Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment
Abstract
:Simple Summary
Abstract
1. Introduction
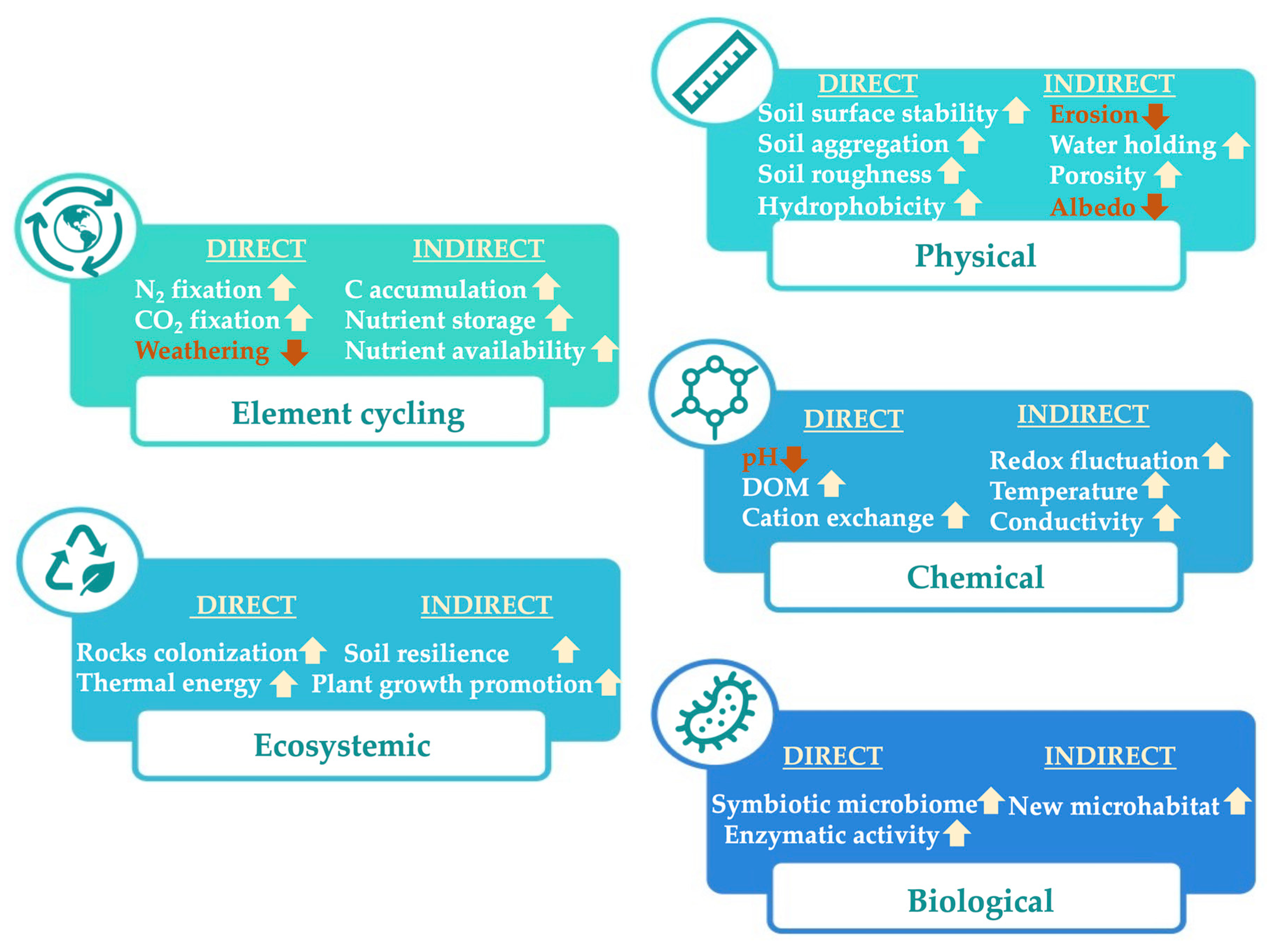
1.1. Biocrust, a Photosynthetic Cell Factory for C Sequestration in Agriculture
1.2. Biocrust, a Photosynthetic Cell Factory for C Sequestration in Agriculture
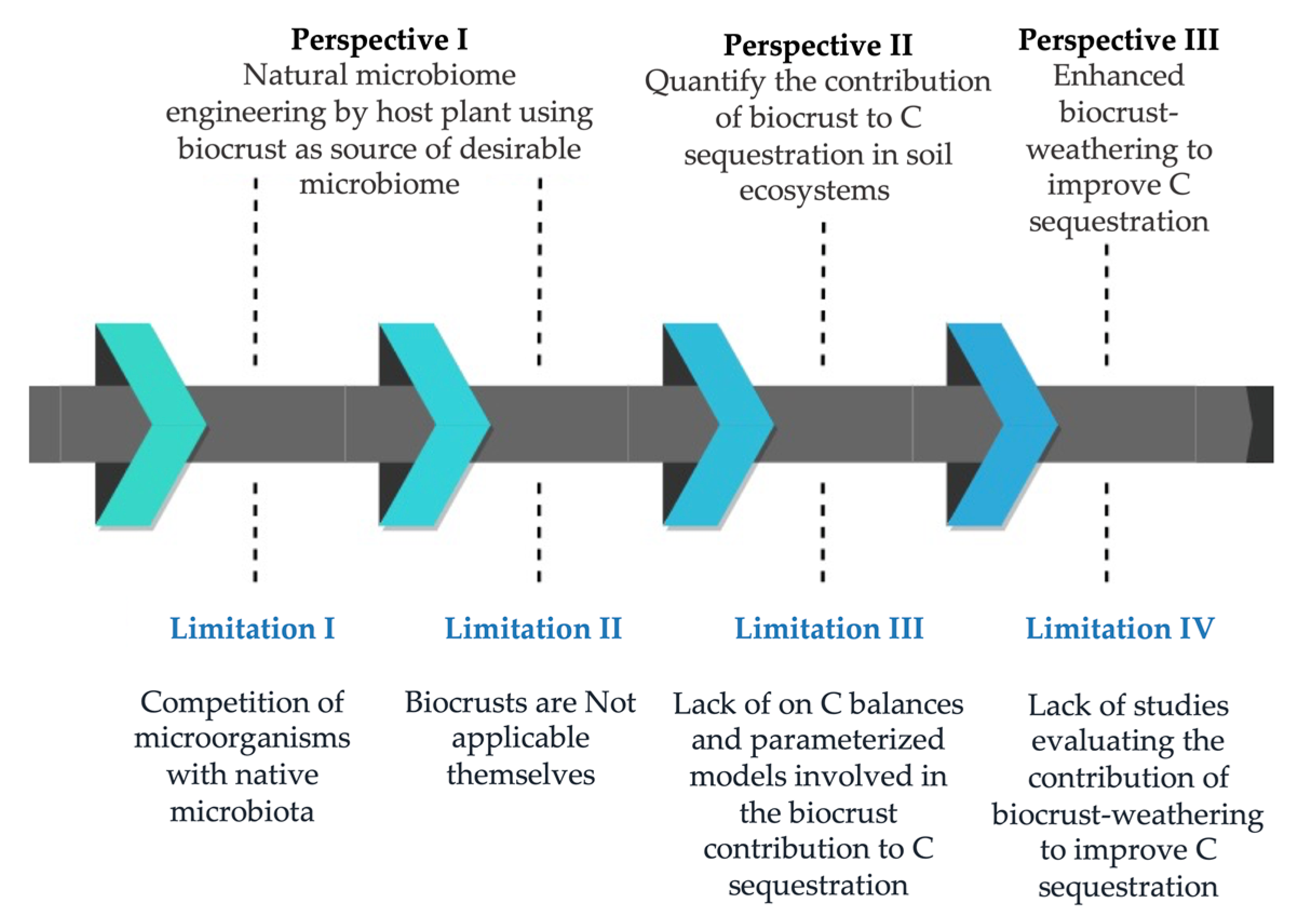
2. Limitations of the Broad-Scale Application of Biocrusts in Agriculture
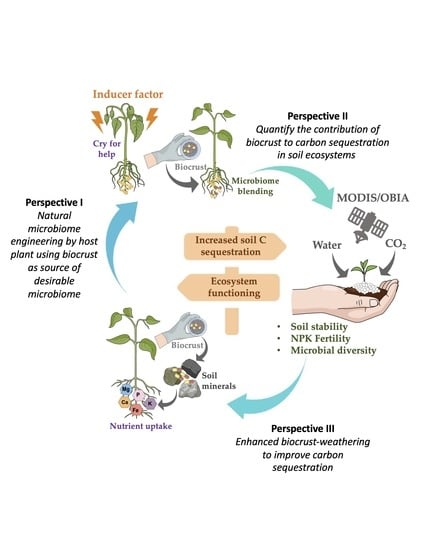
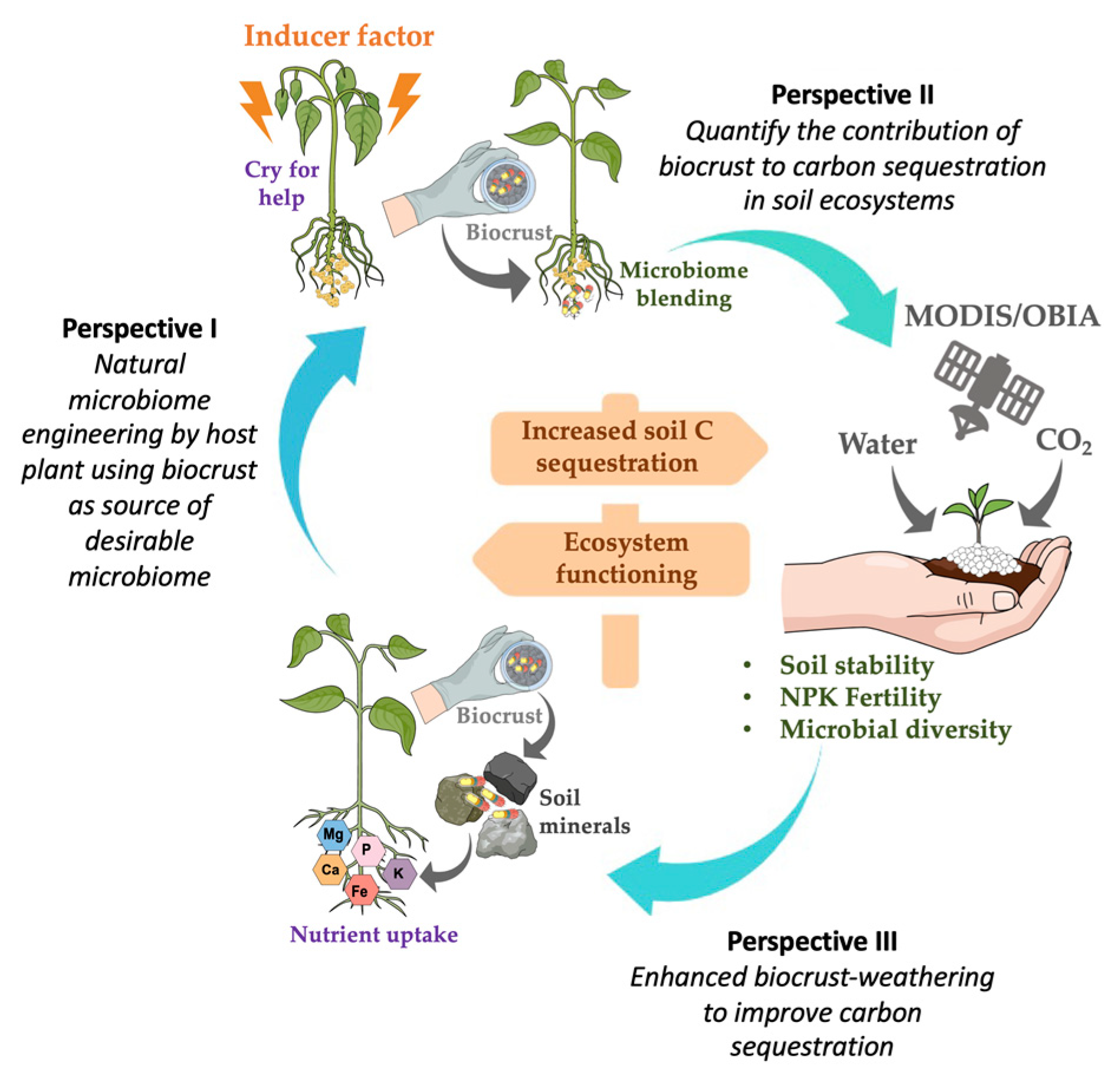
3. Perspectives to Solve Practical Limitations of Biocrust Use for Carbon Sequestration
3.1. Perspective I. Natural Microbiome Engineering by a Host Plant, Using a Biocrust as Source of Desirable Micro-Organisms
3.2. Perspective II. Quantify the Contribution of Biocrusts to Carbon Sequestration in Soils
3.3. Perspective III. Enhanced Biocrust Weathering to Improve Carbon Sequestration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minasny, B.; Malone, B.P.; McBratney, A.; Angers, D.A.; Arrouays, D.; Chambers, A.; Chaplot, V.; Chen, Z.-S.; Cheng, K.; Das, B.S.; et al. Soil carbon 4 per mille. Geoderma 2017, 292, 59–86. [Google Scholar] [CrossRef]
- Rumpel, C.; Amiraslani, F.; Chenu, C.; Cardenas, M.G.; Kaonga, M.; Koutika, L.-S.; Ladha, J.; Madari, B.; Shirato, Y.; Smith, P.; et al. The 4p1000 initiative: Opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 2019, 49, 350–360. [Google Scholar] [CrossRef] [Green Version]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Ortiz, A.M.D.; Outhwaite, C.L.; Dalin, C.; Newbold, T. A review of the interactions between biodiversity, agriculture, climate change, and international trade: Research and policy priorities. One Earth 2021, 4, 88–101. [Google Scholar] [CrossRef]
- Fu, X.; Zhou, Y.; Yang, F.; Ma, L.; Long, H.; Zhong, Y.; Ni, P. A Review of Key Technologies and Trends in the Development of Integrated Heating and Power Systems in Agriculture. Entropy 2021, 23, 260. [Google Scholar] [CrossRef] [PubMed]
- Kallenbach, C.M.; Wallenstein, M.; Schipanksi, M.E.; Grandy, A.S. Managing Agroecosystems for Soil Microbial Carbon Use Efficiency: Ecological Unknowns, Potential Outcomes, and a Path Forward. Front. Microbiol. 2019, 10, 1146. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Bowling, D.R.; Grote, E.E.; Belnap, J. Rain pulse response of soil CO2 exchange by biological soil crusts and grasslands of the semiarid Colorado Plateau, United States. J. Geophys. Res. Space Phys. 2011, 116, G03028. [Google Scholar] [CrossRef] [Green Version]
- Kheirfam, H. Increasing soil potential for carbon sequestration using microbes from biological soil crusts. J. Arid. Environ. 2019, 172, 104022. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Darki, B.Z. Soil conservation in an abandoned agricultural rain-fed land through inoculation of cyanobacteria. CATENA 2019, 187, 104341. [Google Scholar] [CrossRef]
