef]
- Jorge, S.; Ferreira, J.M.; Olsson, I.A.S.; Valentim, A.M. Adult Zebrafish Anesthesia: A Study of Efficacy and Behavioral Recovery of Different Anesthetics. Zebrafish 2021, 18, 330–337. [Google Scholar] [CrossRef]
- Valentim, A.; Félix, L.; Carvalho, L.; Diniz, E.; Antunes, L. A New Anaesthetic Protocol for Adult Zebrafish (Danio rerio): Propofol Combined with Lidocaine. PLoS ONE 2016, 11, e0147747. [Google Scholar] [CrossRef]
- Nordgreen, J.; Tahamtani, F.M.; Janczak, A.M.; Horsberg, T.E. Behavioural Effects of the Commonly Used Fish Anaesthetic Tricaine Methanesulfonate (MS-222) on Zebrafish (Danio rerio) and Its Relevance for the Acetic Acid Pain Test. PLoS ONE 2014, 9, e92116. [Google Scholar] [CrossRef]
- Ehrlich, O.; Karamalakis, A.; Krylov, A.J.; Dudczig, S.; Hassell, K.L.; Jusuf, P.R. Clove Oil and AQUI-S Efficacy for Zebrafish Embryo, Larva, and Adult Anesthesia. Zebrafish 2019, 16, 451–459. [Google Scholar] [CrossRef]
- Davis, D.J.; Klug, J.; Hankins, M.; Doerr, H.M.; Monticelli, S.R.; Song, A.; Gillespie, C.H.; Bryda, E.C. Effects of Clove Oil as a Euthanasia Agent on Blood Collection Efficiency and Serum Cortisol Levels in Danio rerio. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 564–567. [Google Scholar]
- Musk, G.C.; Ezzy, B.J.; Kenchington, L.M.; Hopper, W.A.; Callahan, L.M. A Comparison of Buffered Tricaine Methanesulfonate (MS-222) and Isoeugenol Anesthesia for Caudal Fin Clipping in Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, P.G.; Sneddon, L.U. Exploring the efficacy of immersion analgesics in zebrafish using an integrative approach. Appl. Anim. Behav. Sci. 2017, 187, 93–102. [Google Scholar] [CrossRef]
- Huang, W.-C.; Hsieh, Y.-S.; Chen, I.-H.; Wang, C.-H.; Chang, H.-W.; Yang, C.-C.; Ku, T.-H.; Yeh, S.-R.; Chuang, Y.-J. Combined Use of MS-222 (Tricaine) and Isoflurane Extends Anesthesia Time and Minimizes Cardiac Rhythm Side Effects in Adult Zebrafish. Zebrafish 2010, 7, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Chambel, J.; Pinho, R.; Sousa, R.; Ferreira, T.; Baptista, T.; Severiano, V.; Mendes, S.; Pedrosa, R. The efficacy of MS-222 as anaesthetic agent in four freshwater aquarium fish species. Aquac. Res. 2013, 46, 1582–1589. [Google Scholar] [CrossRef]
- Chen, K.; Wang, C.-Q.; Fan, Y.-Q.; Xie, Y.-S.; Yin, Z.-F.; Xu, Z.-J.; Zhang, H.-L.; Cao, J.-T.; Han, Z.-H.; Wang, Y.; et al. The Evaluation of Rapid Cooling as an Anesthetic Method for the Zebrafish. Zebrafish 2014, 11, 71–75. [Google Scholar] [CrossRef]
