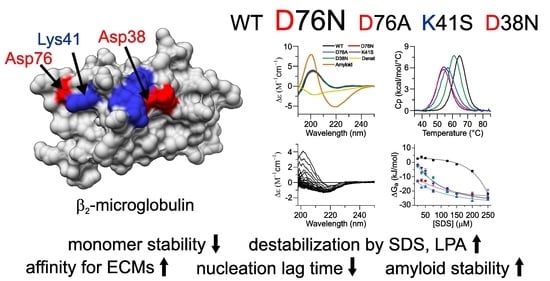
Pathogenic D76N Variant of β2-Microglobulin: Synergy of Diverse Effects in Both the Native and Amyloid States
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Constructs, Protein Expression, and Purification
2.2. Differential Scanning Calorimetry (DSC)
2.3. Circular Dichroism (CD) Spectroscopy
2.4. Thioflavin T Fluorescence Assay
2.5. Equilibrium Monomer Concentrations—Intrinsic Trp Fluorescence
2.6. Conformational Stability of the Native State of β2m Studied by GdnHCl Denaturation
2.7. Conformational Stability of β2m Amyloid Fibrils
2.7.1. Calculation of Amyloid Fibril Stability from the Equilibrium Monomer Concentration
2.7.2. Stability of Amyloid Fibrils against GdnHCl Denaturation
2.8. ELISA Assay for β2m Binding to Extracellular Matrix Proteins
3. Results and Discussion
3.1. The Role of the Ion-Pairs in the Stability of Native β2m
3.2. The Effect of SDS and LPA on the Structure of β2m Monomers
3.3. Interactions of β2m Variants with Extracellular Matrix Proteins
3.4. Amyloid Fibril Formation of β2m Variants Studied by ThT Fluorescence
3.4.1. Amyloid Fibril Growth Induced by SDS and LPA in the Presence of Seeds
3.4.2. The Effect of Polyphosphate and SDS on Nucleation of Amyloid Formation
3.5. Equilibrium Monomer Concentration of the Fibril Solutions
3.6. Stability of β2m Amyloid Fibrils Investigated by GdnHCl Denaturation
3.7. Synergy of Diverse Effects Is behind the Amyloidogenicity of D76N β2m, Causing Hereditary Systemic Amyloidosis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eisenberg, D.; Jucker, M. The amyloid state of proteins in human diseases. Cell 2012, 148, 1188–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepys, M.B. Pathogenesis, diagnosis and treatment of systemic amyloidosis. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 203–210, discussion 210–201. [Google Scholar] [CrossRef] [PubMed]
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatami, A.; Monjazeb, S.; Milton, S.; Glabe, C.G. Familial Alzheimer’s Disease Mutations within the Amyloid Precursor Protein Alter the Aggregation and Conformation of the Amyloid-beta Peptide. J. Biol. Chem. 2017, 292, 3172–3185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic amyloidosis from A (AA) to T (ATTR): A review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef] [PubMed]
- Ma’arfi, F.; Chandra, S.; Fatima, J.E.; Khan, M.Y.; Mir, S.S.; Yusuf, M.A. Probing the Structure-Function relationship and amyloidogenic propensities in natural variants of apolipoprotein A-I. Biochem. Biophys. Rep. 2020, 24, 100815. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Esposto, J.; Golec, C.; Wu, C.; Martic-Milne, S. Aggregation of gelsolin wild-type and G167K/R, N184K, and D187N/Y mutant peptides and inhibition. Mol. Cell. Biochem. 2021, 476, 2393–2408. [Google Scholar] [CrossRef]
- Perni, M.; van der Goot, A.; Limbocker, R.; van Ham, T.J.; Aprile, F.A.; Xu, C.K.; Flagmeier, P.; Thijssen, K.; Sormanni, P.; Fusco, G.; et al. Comparative Studies in the A30P and A53T α-Synuclein C. elegans Strains to Investigate the Molecular Origins of Parkinson’s Disease. Front. Cell Dev. Biol. 2021, 9, 552549. [Google Scholar] [CrossRef]
- Buell, A.K.; Tartaglia, G.G.; Birkett, N.R.; Waudby, C.A.; Vendruscolo, M.; Salvatella, X.; Welland, M.E.; Dobson, C.M.; Knowles, T.P. Position-dependent electrostatic protection against protein aggregation. Chembiochem. A Eur. J. Chem. Biol. 2009, 10, 1309–1312. [Google Scholar] [CrossRef]