- Maier, S.; Tamm, A.; Wu, D.; Caesar, J.; Grube, M.; Weber, B. Photoautotrophic organisms control microbial abundance, diversity, and physiology in different types of biological soil crusts. ISME J. 2018, 12, 1032–1046. [Google Scholar] [CrossRef] [Green Version]
- Szyja, M.; Menezes, A.G.D.S.; Oliveira, F.D.A.; Leal, I.; Tabarelli, M.; Büdel, B.; Wirth, R. Neglected but Potent Dry Forest Players: Ecological Role and Ecosystem Service Provision of Biological Soil Crusts in the Human-Modified Caatinga. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Belnap, J.; Büdel, B.; Lange, O.L. Biological Soil Crusts: Characteristics and Distribution. In Biological Soil Crusts: Structure, Function, and Management; Springer: Berlin/Heidelberg, Germany, 2001; pp. 3–30. [Google Scholar] [CrossRef]
- Felde, V.J.M.N.L.; Chamizo, S.; Felix-Henningsen, P.; Drahorad, S.L. What stabilizes biological soil crusts in the Negev Desert? Plant Soil 2018, 429, 9–18. [Google Scholar] [CrossRef]
- Warren, S.D.; Clair, L.L.S.; Stark, L.R.; Lewis, L.; Pombubpa, N.; Kurbessoian, T.; Stajich, J.E.; Aanderud, Z.T. Reproduction and Dispersal of Biological Soil Crust Organisms. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Baumann, K.; Jung, P.; Samolov, E.; Lehnert, L.W.; Büdel, B.; Karsten, U.; Bendix, J.; Achilles, S.; Schermer, M.; Matus, F.; et al. Biological soil crusts along a climatic gradient in Chile: Richness and imprints of phototrophic microorganisms in phosphorus biogeochemical cycling. Soil Biol. Biochem. 2018, 127, 286–300. [Google Scholar] [CrossRef]
- Chock, T.; Antoninka, A.J.; Faist, A.M.; Bowker, M.A.; Belnap, J.; Barger, N.N. Responses of biological soil crusts to rehabilitation strategies. J. Arid. Environ. 2018, 163, 77–85. [Google Scholar] [CrossRef]
- Adessi, A.; de Carvalho, R.C.; De Philippis, R.; Branquinho, C.; da Silva, J.M. Microbial extracellular polymeric substances improve water retention in dryland biological soil crusts. Soil Biol. Biochem. 2018, 116, 67–69. [Google Scholar] [CrossRef]
- Baumann, K.; Glaser, K.; Mutz, J.-E.; Karsten, U.; MacLennan, A.; Hu, Y.; Michalik, D.; Kruse, J.; Eckhardt, K.-U.; Schall, P.; et al. Biological soil crusts of temperate forests: Their role in P cycling. Soil Biol. Biochem. 2017, 109, 156–166. [Google Scholar] [CrossRef]
- Chilton, A.M.; Neilan, B.A.; Eldridge, D.J. Biocrust morphology is linked to marked differences in microbial community composition. Plant Soil 2017, 429, 65–75. [Google Scholar] [CrossRef]
