Zebrafish Model to Study Angiotensin II-Mediated Pathophysiology
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Ethics Statement
2.3. Angiotensin II injections
2.4. qPCR and Gene Expression Analysis
2.5. Edu Incorporation Assay
2.6. Picrosirius Red Staining
2.7. Cardiomyocyte Cross-Section Area Analysis
2.8. Statistical Analysis
3. Results
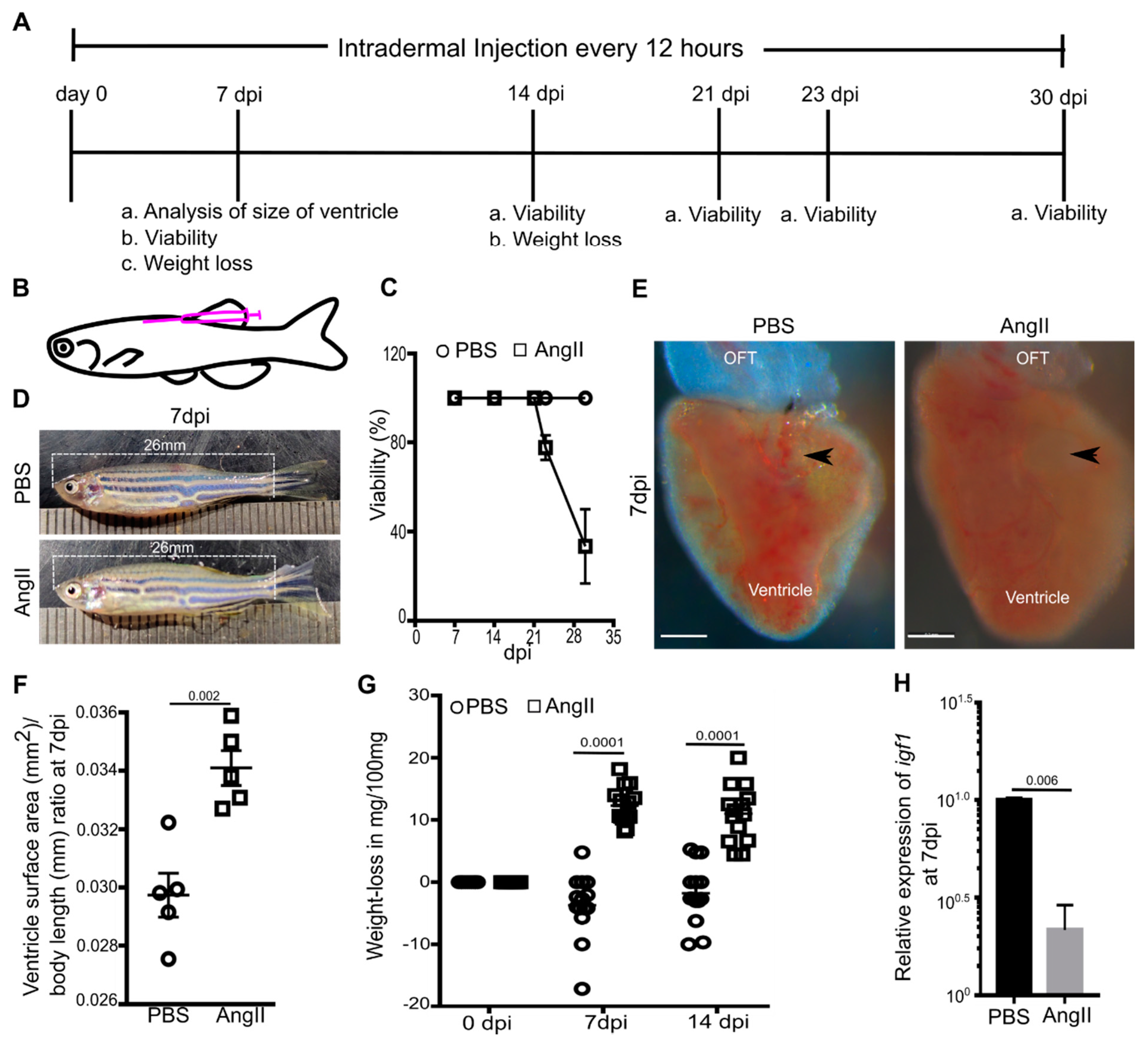
3.1. AngII Induces Cardiac Hypertrophy and Overall Weight Loss in Adult Zebrafish
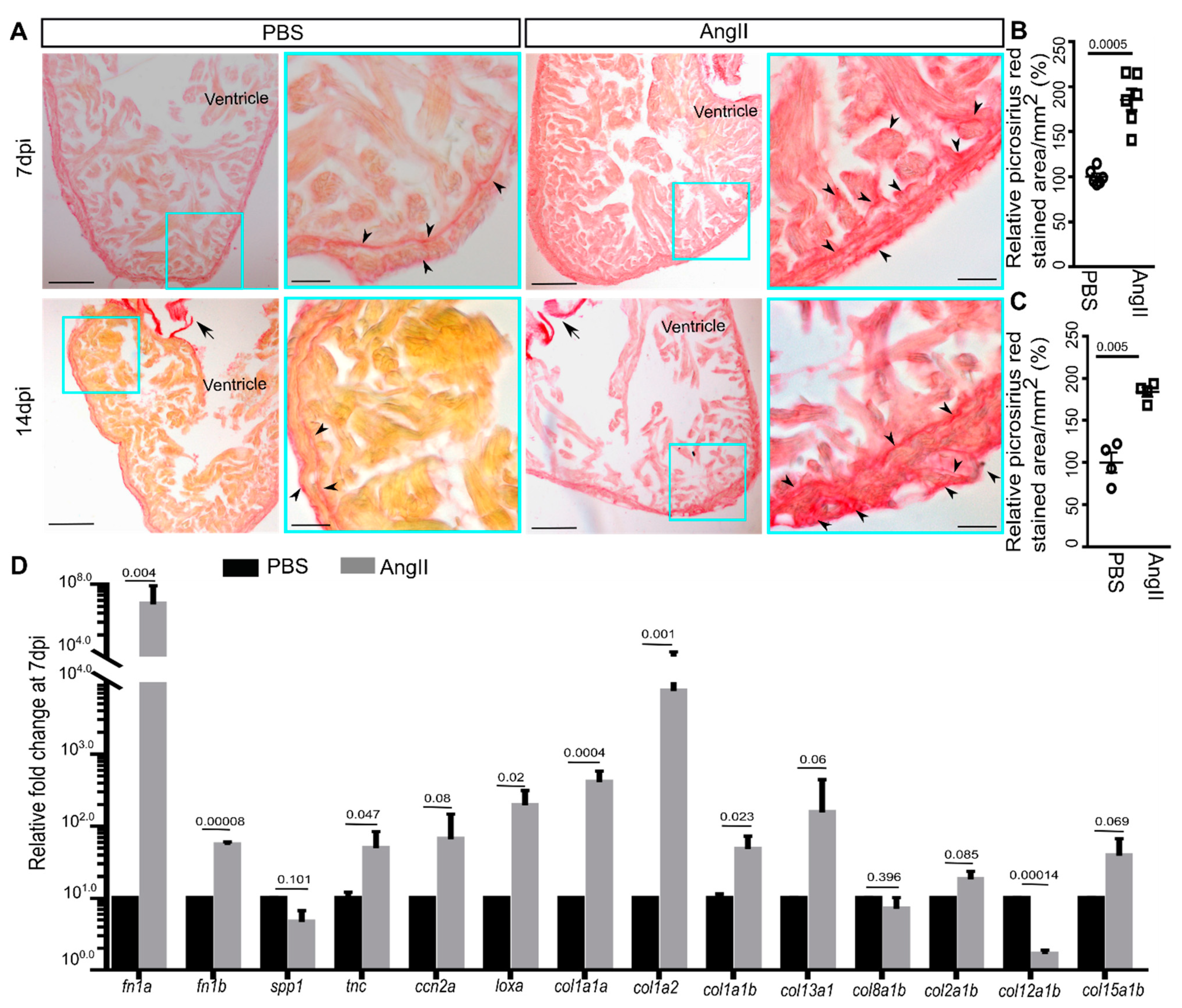
3.2. AngII Injection Leads to Fibrotic Gene Expression and Scarring in Zebrafish Hearts
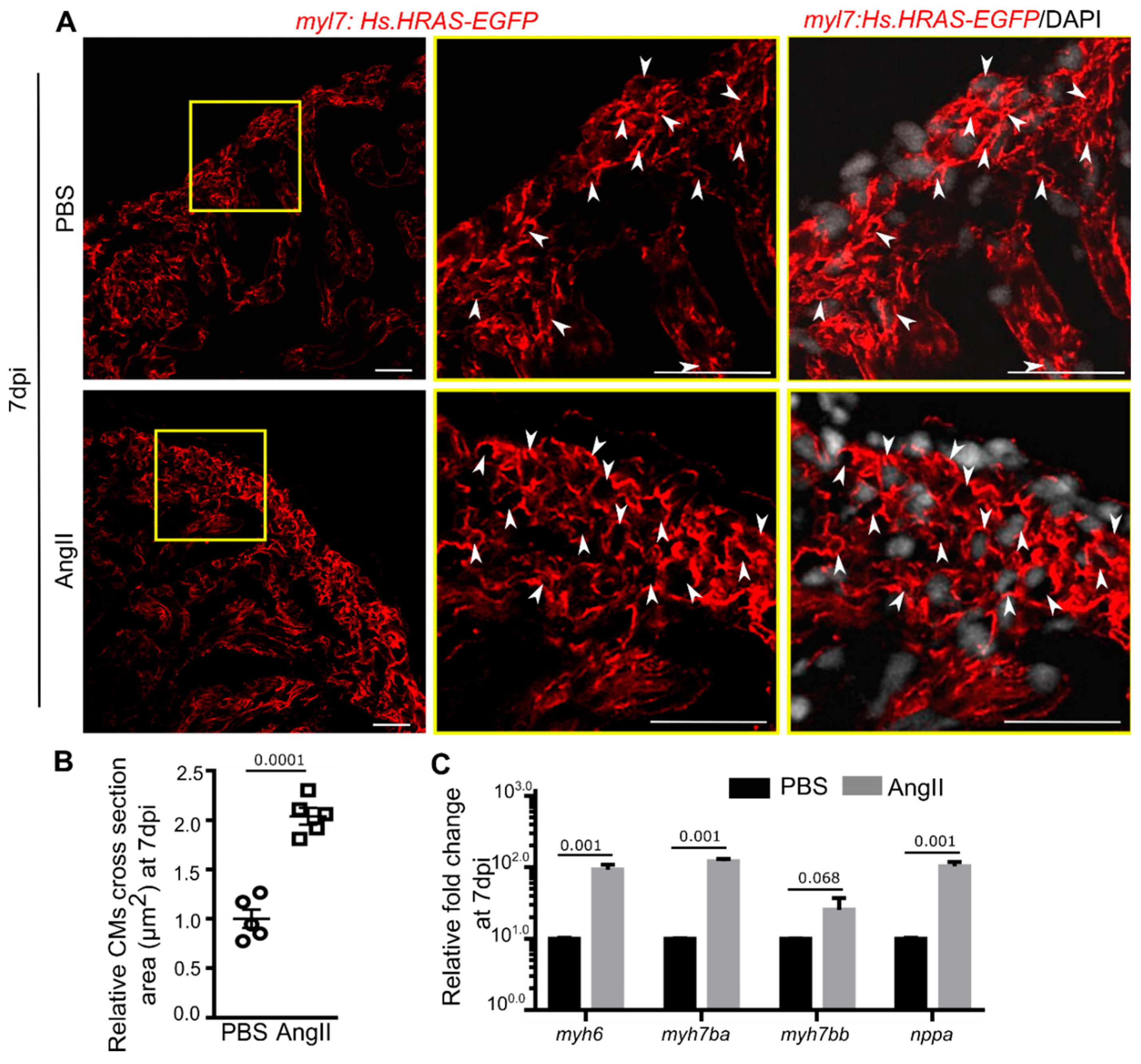
3.3. AngII Injection Induces Cardiomyocyte Hypertrophy in Zebrafish
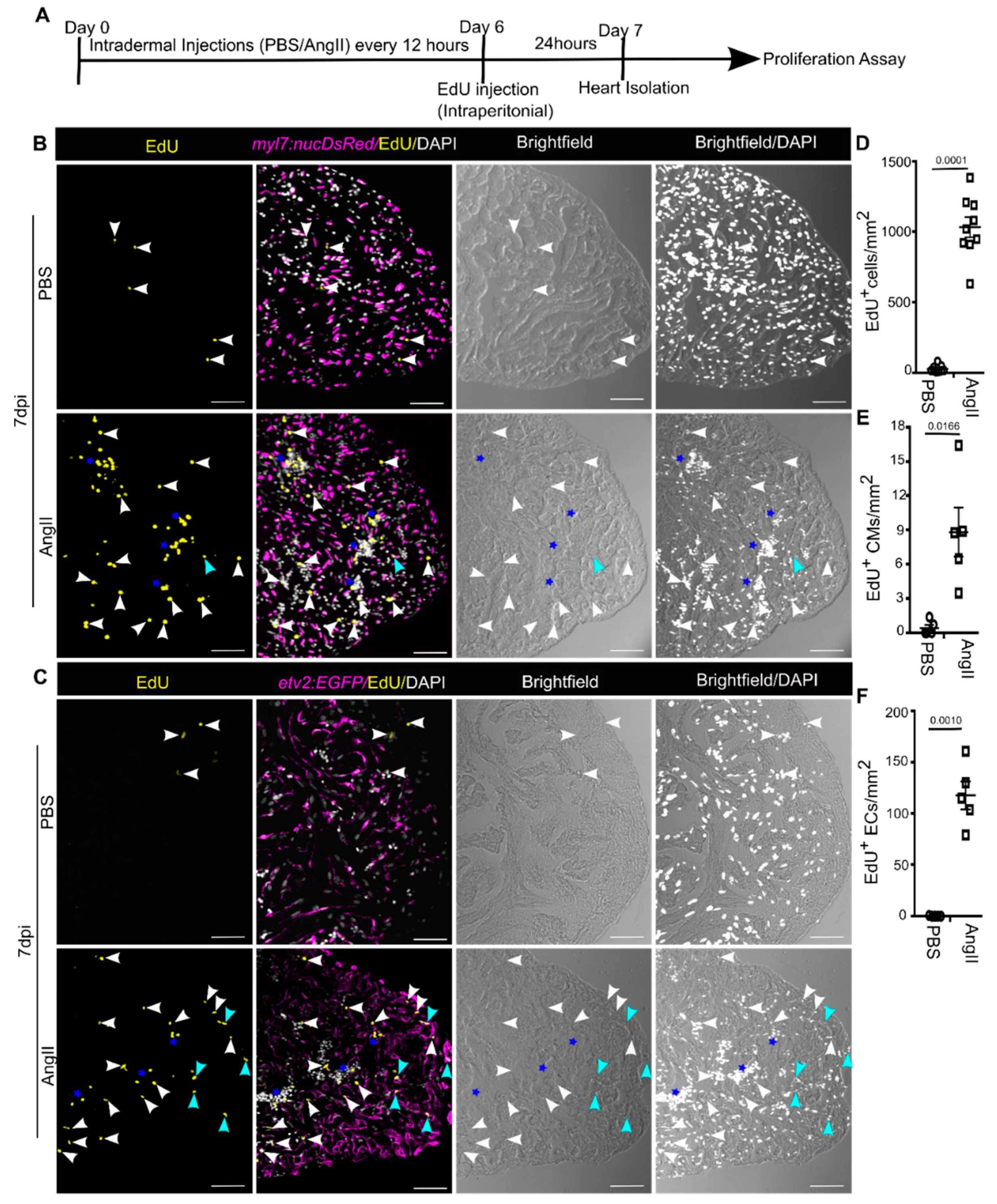
3.4. AngII Injection Promotes Cardiac Cell Proliferation in Adult Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radermacher, J. Hypertonie. Gefässchirurgie 2020, 25, 166–171. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults. J. Am. Coll. Cardiol. 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Oliveros, E.; Patel, H.; Kyung, S.; Fugar, S.; Goldberg, A.; Madan, N.; Williams, K.A. Hypertension in older adults: Assessment, management, and challenges. Clin. Cardiol. 2019, 43, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Beevers, G.; Lip, G.Y.H.; O’Brien, E. ABC of hypertension: The pathophysiology of hypertension. BMJ 2001, 322, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Stefano, T.; Rosa, M.B.; Anna, S. Epidemiology and Pathophysiology of Hypertension—Oxford Medicine. Eur. Soc. Cardiol. 2020, 3, 2377–2388. [Google Scholar] [CrossRef]
- Burnier, M.; Wuerzner, G. Pathophysiology of Hypertension. In Pathophysiology and Pharmacotherapy of Cardiovascular Disease; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2015; pp. 655–683. [Google Scholar]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Ambrosioni, E. Pharmacoeconomics of Hypertension Management. PharmacoEconomics 2001, 19, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Howard, K.; White, S.; Salkeld, G.; McDonald, S.; Craig, J.C.; Chadban, S.; Cass, A. Cost-Effectiveness of Screening and Optimal Management for Diabetes, Hypertension, and Chronic Kidney Disease: A Modeled Analysis. Value Health 2010, 13, 196–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.; Wu, J.; Zhang, Q.; Ye, Y.; Wang, S.; Huang, J.; Liu, H.; Wang, X.; Zhang, W.; Bu, L.; et al. Differential cardiac hypertrophy and signaling pathways in pressure versus volume overload. Am. J. Physiol. Circ. Physiol. 