- Chiti, F.; Stefani, M.; Taddei, N.; Ramponi, G.; Dobson, C.M. Rationalization of the effects of mutations on peptide and protein aggregation rates. Nature 2003, 424, 805–808. [Google Scholar] [CrossRef]
- Ferrao-Gonzales, A.D.; Palmieri, L.; Valory, M.; Silva, J.L.; Lashuel, H.; Kelly, J.W.; Foguel, D. Hydration and packing are crucial to amyloidogenesis as revealed by pressure studies on transthyretin variants that either protect or worsen amyloid disease. J. Mol. Biol. 2003, 328, 963–974. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.; Teoh, C.L.; Yang, S.; Zlatic, C.O.; Rosenes, Z.K.; Gooley, P.R.; Howlett, G.J.; Griffin, M.D. Charge and charge-pair mutations alter the rate of assembly and structural properties of apolipoprotein C-II amyloid fibrils. Biochemistry 2015, 54, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Valleix, S.; Verona, G.; Jourde-Chiche, N.; Nedelec, B.; Mangione, P.P.; Bridoux, F.; Mange, A.; Dogan, A.; Goujon, J.M.; Lhomme, M.; et al. D25V apolipoprotein C-III variant causes dominant hereditary systemic amyloidosis and confers cardiovascular protective lipoprotein profile. Nat. Commun. 2016, 7, 10353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nostrand, W.E.; Melchor, J.P.; Cho, H.S.; Greenberg, S.M.; Rebeck, G.W. Pathogenic effects of D23N Iowa mutant amyloid beta -protein. J. Biol. Chem. 2001, 276, 32860–32866. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Meisl, G.; Frohm, B.; Thulin, E.; Knowles, T.P.J.; Linse, S. On the role of sidechain size and charge in the aggregation of Aβ42 with familial mutations. Proc. Natl. Acad. Sci. USA 2018, 115, E5849–E5858. [Google Scholar] [CrossRef] [Green Version]
- Zibaee, S.; Jakes, R.; Fraser, G.; Serpell, L.C.; Crowther, R.A.; Goedert, M. Sequence Determinants for Amyloid Fibrillogenesis of Human α-Synuclein. J. Mol. Biol. 2007, 374, 454–464. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering Beta-2 Microglobulin as a Biomarker across the Spectrum of Kidney Diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Dong, M.; Wang, X.G. The Implication and Significance of Beta 2 Microglobulin: A Conservative Multifunctional Regulator. Chin. Med. J. 2016, 129, 448–455. [Google Scholar] [CrossRef]
- Gejyo, F.; Yamada, T.; Odani, S.; Nakagawa, Y.; Arakawa, M.; Kunitomo, T.; Kataoka, H.; Suzuki, M.; Hirasawa, Y.; Shirahama, T.; et al. A new form of amyloid protein associated with chronic hemodialysis was identified as β2-microglobulin. Biochem. Biophys. Res. Commun. 1985, 129, 701–706. [Google Scholar] [CrossRef]
- Valleix, S.; Gillmore, J.D.; Bridoux, F.; Mangione, P.P.; Dogan, A.; Nedelec, B.; Boimard, M.; Touchard, G.; Goujon, J.M.; Lacombe, C.; et al. Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N. Engl. J. Med. 2012, 366, 2276–2283. [Google Scholar] [CrossRef]
- Halabelian, L.; Ricagno, S.; Giorgetti, S.; Santambrogio, C.; Barbiroli, A.; Pellegrino, S.; Achour, A.; Grandori, R.; Marchese, L.; Raimondi, S.; et al. Class I major histocompatibility complex, the trojan horse for secretion of amyloidogenic β2-microglobulin. J. Biol. Chem. 2014, 289, 3318–3327. [Google Scholar] [CrossRef] [Green Version]