- Aanderud, Z.T.; Bahr, J.; Robinson, D.M.; Belnap, J.; Campbell, T.; Gill, R.A.; McMILLIAN, B.; Clair, S.S. The Burning of Biocrusts Facilitates the Emergence of a Bare Soil Community of Poorly-Connected Chemoheterotrophic Bacteria With Depressed Ecosystem Services. Front. Ecol. Evol. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Gallardo, A.; Covelo, F.; Prado-Comesaña, A.; Ochoa, V.; Maestre, F.T. Differences in thallus chemistry are related to species-specific effects of biocrust-forming lichens on soil nutrients and microbial communities. Funct. Ecol. 2015, 29, 1087–1098. [Google Scholar] [CrossRef] [Green Version]
- Concostrina-Zubiri, L.; Matos, P.; Giordani, P.; Branquinho, C. Biocrust tissue traits as potential indicators of global change in the Mediterranean. Plant Soil 2017, 429, 159–174. [Google Scholar] [CrossRef]
- Maestre, F.T.; Escolar, C.; de Guevara, M.L.; Quero, J.L.; Lázaro, R.; Delgado-Baquerizo, M.; Ochoa, V.; Berdugo, M.; Gozalo, B.; Gallardo, A. Changes in biocrust cover drive carbon cycle responses to climate change in drylands. Glob. Chang. Biol. 2013, 19, 3835–3847. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Maestre, F.T.; Eldridge, D.J.; Bowker, M.A.; Jeffries, T.C.; Singh, B.K. Biocrust-forming mosses mitigate the impact of aridity on soil microbial communities in drylands: Observational evidence from three continents. New Phytol. 2018, 220, 824–835. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, M.-H.; Ding, G.-D.; He, Y.; Liu, W.; Wang, C.-Y. Soil biocrusts reduce seed germination and contribute to the decline in Artemisia ordosica Krasch. shrub populations in the Mu Us Sandy Land of North China. Glob. Ecol. Conserv. 2021, 26, e01467. [Google Scholar] [CrossRef]
- Chamizo, S.; Cantón, Y.; Miralles, I.; Domingo, F. Biological soil crust development affects physicochemical characteristics of soil surface in semiarid ecosystems. Soil Biol. Biochem. 2012, 49, 96–105. [Google Scholar] [CrossRef]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2019, 225, 1440–1446. [Google Scholar] [CrossRef] [PubMed]
- Chamizo, S.; Adessi, A.; Torzillo, G.; De Philippis, R. Exopolysaccharide Features Influence Growth Success in Biocrust-forming Cyanobacteria, Moving From Liquid Culture to Sand Microcosms. Front. Microbiol. 2020, 11, 568224. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.S.; Matsui, M.; Abdullah, A.A.-A. Cyanobacteria: Photoautotrophic Microbial Factories for the Sustainable Synthesis of Industrial Products. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Lu, X. Engineering cyanobacteria chassis cells toward more efficient photosynthesis. Curr. Opin. Biotechnol. 2019, 62, 1–6. [Google Scholar] [CrossRef]