2017, 314, H552–H562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugden, H.P.; Clerk, A. Cellular mechanisms of cardiac hypertrophy. J. Mol. Med. 1998, 76, 725–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Rockey, D.C.; Bell, P.D.; Hill, J.A. Fibrosis—A Common Pathway to Organ Injury and Failure. N. Engl. J. Med. 2015, 372, 1138–1149. [Google Scholar] [CrossRef]
- Zeisberg, M.; Kalluri, R. Cellular Mechanisms of Tissue Fibrosis. 1. Common and organ-specific mechanisms associated with tissue fibrosis. Am. J. Physiol. 2013, 304, C216–C225. [Google Scholar] [CrossRef] [Green Version]
- Leong, X.-F.; Ng, C.-Y.; Jaarin, K. Animal Models in Cardiovascular Research: Hypertension and Atherosclerosis. BioMed Res. Int. 2015, 2015, 528757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doggrell, S.A. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc. Res. 1998, 39, 89–105. [Google Scholar] [CrossRef]
- Trippodo, N.C.; Frohlich, E.D. Similarities of genetic (spontaneous) hypertension. Man and rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Dahl, L.K.; Heine, M.; Tassinari, L. Role of Genetic Factors in Susceptibility to Experimental Hypertension due to Chronic Excess Salt Ingestion. Nat. Cell Biol. 1962, 194, 480–482. [Google Scholar] [CrossRef]
- Sun, Z.-J.; Zhang, Z.-E. Historic perspectives and recent advances in major animal models of Hypertension1. Acta Pharmacol. Sin. 2005, 26, 295–301. [Google Scholar] [CrossRef]
- Fanelli, C.; Zatz, R. Linking Oxidative Stress, the Renin-Angiotensin System, and Hypertension. Hypertension 2011, 57, 373–374. [Google Scholar] [CrossRef] [Green Version]
- Zhuravleva, M.V.; Luchinina, E.V.; Shelekhova, T.V.; Serebrova, S.Y.; Belkov, S.A.; Dmitriev, A.I.; Gorodetskaya, G.I. Combined Therapy of Arterial Hypertension. The Opinion of a Clinical Pharmacologist. Ration Pharm. Cardiol. 2020, 16, 601–606. [Google Scholar] [CrossRef]