- Mangione, P.P.; Esposito, G.; Relini, A.; Raimondi, S.; Porcari, R.; Giorgetti, S.; Corazza, A.; Fogolari, F.; Penco, A.; Goto, Y.; et al. Structure, folding dynamics, and amyloidogenesis of D76N β2-microglobulin: Roles of shear flow, hydrophobic surfaces, and alpha-crystallin. J. Biol. Chem. 2013, 288, 30917–30930. [Google Scholar] [CrossRef] [Green Version]
- Cornwell, O.; Ault, J.R.; Bond, N.J.; Radford, S.E.; Ashcroft, A.E. Investigation of D76N β2-Microglobulin Using Protein Footprinting and Structural Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2021, 32, 1583–1592. [Google Scholar] [CrossRef]
- De Rosa, M.; Barbiroli, A.; Giorgetti, S.; Mangione, P.P.; Bolognesi, M.; Ricagno, S. Decoding the Structural Bases of D76N ss2-Microglobulin High Amyloidogenicity through Crystallography and Asn-Scan Mutagenesis. PLoS ONE 2015, 10, e0144061. [Google Scholar] [CrossRef] [Green Version]
- Faravelli, G.; Raimondi, S.; Marchese, L.; Partridge, F.A.; Soria, C.; Mangione, P.P.; Canetti, D.; Perni, M.; Aprile, F.A.; Zorzoli, I.; et al. C. elegans expressing D76N β2-microglobulin: A model for in vivo screening of drug candidates targeting amyloidosis. Sci. Rep. 2019, 9, 19960. [Google Scholar] [CrossRef]
- Leri, M.; Bemporad, F.; Oropesa-Nunez, R.; Canale, C.; Calamai, M.; Nosi, D.; Ramazzotti, M.; Giorgetti, S.; Pavone, F.S.; Bellotti, V.; et al. Molecular insights into cell toxicity of a novel familial amyloidogenic variant of β2-microglobulin. J. Cell. Mol. Med. 2016, 20, 1443–1456. [Google Scholar] [CrossRef]
- Natalello, A.; Mangione, P.P.; Giorgetti, S.; Porcari, R.; Marchese, L.; Zorzoli, I.; Relini, A.; Ami, D.; Faravelli, G.; Valli, M.; et al. Co-fibrillogenesis of Wild-type and D76N β2-Microglobulin: The Crucial Role of Fibrillar Seeds. J. Biol. Chem. 2016, 291, 9678–9689. [Google Scholar] [CrossRef] [Green Version]
- Le Marchand, T.; de Rosa, M.; Salvi, N.; Sala, B.M.; Andreas, L.B.; Barbet-Massin, E.; Sormanni, P.; Barbiroli, A.; Porcari, R.; Sousa Mota, C.; et al. Conformational dynamics in crystals reveal the molecular bases for D76N beta-2 microglobulin aggregation propensity. Nat. Commun. 2018, 9, 1658. [Google Scholar] [CrossRef] [Green Version]
- Iadanza, M.G.; Silvers, R.; Boardman, J.; Smith, H.I.; Karamanos, T.K.; Debelouchina, G.T.; Su, Y.; Griffin, R.G.; Ranson, N.A.; Radford, S.E. The structure of a β2-microglobulin fibril suggests a molecular basis for its amyloid polymorphism. Nat. Commun. 2018, 9, 4517. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, D.; Nomura, R.; Mangione, P.P.; Hasegawa, K.; Okoshi, T.; Porcari, R.; Bellotti, V.; Naiki, H. Multifaceted anti-amyloidogenic and pro-amyloidogenic effects of C-reactive protein and serum amyloid P component in vitro. Sci. Rep. 2016, 6, 29077. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, S.; Porcari, R.; Mangione, P.P.; Verona, G.; Marcoux, J.; Giorgetti, S.; Taylor, G.W.; Ellmerich, S.; Ballico, M.; Zanini, S.; et al. A specific nanobody prevents amyloidogenesis of D76N beta2-microglobulin in vitro and modifies its tissue distribution in vivo. Sci. Rep. 2017, 7, 46711. [Google Scholar] [CrossRef] [Green Version]
- Relini, A.; De Stefano, S.; Torrassa, S.; Cavalleri, O.; Rolandi, R.; Gliozzi, A.; Giorgetti, S.; Raimondi, S.; Marchese, L.; Verga, L.; et al. Heparin strongly enhances the formation of β2-microglobulin amyloid fibrils in the presence of type I collagen. J. Biol. Chem. 2008, 283, 4912–4920. [Google Scholar] [CrossRef] [Green Version]