- Moore, K.A.; Altus, S.; Tay, J.W.; Meehl, J.B.; Johnson, E.B.; Bortz, D.M.; Cameron, J.C. Mechanical regulation of photosynthesis in cyanobacteria. Nat. Microbiol. 2020, 5, 757–767. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018, 34, 811–827. [Google Scholar] [CrossRef]
- Kumar, B.M.; Nair, P.R. Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges; Springer: Berlin/Heidelberg, Germany, 2011; Volume 8. [Google Scholar]
- Jochum, M.D.; McWilliams, K.L.; Pierson, E.A.; Jo, Y.-K. Host-mediated microbiome engineering (HMME) of drought tolerance in the wheat rhizosphere. PLoS ONE 2019, 14, e0225933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panke-Buisse, K.; Poole, A.; Goodrich, J.K.; Ley, R.; Kao-Kniffin, J. Selection on soil microbiomes reveals reproducible impacts on plant function. ISME J. 2014, 9, 980–989. [Google Scholar] [CrossRef]
- Mueller, U.; Sachs, J. Engineering Microbiomes to Improve Plant and Animal Health. Trends Microbiol. 2015, 23, 606–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, R.; Durán, P. Natural Holobiome Engineering by Using Native Extreme Microbiome to Counteract the Climate Change Effects. Front. Bioeng. Biotechnol. 2020, 8, 568. [Google Scholar] [CrossRef]
- Qiu, Z.; Egidi, E.; Liu, H.; Kaur, S.; Singh, B.K. New frontiers in agriculture productivity: Optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 2019, 37, 107371. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Sadowsky, M.J.; Viscardi, S.; Barra, P.J.; Mora, M.D.L.L. Engineering Multigenerational Host-Modulated Microbiota against Soilborne Pathogens in Response to Global Climate Change. Biology 2021, 10, 865. [Google Scholar] [CrossRef]
- Durán, P.; Tortella, G.; Viscardi, S.; Barra, P.J.; Carrión, V.J.; Mora, M.L.; Pozo, M.J. Microbial community composition in take-all suppressive soils. Front. Microbiol. 2018, 9, 2198. [Google Scholar] [CrossRef] [PubMed]
- Harkes, P.; van Steenbrugge, J.; Elsen, S.J.J.V.D.; Suleiman, A.K.A.; De Haan, J.J.; Holterman, M.H.M.; Helder, J. Shifts in the Active Rhizobiome Paralleling Low Meloidogyne chitwoodi Densities in Fields Under Prolonged Organic Soil Management. Front. Plant Sci. 2020, 10, 1697. [Google Scholar] [CrossRef]
- Wang, N.; Wang, L.; Zhu, K.; Hou, S.; Chen, L.; Mi, D.; Gui, Y.; Qi, Y.; Jiang, C.; Guo, J.-H. Plant Root Exudates Are Involved in Bacillus cereus AR156 Mediated Biocontrol Against Ralstonia solanacearum. Front. Microbiol. 2019, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Carrión, V.J.; Cordovez, V.; Tyc, O.; Etalo, D.W.; De Bruijn, I.; De Jager, V.C.L.; Medema, M.H.; Eberl, L.; Raaijmakers, J.M. Involvement of Burkholderiaceae and sulfurous volatiles in disease-suppressive soils. ISME J. 2018, 12, 2307–2321. [Google Scholar] [CrossRef] [Green Version]