- Amend, D.F.; Goven, B.A.; Elliot, D.G. Etomidate: Effective Dosages for a New Fish Anesthetic. Trans. Am. Fish. Soc. 1982, 111, 337–341. [Google Scholar] [CrossRef]
- Martins, T.; Diniz, E.; Félix, L.M.; Antunes, L. Evaluation of anaesthetic protocols for laboratory adult zebrafish (Danio rerio). PLoS ONE 2018, 13, e0197846. [Google Scholar] [CrossRef] [Green Version]
- Martins, T.; Valentim, A.; Pereira, N.M.; Antunes, L.M. Anaesthesia and analgesia in laboratory adult zebrafish: A question of refinement. Lab. Anim. 2016, 50, 476–488. [Google Scholar] [CrossRef]
- Sneddon, L.U. Clinical Anesthesia and Analgesia in Fish. J. Exot. Pet Med. 2012, 21, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Zahl, I.H.; Kiessling, A.; Samuelsen, O.B.; Olsen, R.E. Anesthesia induces stress in Atlantic salmon (Salmo salar), Atlantic cod (Gadus morhua) and Atlantic halibut (Hippoglossus hippoglossus). Fish Physiol. Biochem. 2010, 36, 719–730. [Google Scholar] [CrossRef]
- Blessing, J.J.; Marshall, J.C.; Balcombe, S. Humane killing of fishes for scientific research: A comparison of two methods. J. Fish Biol. 2010, 76, 2571–2577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pounder, K.C.; Mitchell, J.L.; Thomson, J.S.; Pottinger, T.; Sneddon, L. Physiological and behavioural evaluation of common anaesthesia practices in the rainbow trout. Appl. Anim. Behav. Sci. 2018, 199, 94–102. [Google Scholar] [CrossRef]
- Fagerberg, J.H.; Al-Tikriti, Y.; Ragnarsson, G.; Bergström, C.A. Ethanol Effects on Apparent Solubility of Poorly Soluble Drugs in Simulated Intestinal Fluid. Mol. Pharm. 2012, 9, 1942–1952. [Google Scholar] [CrossRef]
- Pittman, J.T.; Ichikawa, K.M. iPhone® applications as versatile video tracking tools to analyze behavior in zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2013, 106, 137–142. [Google Scholar] [CrossRef]
- Pittman, J.T.; Lott, C.S. Startle response memory and hippocampal changes in adult zebrafish pharmacologically-induced to exhibit anxiety/depression-like behaviors. Physiol. Behav. 2014, 123, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Berberoglu, M.A.; Guo, S. Preference for ethanol in zebrafish following a single exposure. Behav. Brain Res. 2011, 217, 128–133. [Google Scholar] [CrossRef] [Green Version]

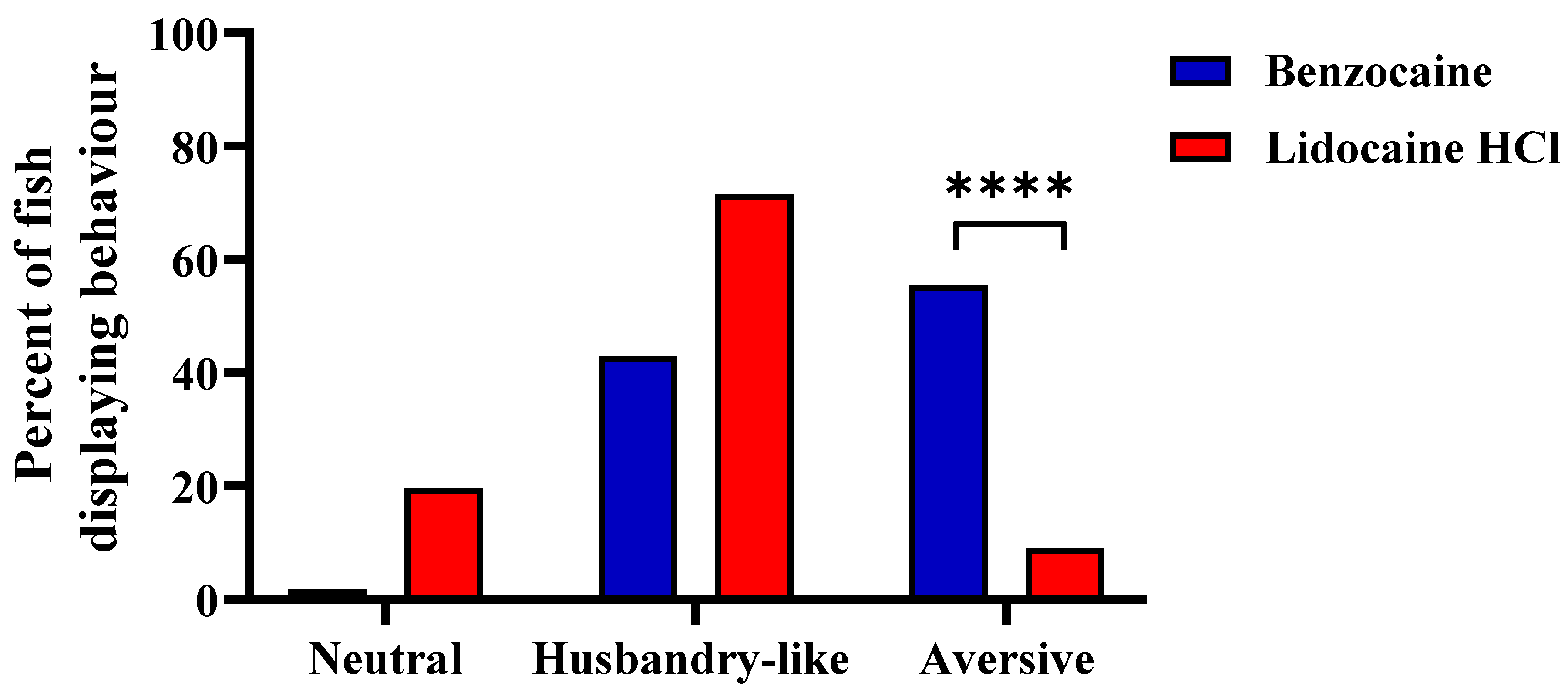
| Generic Name | Active Chemical | Product Name | Manufacturer |
|---|---|---|---|
| Isoeugenol | 2-Methoxy-4-(prop-1-en-1-yl)phenol | AQUI-S VET (540 mg/mL) | SCAN AQUA (Norway) |