- So, M.; Hata, Y.; Naiki, H.; Goto, Y. Heparin-induced amyloid fibrillation of beta2 -microglobulin explained by solubility and a supersaturation-dependent conformational phase diagram. Protein Sci. A Publ. Protein Soc. 2017, 26, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Giorgetti, S.; Rossi, A.; Mangione, P.; Raimondi, S.; Marini, S.; Stoppini, M.; Corazza, A.; Viglino, P.; Esposito, G.; Cetta, G.; et al. Beta2-microglobulin isoforms display an heterogeneous affinity for type I collagen. Protein Sci. A Publ. Protein Soc. 2005, 14, 696–702. [Google Scholar] [CrossRef]
- Ookoshi, T.; Hasegawa, K.; Ohhashi, Y.; Kimura, H.; Takahashi, N.; Yoshida, H.; Miyazaki, R.; Goto, Y.; Naiki, H. Lysophospholipids induce the nucleation and extension of beta2-microglobulin-related amyloid fibrils at a neutral pH. Nephrol. Dial. Transplant. 2008, 23, 3247–3255. [Google Scholar] [CrossRef] [Green Version]
- Pal-Gabor, H.; Gombos, L.; Micsonai, A.; Kovacs, E.; Petrik, E.; Kovacs, J.; Graf, L.; Fidy, J.; Naiki, H.; Goto, Y.; et al. Mechanism of lysophosphatidic acid-induced amyloid fibril formation of β(2)-microglobulin in vitro under physiological conditions. Biochemistry 2009, 48, 5689–5699. [Google Scholar] [CrossRef]
- Chiba, T.; Hagihara, Y.; Higurashi, T.; Hasegawa, K.; Naiki, H.; Goto, Y. Amyloid fibril formation in the context of full-length protein: Effects of proline mutations on the amyloid fibril formation of beta2-microglobulin. J. Biol. Chem. 2003, 278, 47016–47024. [Google Scholar] [CrossRef] [Green Version]
- Pace, C.N. Measuring and increasing protein stability. Trends Biotechnol. 1990, 8, 93–98. [Google Scholar] [CrossRef]
- Baldwin, A.J.; Knowles, T.P.; Tartaglia, G.G.; Fitzpatrick, A.W.; Devlin, G.L.; Shammas, S.L.; Waudby, C.A.; Mossuto, M.F.; Meehan, S.; Gras, S.L.; et al. Metastability of native proteins and the phenomenon of amyloid formation. J. Am. Chem. Soc. 2011, 133, 14160–14163. [Google Scholar] [CrossRef]
- Kardos, J.; Micsonai, A.; Pal-Gabor, H.; Petrik, E.; Graf, L.; Kovacs, J.; Lee, Y.H.; Naiki, H.; Goto, Y. Reversible heat-induced dissociation of beta2-microglobulin amyloid fibrils. Biochemistry 2011, 50, 3211–3220. [Google Scholar] [CrossRef]
- Narimoto, T.; Sakurai, K.; Okamoto, A.; Chatani, E.; Hoshino, M.; Hasegawa, K.; Naiki, H.; Goto, Y. Conformational stability of amyloid fibrils of beta2-microglobulin probed by guanidine-hydrochloride-induced unfolding. FEBS Lett. 2004, 576, 313–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oosawa, F.; Kasai, M. A theory of linear and helical aggregations of macromolecules. J. Mol. Biol. 1962, 4, 10–21. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Karlin, S. Clusters of charged residues in protein three-dimensional structures. Proc. Natl. Acad. Sci. USA 1996, 93, 8350–8355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micsonai, A.; Bulyaki, E.; Kardos, J. BeStSel: From Secondary Structure Analysis to Protein Fold Prediction by Circular Dichroism Spectroscopy. Methods Mol. Biol. 2021, 2199, 175–189. [Google Scholar] [CrossRef]
- Micsonai, A.; Wien, F.; Bulyaki, E.; Kun, J.; Moussong, E.; Lee, Y.H.; Goto, Y.; Refregiers, M.; Kardos, J. BeStSel: A web server for accurate protein secondary structure prediction and fold recognition from the circular dichroism spectra. Nucleic Acids Res. 2018, 46, W315–W322. [Google Scholar] [CrossRef]