- Olanrewaju, O.S.; Ayangbenro, A.S.; Glick, B.R.; Babalola, O.O.; Ayangbenro, A. Plant health: Feedback effect of root exudates-rhizobiome interactions. Appl. Microbiol. Biotechnol. 2018, 103, 1155–1166. [Google Scholar] [CrossRef] [Green Version]
- Orozco-Mosqueda, M.D.C.; Rocha-Granados, M.D.C.; Glick, B.R.; Santoyo, G. Microbiome engineering to improve biocontrol and plant growth-promoting mechanisms. Microbiol. Res. 2018, 208, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.; Pieterse, C.; de Jonge, R.; Berendsen, R. The Soil-Borne Legacy. Cell 2018, 172, 1178–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Rolfe, S.A.; Griffiths, J.; Ton, J. Crying out for help with root exudates: Adaptive mechanisms by which stressed plants assemble health-promoting soil microbiomes. Curr. Opin. Microbiol. 2019, 49, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Maheswari, M.; Desai, S.; Gopinath, K.; Venkateswarlu, B. Elevated CO2: Plant associated microorganisms and carbon sequestration. Appl. Soil Ecol. 2015, 95, 73–85. [Google Scholar] [CrossRef]
- Musche, M.; Adamescu, M.; Angelstam, P.; Bacher, S.; Bäck, J.; Buss, H.L.; Duffy, C.; Flaim, G.; Gaillardet, J.; Giannakis, G.V.; et al. Research questions to facilitate the future development of European long-term ecosystem research infrastructures: A horizon scanning exercise. J. Environ. Manag. 2019, 250, 109479. [Google Scholar] [CrossRef]
- Prăvălie, R. Drylands extent and environmental issues. A global approach. Earth-Sci. Rev. 2016, 161, 259–278. [Google Scholar] [CrossRef]
- Ahlström, A.; Raupach, M.R.; Schurgers, G.; Smith, B.; Arneth, A.; Jung, M.; Reichstein, M.; Canadell, J.G.; Friedlingstein, P.; Jain, A.K.; et al. The dominant role of semi-arid ecosystems in the trend and variability of the land CO 2 sink. Science 2015, 348, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Chapin, F.S. Effects of Plant Traits on Ecosystem and Regional Processes: A Conceptual Framework for Predicting the Consequences of Global Change. Ann. Bot. 2003, 91, 455–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavorel, S. Plant functional effects on ecosystem services. J. Ecol. 2012, 101, 4–8. [Google Scholar] [CrossRef]
- Colica, G.; Li, H.; Rossi, F.; Li, D.; Liu, Y.; De Philippis, R. Microbial secreted exopolysaccharides affect the hydrological behavior of induced biological soil crusts in desert sandy soils. Soil Biol. Biochem. 2014, 68, 62–70. [Google Scholar] [CrossRef]
- Kheirfam, H.; Sadeghi, S.H.; Homaee, M.; Darki, B.Z. Quality improvement of an erosion-prone soil through microbial enrichment. Soil Tillage Res. 2017, 165, 230–238. [Google Scholar] [CrossRef]
- Miralles-Mellado, I.; Cantón, Y.; Solé-Benet, A. Two-dimensional porosity of crusted silty soils: Indicators of soil quality in semiarid rangelands? Soil Sci. Soc. Am. J. 2011, 75, 1330–1342. [Google Scholar] [CrossRef] [Green Version]