| Clove Oil | 2-Methoxy-4-(prop-2-en-1-yl)phenol (i.e., eugenol) and others unidentified | Clove Oil | Boots (UK) |
| 2-PE | 2-Phenoxyethanol | Aqua-Sed | VETARK (UK) |
| Tricaine | Ethyl 3-aminobenzoate methanesulfonate | Tricaine Pharmaq 1000 mg/g | PHARMAQ (UK) |
| Benzocaine | Ethyl 4-aminobenzoate | Benzocaine (06952) | SIGMA-ALDRICH (USA) |
| Lidocaine HCl | 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide hydrochloride monohydrate | Lidocaine hydrochloride monohydrate (L5647) | SIGMA-ALDRICH (USA) |
| Etomidate | (R)-1-(α-Methylbenzyl)imidazole-5-carboxylic acid ethyl ester | Etomidate CRS (E2503000) | European Pharmacopoeia (EP) Reference Standard |
| Agent | Dose | pKa |
|---|---|---|
| Isoeugenol | 540 mg/L SW | 10.01 |
| Clove Oil | 0.1% (1:10 in EtOH, then 10 mL/L SW) | N/A |
| 2-PE | 3 mL/L SW | 15.1 |
| Tricaine a | 0.5 g/L + 1 g/L NaHCO3 SW | 2.89 |
| Tricaine b | 1 g/L + 2 g/L NaHCO3 SW | 2.89 |
| Benzocaine | 1 g/L (20 g/L in EtOH, then 50 mL/L SW) | 2.78 |
| Lidocaine HCl | 1 g/L + 2 g/L NaHCO3 SW | 7.75 |
| Etomidate | 50 mg/L (10 mg/mL in EtOH, then 5 mL/L SW) | 4.25 |
| Treatment | pH | Seconds to Immobility | n | |
|---|---|---|---|---|
| Mean (±SD) | Median | |||
| Tris (0.2 mL/L) | 7.0 | 65.5 (±21.11) | 63 | 10 |
| Tris (2 mL/L) | 7.9 | 20 (±10.12) | 18 | 57 |
| Tris (4 mL/L) | 8.0 | 11.93 (±5.93) | 10 | 41 |
| NaHCO3 (1 g/L) | 7.7 | 12.5 (±4.91) | 12 | 38 |
| NaHCO3 (2 g/L) | 7.9 | 10.76 (±4.54) | 10 | 51 |
| NaHCO3 (3 g/L) | 8.0 | 10.32 (±3.97) | 10 | 37 |
| NaHCO3 (2 g/L) + 50 mL/L EtOH | 8.0 | 9.25 (±3.07) | 9 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Krogh, K.; Higgins, J.; Saavedra Torres, Y.; Mocho, J.-P. Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose. Biology 2021, 10, 1133. https://doi.org/10.3390/biology10111133
von Krogh K, Higgins J, Saavedra Torres Y, Mocho J-P. Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose. Biology. 2021; 10(11):1133. https://doi.org/10.3390/biology10111133
Chicago/Turabian Stylevon Krogh, Kristine, Joseph Higgins, Yolanda Saavedra Torres, and Jean-Philippe Mocho. 2021. "Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose" Biology 10, no. 11: 1133. https://doi.org/10.3390/biology10111133
APA Stylevon Krogh, K., Higgins, J., Saavedra Torres, Y., & Mocho, J.-P. (2021). Screening of Anaesthetics in Adult Zebrafish (Danio rerio) for the Induction of Euthanasia by Overdose. Biology, 10(11), 1133. https://doi.org/10.3390/biology10111133