- Kardos, J.; Yamamoto, K.; Hasegawa, K.; Naiki, H.; Goto, Y. Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J. Biol. Chem. 2004, 279, 55308–55314. [Google Scholar] [CrossRef] [Green Version]
- Makhatadze, G.I.; Privalov, P.L. Energetics of protein structure. Adv. Protein Chem. 1995, 47, 307–425. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hasegawa, K.; Yamaguchi, I.; Tsutsumi, S.; Kardos, J.; Goto, Y.; Gejyo, F.; Naiki, H. Low concentrations of sodium dodecyl sulfate induce the extension of beta 2-microglobulin-related amyloid fibrils at a neutral pH. Biochemistry 2004, 43, 11075–11082. [Google Scholar] [CrossRef]
- Naiki, H.; Yamamoto, S.; Hasegawa, K.; Yamaguchi, I.; Goto, Y.; Gejyo, F. Molecular interactions in the formation and deposition of beta2-microglobulin-related amyloid fibrils. Amyloid 2005, 12, 15–25. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kazama, J.J.; Narita, I.; Naiki, H.; Gejyo, F. Recent progress in understanding dialysis-related amyloidosis. Bone 2009, 45 (Suppl. S1), S39–S42. [Google Scholar] [CrossRef]
- Jirgensons, B. Conformational transitions of non-helical proteins effected by dodecyl sulfate. Circular dichroism of alpha1-acid glycoprotein, Bence Jones protein, carbonic anhydrase B, deoxyribonuclease, pepsinogen, and plasminogen. Biochim. Biophys. Acta 1976, 434, 58–68. [Google Scholar] [CrossRef]
- Relini, A.; Canale, C.; De Stefano, S.; Rolandi, R.; Giorgetti, S.; Stoppini, M.; Rossi, A.; Fogolari, F.; Corazza, A.; Esposito, G.; et al. Collagen plays an active role in the aggregation of β2-microglobulin under physiopathological conditions of dialysis-related amyloidosis. J. Biol. Chem. 2006, 281, 16521–16529. [Google Scholar] [CrossRef] [Green Version]
- Stoppini, M.; Bellotti, V. Systemic amyloidosis: Lessons from β2-microglobulin. J. Biol. Chem. 2015, 290, 9951–9958. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.M.; Yamaguchi, K.; So, M.; Sasahara, K.; Ito, T.; Yamamoto, S.; Narita, I.; Kardos, J.; Naiki, H.; Goto, Y. Possible mechanisms of polyphosphate-induced amyloid fibril formation of β2-microglobulin. Proc. Natl. Acad. Sci. USA 2019, 116, 12833–12838. [Google Scholar] [CrossRef] [Green Version]
- Ikenoue, T.; Lee, Y.H.; Kardos, J.; Saiki, M.; Yagi, H.; Kawata, Y.; Goto, Y. Cold denaturation of alpha-synuclein amyloid fibrils. Angew. Chem. 2014, 53, 7799–7804. [Google Scholar] [CrossRef]
- Ikenoue, T.; Lee, Y.H.; Kardos, J.; Yagi, H.; Ikegami, T.; Naiki, H.; Goto, Y. Heat of supersaturation-limited amyloid burst directly monitored by isothermal titration calorimetry. Proc. Natl. Acad. Sci. USA 2014, 111, 6654–6659. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, K.; Tomiyama, R. Enhanced accessibility and hydrophobicity of amyloidogenic intermediates of the beta2-microglobulin D76N mutant revealed by high-pressure experiments. J. Biol. Chem. 2021, 296, 100333. [Google Scholar] [CrossRef]
- Sakurai, K.; Tomiyama, R.; Shiraki, T.; Yonezawa, Y. Loosening of Side-Chain Packing Associated with Perturbations in Peripheral Dynamics Induced by the D76N Mutation of β2-Microglobulin Revealed by Pressure-NMR and Molecular Dynamic Simulations. Biomolecules 2019, 9, 491. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.I.; Guthertz, N.; Cawood, E.E.; Maya-Martinez, R.; Breeze, A.L.; Radford, S.E. The role of the IT-state in D76N beta2-microglobulin amyloid assembly: A crucial intermediate or an innocuous bystander? J. Biol. Chem. 2020, 295, 12474–12484. [Google Scholar] [CrossRef]