- Chamizo, S.; Cantón, Y.; Domingo, F.; Belnap, J. Evaporative losses from soils covered by physical and different types of biological soil crusts. Hydrol. Process. 2011, 27, 324–332. [Google Scholar] [CrossRef]
- Sadeghi, S.H.; Kheirfam, H.; Homaee, M.; Darki, B.Z.; Vafakhah, M. Improving runoff behavior resulting from direct inoculation of soil micro-organisms. Soil Tillage Res. 2017, 171, 35–41. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Oses, R.; Torres-Díaz, C.; Atala, C.; Zurita-Silva, A.; Ruiz-Lara, S. Root-endophytes improve the ecophysiological performance and production of an agricultural species under drought condition. AoB Plants 2016, 8, plw062. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, E.; Chamizo, S.; Roncero-Ramos, B.; Roman, R.; Canton, Y. Runoff from biocrust: A vital resource for vegetation performance on Mediterranean steppes. Ecohydrology 2018, 11, e1977. [Google Scholar] [CrossRef]
- Samolov, E.; Baumann, K.; Büdel, B.; Jung, P.; Leinweber, P.; Mikhailyuk, T.; Karsten, U.; Glaser, K. Biodiversity of Algae and Cyanobacteria in Biological Soil Crusts Collected Along a Climatic Gradient in Chile Using an Integrative Approach. Microorganisms 2020, 8, 1047. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler-Robinson, E.; Nuanez, M.; Litvak, M.E. Biocrust contribution to ecosystem carbon fluxes varies along an elevational gradient. Ecosphere 2018, 9, e02315. [Google Scholar] [CrossRef]
- Heindel, R.C.; Governali, F.C.; Spickard, A.M.; Virginia, R.A. The Role of Biological Soil Crusts in Nitrogen Cycling and Soil Stabilization in Kangerlussuaq, West Greenland. Ecosystems 2018, 22, 243–256. [Google Scholar] [CrossRef]
- Zhang, S.; Bai, X.; Zhao, C.; Tan, Q.; Luo, G.; Wang, J.; Li, Q.; Wu, L.; Chen, F.; Li, C.; et al. Global CO 2 Consumption by Silicate Rock Chemical Weathering: Its Past and Future. Earth’s Futur. 2021, 9, e2020EF001938. [Google Scholar] [CrossRef]
- Kelemen, P.B.; McQueen, N.; Wilcox, J.; Renforth, P.; Dipple, G.; Vankeuren, A.P. Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chem. Geol. 2020, 550, 119628. [Google Scholar] [CrossRef]
- Maher, K.; Steefel, C.I.; White, A.F.; Stonestrom, D. The role of reaction affinity and secondary minerals in regulating chemical weathering rates at the Santa Cruz Soil Chronosequence, California. Geochim. Cosmochim. Acta 2009, 73, 2804–2831. [Google Scholar] [CrossRef] [Green Version]