- Sever, P.S.; Gradman, A.H.; Azizi, M. Managing cardiovascular and renal risk: The potential of direct renin inhibition. J. Renin-Angiotensin-Aldosterone Syst. 2009, 10, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Abadir, P.M. The Frail Renin-Angiotensin System. Clin. Geriatr. Med. 2011, 27, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badyal, D.K.; Lata, H.; Dadhich, A.P. Animal Models of Hypertension and Effect of Drugs. Indian J. Pharmacol. 2003, 35, 349–362. [Google Scholar]
- Granger, J.P.; Schnackenberg, C.G. Renal mechanisms of angiotensin II-induced hypertension. Semin. Nephrol. 2000, 2, 417–425. [Google Scholar] [PubMed]
- Paul, M.; Mehr, A.P.; Kreutz, R. Physiology of Local Renin-Angiotensin Systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.A.; Fattah, C.; Loughrey, C.; Milligan, G.; Nicklin, S.A. Angiotensin-(1–7) and angiotensin-(1–9): Function in cardiac and vascular remodelling. Clin. Sci. 2014, 126, 815–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.M.; Campanile, C.P.; Trachte, G.J.; Peach, M.J. Identification and characterization of the rabbit angiotensin II myocardial receptor. Circ. Res. 1984, 54, 286–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.M.; Singer, H.A. Identification and characterization of guinea pig angiotensin II ventricular and atrial receptors: Coupling to inositol phosphate production. Circ. Res. 1988, 62, 896–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogg, H.; Schmid, A.; de Gasparo, M. Identification and characterization of angiotensin II receptor subtypes in rabbit ventricular myocardium. Biochem. Biophys. Res. Commun. 1990, 173, 416–422. [Google Scholar] [CrossRef]
- Sadoshima, J.; Izumo, S. Molecular characterization of angiotensin I—Induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ. Res. 1993, 73, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Samyn, M.E.; Petershack, J.A.; Bedell, K.A.; Mathews, M.S.; Segar, J.L. Ontogeny and Regulation of Cardiac Angiotensin Types 1 and 2 Receptors during Fetal Life in Sheep. Pediatric Res. 1998, 44, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Matsubara, H. Pathophysiological Role of Angiotensin II Type 2 Receptor in Cardiovascular and Renal Diseases. Circ. Res. 1998, 83, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, N.; Matsubara, H.; Nozawa, Y.; Mori, Y.; Murasawa, S.; Kijima, K.; Maruyama, K.; Masaki, H.; Tsutumi, Y.; Shibazaki, Y.; et al. Angiotensin Type 2 Receptors Are Reexpressed by Cardiac Fibroblasts From Failing My-opathic Hamster Hearts and Inhibit Cell Growth and Fibrillar Collagen Metabolism. Circulation 1997, 96, 3954–3962. [Google Scholar] [CrossRef]
- Bryda, E.C. The Mighty Mouse: The Impact of Rodents on Advances in Biomedical Research. Mo. Med. 2013, 110, 207–211. [Google Scholar] [PubMed]
- Szpirer, C. Rat models of human diseases and related phenotypes: A systematic inventory of the causative genes. J. Biomed. Sci. 2020, 27, 84. [Google Scholar] [CrossRef] [PubMed]
- Vodička, P.; Smetana, J.K.; Dvořánková, B.; Emerick, T.; Xu, Y.Z.; Ourednik, J.; Ourednik, V.; Motlík, J. The Miniature Pig as an Animal Model in Biomedical Research. Ann. N. Y. Acad. Sci. 2005, 1049, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Esteves, P.J.; Abrantes, J.; Baldauf, H.-M.; BenMohamed, L.; Chen, Y.; Christensen, N.; González-Gallego, J.; Giacani, L.; Hu, J.; Kaplan, G.; et al. The wide utility of rabbits as models of human diseases. Exp. Mol. Med. 2018, 50, 66. [Google Scholar] [CrossRef]
- Khan, A.; Waqar, K.; Shafique, A.; Irfan, R.; Gul, A. In Vitro and In Vivo Animal Models. In Omics Technologies and Bio-Engineering; Elsevier BV: Amsterdam, Netherlands, 2018; pp. 431–448. [Google Scholar]
- Meyers, J.R. Zebrafish: Development of a Vertebrate Model Organism. Curr. Protoc. Essent. Lab. Tech. 2018, 16, e19. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, S.; Mullins, L.; Buckley, C.; Rider, S.; Mullins, J. Investigating the RAS can be a fishy business: Interdisciplinary opportunities using Zebrafish. Clin. Sci. 2018, 132, 2469–2481. [Google Scholar] [CrossRef] [Green Version]
- Kumai, Y.; Bernier, N.J.; Perry, S.F. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J. Endocrinol. 2014, 220, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Filice, M.; Barca, A.; Amelio, D.; Leo, S.; Mazzei, A.; Del Vecchio, G.; Verri, T.; Cerra, M.C.; Imbrogno, S. Morpho-functional remodelling of the adult zebrafish (Danio rerio) heart in response to waterborne angiotensin II exposure. Gen. Comp. Endocrinol. 2021, 301, 113663. [Google Scholar] [CrossRef]