- Naiki, H.; Okoshi, T.; Ozawa, D.; Yamaguchi, I.; Hasegawa, K. Molecular pathogenesis of human amyloidosis: Lessons from beta2 -microglobulin-related amyloidosis. Pathol. Int. 2016, 66, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Yamaguchi, I.; Hasegawa, K.; Tsutsumi, S.; Goto, Y.; Gejyo, F.; Naiki, H. Glycosaminoglycans enhance the trifluoroethanol-induced extension of beta 2-microglobulin-related amyloid fibrils at a neutral pH. J. Am. Soc. Nephrol. 2004, 15, 126–133. [Google Scholar] [CrossRef]
- Hasegawa, K.; Tsutsumi-Yasuhara, S.; Ookoshi, T.; Ohhashi, Y.; Kimura, H.; Takahashi, N.; Yoshida, H.; Miyazaki, R.; Goto, Y.; Naiki, H. Growth of β2-microglobulin-related amyloid fibrils by non-esterified fatty acids at a neutral pH. Biochem. J. 2008, 416, 307–315. [Google Scholar] [CrossRef]
- Fogolari, F.; Corazza, A.; Yarra, V.; Jalaru, A.; Viglino, P.; Esposito, G. Bluues: A program for the analysis of the electrostatic properties of proteins based on generalized Born radii. BMC Bioinform. 2012, 13 (Suppl. S4), S18. [Google Scholar] [CrossRef] [Green Version]








| Protein | Tm (°C) a | ΔHv (kJ/mol) b | Thermal Denat. ΔGN-D (kJ/mol) c | GdnHCl Denat. ΔGN-D (kJ/mol) d |
|---|---|---|---|---|
| WT | 65.5 (±0.02) | 367.0 (±1.4) | 24.0 (±0.5) | 18.9 (±0.8) |
| D76N | 55.9 (±0.03) | 283.2 (±2.5) | 13.2 (±1.6) | 12.0 (±0.5) |
| D76A | 58.3 (±0.04) | 283.1 (±3.2) | 14.3 (±0.2) | 11.1 (±1.3) |
| K41S | 55.8 (±0.07) | 311.9 (±7.3) | 14.8 (±0.5) | 11.3 (±1.0) |
| D38N | 61.3 (±0.09) | 329.0 (±2.7) | 18.8 (±0.3) | 16.9 (±1.7) |
| 250 μM SDS | 300 μM LPA | |||
|---|---|---|---|---|
| ΔGa0 (kJ/mol) | Cm (M) | ΔGa0 (kJ/mol) | Cm (M) | |
| WT | –17.3 (±0.5) | 3.5 | –11.7 (±0.2) | 3.5 |
| D76N | –22.1 (±0.3) | 4.8 | –23.6 (±0.7) | 4.8 |
| D76A | –24.2 (±1.8) | 4.7 | –25.6(±3.2) | 4.7 |
| K41S | –15.1 (±0.6) | 3.6 | –12.5 (±0.3) | 3.6 |
| D38N | –19.2 (±3.0) | 4.9 | –24.8 (±2.0) | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulyáki, É.; Kun, J.; Molnár, T.; Papp, A.; Micsonai, A.; Vadászi, H.; Márialigeti, B.; Kovács, A.I.; Gellén, G.; Yamaguchi, K.; et al. Pathogenic D76N Variant of β2-Microglobulin: Synergy of Diverse Effects in Both the Native and Amyloid States. Biology 2021, 10, 1197. https://doi.org/10.3390/biology10111197
Bulyáki É, Kun J, Molnár T, Papp A, Micsonai A, Vadászi H, Márialigeti B, Kovács AI, Gellén G, Yamaguchi K, et al. Pathogenic D76N Variant of β2-Microglobulin: Synergy of Diverse Effects in Both the Native and Amyloid States. Biology. 2021; 10(11):1197. https://doi.org/10.3390/biology10111197
Chicago/Turabian StyleBulyáki, Éva, Judit Kun, Tamás Molnár, Alexandra Papp, András Micsonai, Henrietta Vadászi, Borbála Márialigeti, Attila István Kovács, Gabriella Gellén, Keiichi Yamaguchi, and et al. 2021. "Pathogenic D76N Variant of β2-Microglobulin: Synergy of Diverse Effects in Both the Native and Amyloid States" Biology 10, no. 11: 1197. https://doi.org/10.3390/biology10111197
APA StyleBulyáki, É., Kun, J., Molnár, T., Papp, A., Micsonai, A., Vadászi, H., Márialigeti, B., Kovács, A. I., Gellén, G., Yamaguchi, K., Lin, Y., So, M., Józsi, M., Schlosser, G., Lee, Y. -H., Liliom, K., Goto, Y., & Kardos, J. (2021). Pathogenic D76N Variant of β2-Microglobulin: Synergy of Diverse Effects in Both the Native and Amyloid States. Biology, 10(11), 1197. https://doi.org/10.3390/biology10111197