- Perez-Fodich, A.; Derry, L.A. Organic acids and high soil CO2 drive intense chemical weathering of Hawaiian basalts: Insights from reactive transport models. Geochim. Cosmochim. Acta 2019, 249, 173–198. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, Y.; Li, Y.; Wei, W.; Zhang, J.; Wu, N. The variation of morphological features and mineralogical components of biological soil crusts in the Gurbantunggut Desert of Northwestern China. Environ. Earth Sci. 2008, 57, 1135–1143. [Google Scholar] [CrossRef]
- Suchet, P.A.; Probst, J. Modelling of atmospheric CO2 consumption by chemical weathering of rocks: Application to the Garonne, Congo and Amazon basins. Chem. Geol. 1993, 107, 205–210. [Google Scholar] [CrossRef]
- Finlay, R.D.; Mahmood, S.; Rosenstock, N.; Bolou-Bi, E.B.; Köhler, S.J.; Fahad, Z.; Rosling, A.; Wallander, H.; Belyazid, S.; Bishop, K. Biological weathering and its consequences at different spatial levels–from nanoscale to global scale. Biogeosciences 2020, 17, 1507–1533. [Google Scholar] [CrossRef] [Green Version]
- Beraldi-Campesi, H.; Hartnett, H.; Anbar, A.; Gordon, G.; Garcia-Pichel, F. Effect of biological soil crusts on soil elemental concentrations: Implications for biogeochemistry and as traceable biosignatures of ancient life on land. Geobiology 2009, 7, 348–359. [Google Scholar] [CrossRef]
- Celle, H. Caractérisation des Précipitations sur le Pourtour de la Méditerranée Occidentale: Approche Isotopique et Chimique. Ph.D. Thesis, Université d’Avignon et des, Pays de Vaucluse, France, 2000. [Google Scholar]
- Sommer, U.; Sommer, F. Cladocerans versus copepods: The cause of contrasting top–down controls on freshwater and marine phytoplankton. Oecologia 2005, 147, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, K.; Pustovoytov, K.; Kuzyakov, Y. Pedogenic carbonates: Forms and formation processes. Earth-Sci. Rev. 2016, 157, 1–17. [Google Scholar] [CrossRef]
- Craig, H. Isotopic Variations in Meteoric Waters. Science 1961, 133, 1702–1703. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Buss, H. Natural Weathering Rates of Silicate Minerals. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 115–155. [Google Scholar] [CrossRef]
- Gruber, C.; Zhu, C.; Georg, B.; Zakon, Y.; Ganor, J. Resolving the gap between laboratory and field rates of feldspar weathering. Geochim. Cosmochim. Acta 2014, 147, 90–106. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.; Eiriksdottir, E.S.; Kardjilov, M.I.; Gisladottir, G.; Sigfusson, B.; Snorrason, A.; Elefsen, S.; Hardardottir, J.; Torssander, P.; et al. Direct evidence of the feedback between climate and weathering. Earth Planet. Sci. Lett. 2009, 277, 213–222. [Google Scholar] [CrossRef]
- Beaulieu, E.; Godderis, Y.; Donnadieu, Y.; Labat, D.; Roelandt, C. High sensitivity of the continental-weathering carbon dioxide sink to future climate change. Nat. Clim. Chang. 2012, 2, 346–349. [Google Scholar] [CrossRef]