- Proulx, K.; Lu, A.; Sumanas, S. Cranial vasculature in zebrafish forms by angioblast cluster-derived angiogenesis. Dev. Biol. 2010, 348, 34–46. [Google Scholar] [CrossRef]
- Mably, J.D.; Burns, C.; Chen, J.-N.; Fishman, M.C.; Mohideen, M.-A.P. heart of glass Regulates the Concentric Growth of the Heart in Zebrafish. Curr. Biol. 2003, 13, 2138–2147. [Google Scholar] [CrossRef]
- D’Amico, L.; Scott, I.; Jungblut, B.; Stainier, D. A Mutation in Zebrafish hmgcr1b Reveals a Role for Isoprenoids in Vertebrate Heart-Tube Formation. Curr. Biol. 2007, 17, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Grimm, P.C.; Nickerson, P.; Gough, J.; McKenna, R.; Stern, E.; Jeffery, J.; Rush, D.N. Computerized Image Analysis of Sirius Red–Stained Renal Allograft Biopsies as a Surrogate Marker to Predict Long-Term Allograft Function. J. Am. Soc. Nephrol. 2003, 14, 1662–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, C.; Kontarakis, Z.; Kaur, H.; Rayrikar, A.; Mukherjee, D.; Stainier, D.Y.R. The zebrafish ventricle: A hub of cardiac endothelial cells for in vitro cell behavior studies. Sci. Rep. 2017, 7, 2687. [Google Scholar] [CrossRef] [Green Version]
- Baker, K.M.; Chernin, M.I.; Schreiber, T.; Sanghi, S.; Haiderzaidi, S.; Booz, G.W.; Dostal, D.E.; Kumar, R. Evidence of a novel intracrine mechanism in angiotensin II-induced cardiac hypertrophy. Regul. Pept. 2004, 120, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Sadoshima, J.; Xu, Y.; Slayter, H.S.; Izumo, S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell 1993, 75, 977–984. [Google Scholar] [CrossRef]
- Rosenkranz, S. TGF-β1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [Green Version]
- Kukida, M.; Sawada, H.; Ohno-Urabe, S.; Howatt, D.A.; Moorleghen, J.J.; Poglitsch, M.; Daugherty, A.; Lu, H.S. Effects of Endogenous Angiotensin II on Abdominal Aortic Aneurysms and Atherosclerosis in Angiotensin II–Infused Mice. J. Am. Heart Assoc. 2021, 10, e020467. [Google Scholar] [CrossRef]
- Byrne, J.A.; Grieve, D.J.; Bendall, J.K.; Li, J.-M.; Gove, C.; Lambeth, J.D.; Cave, A.C.; Shah, A.M. Contrasting Roles of NADPH Oxidase Isoforms in Pressure-Overload Versus Angiotensin II–Induced Cardiac Hypertrophy. Circ. Res. 2003, 93, 802–805. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Kurz, S.; Münzel, T.; Tarpey, M.; Freeman, B.A.; Griendling, K.; Harrison, D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996, 97, 1916–1923. [Google Scholar] [CrossRef] [Green Version]
- Keidar, S.; Attias, J. Angiotensin II Injection into Mice Increases the Uptake of Oxidized LDL by Their Macrophages via a Proteoglycan-Mediated Pathway. Biochem. Biophys. Res. Commun. 1997, 239, 63–67. [Google Scholar] [CrossRef]
- Brink, M.; Wellen, J.; Delafontaine, P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J. Clin. Investig. 1996, 97, 2509–2516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brink, M.; Price, S.R.; Chrast, J.; Bailey, J.L.; Anwar, A.; Mitch, W.E.; Delafontaine, P. Angiotensin II Induces Skeletal Muscle Wasting through Enhanced Protein Degradation and Down-Regulates Autocrine Insulin-Like Growth Factor I. Endocrinology 2001, 142, 1489–1496. [Google Scholar] [CrossRef]
- Schorb, W.; Booz, G.W.; Dostal, D.E.; Conrad, K.M.; Chang, K.C.; Baker, K.M. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ. Res. 1993, 72, 1245–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, D.C.; Chobanian, A.V.; Brecher, P. Angiotensin II induces fibronectin expression associated with cardiac fibrosis in the rat. Circ. Res. 1994, 74, 727–739. [Google Scholar] [CrossRef] [Green Version]
- Haudek, S.B.; Cheng, J.; Du, J.; Wang, Y.; Hermosillo-Rodriguez, J.; Trial, J.; Taffet, G.E.; Entman, M.L. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J. Mol. Cell. Cardiol. 2010, 49, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Karsdal, M.A.; Nielsen, S.H.; Leeming, D.J.; Langholm, L.L.; Nielsen, M.J.; Manon-Jensen, T.; Siebuhr, A.; Gudmann, N.S.; Ronnow, S.; Sand, J.M.; et al. The good and the bad collagens of fibrosis—Their role in signaling and organ function. Adv. Drug Deliv. Rev. 2017, 121, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, N.; Hashizume, R.; Kanayama, K.; Hara, M.; Suzuki, Y.; Nishioka, T.; Hiroe, M.; Yoshida, T.; Imanaka-Yoshida, K. Tenascin-C May Accelerate Cardiac Fibrosis by Activating Macrophages via the Integrin αVβ3/Nuclear Factor–κB/Interleukin-6 Axis. Hypertension 2015, 66, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Ashizawa, N.; Graf, K.; Do, Y.S.; Nunohiro, T.; Giachelli, C.M.; Meehan, W.P.; Tuan, T.L.; Hsueh, W.A. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J. Clin. Investig. 1996, 98, 2218–2227. [Google Scholar] [CrossRef] [Green Version]