- Donnini, M.; Frondini, F.; Probst, J.-L.; Probst, A.; Cardellini, C.; Marchesini, I.; Guzzetti, F. Chemical weathering and consumption of atmospheric carbon dioxide in the Alpine region. Glob. Planet. Chang. 2016, 136, 65–81. [Google Scholar] [CrossRef] [Green Version]
| Organism Group | Climate | Soils | References |
|---|---|---|---|
| Cyanobacteria | Semi-arid cold | Alkaline, loamy clay soil | [9] |
| Dry sub-humid coastal area | Nd * | [18] | |
| Arid | Nd | [16] | |
| Semi-arid | Nd | [16] | |
| Semi-arid | Sandy loams | [8] | |
| Mediterranean | Nd | [19] | |
| Humid | Nd | [19] | |
| Semi-arid | Oligotrophic | [20] | |
| Cyanobacteria/lichens | Cold desert | shrub interspaces | [21] |
| Cyanobacteria/moss | Cold desert | beneath Artemisia tridentata | [21] |
| Bacteria (diazotrophs and chemoheterotrophs) | Semi-arid | Oligotrophic | [20] |
| Cold desert | Burnt soils | [21] | |
| Semi-arid cold | Alkaline, loamy clay sil | [10] | |
| Cold desert | Burnt soils | [21] | |
| Green algae | Arid | Nd | [16] |
| Semi-arid | Nd | ||
| Mediterranean | Nd | ||
| Humid | Nd | ||
| Lichens | Mediterranean semi-arid | nd | [22] |
| Semi-arid to dry sub-humid | Arenitic | [23] | |
| Dolomitic | |||
| Arenic fluvisols | |||
| Leptic chernozems | |||
| Luvic phaeozems | |||
| Calcaric cambisols/regosols/leptosols | |||
| Semi-arid | nd | [24] | |
| Mosses | Semi-arid; arid | nd | [22,25] |
| Semi-arid to dry sub-humid | Arenitic | [23] | |
| Luvic litosols | |||
| Leptic chernozems | |||
| Litosols | |||
| Leptic chernozems | |||
| Litosols | |||
| Dolomitic | |||
| Calcic luvisols; humic regosols | |||
| Arenic fluvisolsLeptic chernozems | |||
| Luvic phaeozems | |||
| Calcaric cambisols/regosols/leptosols | |||
| Calcaric regosols |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duran, P.; Mora, M.d.l.L.; Matus, F.; Barra, P.J.; Jofré, I.; Kuzyakov, Y.; Merino, C. Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment. Biology 2021, 10, 1190. https://doi.org/10.3390/biology10111190
Duran P, Mora MdlL, Matus F, Barra PJ, Jofré I, Kuzyakov Y, Merino C. Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment. Biology. 2021; 10(11):1190. https://doi.org/10.3390/biology10111190
Chicago/Turabian StyleDuran, Paola, María de la Luz Mora, Francisco Matus, Patricio Javier Barra, Ignacio Jofré, Yakov Kuzyakov, and Carolina Merino. 2021. "Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment" Biology 10, no. 11: 1190. https://doi.org/10.3390/biology10111190
APA StyleDuran, P., Mora, M. d. l. L., Matus, F., Barra, P. J., Jofré, I., Kuzyakov, Y., & Merino, C. (2021). Biological Crusts to Increase Soil Carbon Sequestration: New Challenges in a New Environment. Biology, 10(11), 1190. https://doi.org/10.3390/biology10111190