- Collins, A.R.; Schnee, J.; Wang, W.; Kim, S.; Fishbein, M.C.; Bruemmer, D.; Law, R.E.; Nicholas, S.; Ross, R.; Hsueh, W.A. Osteopontin modulates angiotensin II—Induced fibrosis in the intact murine heart. J. Am. Coll. Cardiol. 2004, 43, 1698–1705. [Google Scholar] [CrossRef] [Green Version]
- Galán, M.; Varona, S.; Guadall, A.; Orriols, M.; Navas, M.; Aguiló, S.; de Diego, A.; Navarro, M.A.; García-Dorado, D.; Rodríguez-Sinovas, A.; et al. Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II–induced hypertrophy. FASEB J. 2017, 31, 3787–3799. [Google Scholar] [CrossRef]
- Vikstrom, K.L.; Bohlmeyer, T.; Factor, S.M.; Leinwand, L.A. Hypertrophy, pathology, and molecular markers of cardiac pathogenesis. Circ. Res. 1998, 82, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Sartore, S.; Gorza, L.; Schiaffino, S. Fetal myosin heavy chains in regenerating muscle. Nat. Cell Biol. 1982, 298, 294–296. [Google Scholar] [CrossRef]
- Otani, A.; Takagi, H.; Suzuma, K.; Honda, Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ. Res. 1998, 82, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Munzenmaier, D.H.; Greene, A.S. Opposing Actions of Angiotensin II on Microvascular Growth and Arterial Blood Pressure. Hypertension 1996, 27, 760–765. [Google Scholar] [CrossRef] [PubMed]
- See, W.A.; McDermott, T.; Xia, Q.; Williams, R.D. A Continuous Intravesical Drug Delivery System for the Rat. J. Urol. 1991, 145, 596–599. [Google Scholar] [CrossRef]
- Shen, C.; Zhou, J.; Wang, X.; Yu, X.-Y.; Liang, C.; Liu, B.; Pan, X.; Zhao, Q.; Song, J.L.; Wang, J.; et al. Angiotensin-II-induced Muscle Wasting is Mediated by 25-Hydroxycholesterol via GSK3β Signaling Pathway. EBioMedicine 2017, 16, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Marro, J.; Pfefferli, C.; Charles, A.-S.D.P.; Bise, T.; Jaźwińska, A. Collagen XII Contributes to Epicardial and Connective Tissues in the Zebrafish Heart during Ontogenesis and Regeneration. PLoS ONE 2016, 11, e0165497. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Wagh, G.; Mokalled, M.H.; Kontarakis, Z.; Dickson, A.L.; Rayrikar, A.; Günther, S.; Poss, K.D.; Stainier, D.Y.R.; Patra, C. Ccn2a/Ctgfa is an injury-induced matricellular factor that promotes cardiac regeneration in zebrafish. Development 2021, 148, dev193219. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Samuel, J.L.; Sassoon, D.; Lompré, A.M.; Garner, I.; Marotte, F.; Buckingham, M.; Rappaport, L.; Schwartz, K. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload—Induced cardiac hypertrophy demonstrated by in situ hybridization. Circ. Res. 1989, 64, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Whalen, R.G.; Sell, S.M.; Butler-Browne, G.S.; Schwartz, K.; Bouveret, P.; Pinset-Härström, I.; Pinset-Härstöm, I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nat. Cell Biol. 1981, 292, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Nag, A.C. Study of non-muscle cells of the adult mammalian heart: A fine structural analysis and distribution. Cytobios 1980, 28, 41–61. [Google Scholar] [PubMed]
- Banerjee, I.; Fuseler, J.W.; Price, R.L.; Borg, T.K.; Baudino, T.A. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am. J. Physiol. Circ. Physiol. 2007, 293, H1883–H1891. [Google Scholar] [CrossRef] [Green Version]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Sallin, P.; Charles, A.-S.D.P.; Duruz, V.; Pfefferli, C.; Jaźwińska, A. A dual epimorphic and compensatory mode of heart regeneration in zebrafish. Dev. Biol. 2015, 399, 27–40. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, B.; Wagh, G.; Kaur, H.; Patra, C. Zebrafish Model to Study Angiotensin II-Mediated Pathophysiology. Biology 2021, 10, 1177. https://doi.org/10.3390/biology10111177
Joshi B, Wagh G, Kaur H, Patra C. Zebrafish Model to Study Angiotensin II-Mediated Pathophysiology. Biology. 2021; 10(11):1177. https://doi.org/10.3390/biology10111177
Chicago/Turabian StyleJoshi, Bhagyashri, Ganesh Wagh, Harmandeep Kaur, and Chinmoy Patra. 2021. "Zebrafish Model to Study Angiotensin II-Mediated Pathophysiology" Biology 10, no. 11: 1177. https://doi.org/10.3390/biology10111177
APA StyleJoshi, B., Wagh, G., Kaur, H., & Patra, C. (2021). Zebrafish Model to Study Angiotensin II-Mediated Pathophysiology. Biology, 10(11), 1177. https://doi.org/10.3390/biology10111177





