Simple Summary
A subset of carcinomas that arise in the head and neck region show a viral etiology. In fact, a subgroup of oropharyngeal cancers are caused by some types of human papillomavirus (HPV), so-called high-risk (HR)-HPVs, whereas undifferentiated nasopharyngeal carcinomas are etiologically related to Epstein–Barr virus (EBV). However, studies have reported the presence of both HR-HPV and EBV in some types of head and neck cancers. In this review, we discuss the potential contribution and role of HR-HPV/EBV coinfection in head and neck carcinogenesis, as well as the mechanisms that are potentially involved. In addition, HR-HPV/EBV interaction models are proposed.
Abstract
High-risk human papillomaviruses (HR-HPVs) and Epstein–Barr virus (EBV) are recognized oncogenic viruses involved in the development of a subset of head and neck cancers (HNCs). HR-HPVs are etiologically associated with a subset of oropharyngeal carcinomas (OPCs), whereas EBV is a recognized etiological agent of undifferentiated nasopharyngeal carcinomas (NPCs). In this review, we address epidemiological and mechanistic evidence regarding a potential cooperation between HR-HPV and EBV for HNC development. Considering that: (1) both HR-HPV and EBV infections require cofactors for carcinogenesis; and (2) both oropharyngeal and oral epithelium can be directly exposed to carcinogens, such as alcohol or tobacco smoke, we hypothesize possible interaction mechanisms. The epidemiological and experimental evidence suggests that HR-HPV/EBV cooperation for developing a subset of HNCs is plausible and warrants further investigation.
1. Introduction
Head and neck cancers (HNCs) encompass tumors of a variety of subsites, including the lips, oral cavity, nasopharynx, oropharynx, hypopharynx, larynx, and salivary glands. Head and neck squamous cell carcinomas (HNSCCs) constitute more than 90% of all HNCs [1]. These malignancies were the sixth leading cancer worldwide, with 931,931 new cases in 2020, excluding non-melanoma skin neoplasms. Additionally, HNCs represented the seventh highest cause of cancer-related deaths in the same year, with 467,125 deaths [2]. Alcohol consumption and tobacco smoking are the two most important risk factors for HNC development. Infection with oncogenic viruses, such as the human papillomavirus (HPV) or the Epstein–Barr virus (EBV), have been reported to be associated with the development and progression of these neoplasms. In fact, high-risk (HR)-HPV infection is a risk factor for developing a subset of oropharyngeal tumors, including the tonsils and the base of the tongue [3]. Similarly, EBV infection is related to the development of nasopharyngeal (NPC) and salivary gland cancers [4]. However, neither HR-HPV nor EBV infection alone is a sufficient condition for tumorigenesis. In most immunocompetent subjects, HPV infection is ultimately controlled [5], whereas EBV is present during the lifetime of the individual without any clinical manifestation, all of which suggesting that additional cofactors are required for promoting HR-HPV or EBV-driven cancers. Indeed, some studies have reported the simultaneous presence of HR-HPV and EBV in HNCs [6,7,8], proposing that HR-HPV/EBV coinfection could play a fundamental role in the development of these malignancies [6,9,10]. However, potential mechanisms involved in such HPV/EBV cooperation need further clarification. Here, we review the current literature regarding HR-HPV/EBV coinfection in HNSCCs, as well as its potential contribution to carcinogenesis and the progression of these tumors. Finally, we propose a mechanism by which HR-HPV-mediated alterations facilitate EBV infection in epithelial tissues.
2. Human Papillomavirus and Epstein–Barr Virus Tropism
Epithelial tissues are susceptible and permissive to both HPV (cutaneous and mucosal) [11] and EBV (mucosal) infections [12]. Basal epithelial cells are susceptible to HPV infection, although only upper highly differentiated cells in the stratified epithelium are permissive [13,14]. HPV entry into epithelial cells requires the involvement of heparan sulphate proteoglycans (HSPGs) for initial tethering to basal cells [15]. Integrins (α6 and α3) and CD151 are also required to form an “entry receptor complex” with annexin A2, CD63, and EGFR/GFR (reviewed in [16]). Interestingly, multiple studies have reported that HPV can regulate integrin levels. For instance, Werner et al. (2011) reported increased levels of αv, α5, β1, β4, and β6 integrins in HPV-positive cervical cells when compared with non-tumor cells, which was attributable to HPV infection [17]. Overexpression of HPV16 E6*, the smaller splice isoform of the E6 oncogene, increased the levels of the β1 integrin in cervical cancer cells [18]. A 2.44-fold increase in the expression of the ITGB5 gene, which encodes the β5 integrin subunit, was reported in cervical cells upon L2 overexpression [19]. In contrast, it was reported that HPV38 E6/E7 proteins downregulate the expression of αvβ8 integrin in human keratinocytes [20].
EBV exhibits a similar epithelial tropism, although EBV cell entry occurs through both apical and basolateral routes in mucosal epithelia [21], and this virus shows an additional and preferential tropism for B cells, which constitute the EBV reservoir [22]. EBV lytic infections occur in highly differentiated cells, as demonstrated by Temple et al. (2014) [23]. EBV entry to epithelial cells is mediated by the ephrin receptor tyrosine kinase A2 (EphA2). Furthermore, the increased expression of EphA2 leads to a more efficient EBV infection of epithelial cells [24,25]. Of note, elevated EphA2 expression was associated with progression and poor prognosis in HNSCC patients [26]. Notably, some EBV-encoded proteins (e.g., LMP1) can regulate the expression of the Eph family [27]. Studies by Chesnokova et al. suggested that the interaction of EBV glycoproteins gH/gL with αvβ5, αvβ6, and αvβ8 integrins is associated with EBV fusion with gastric carcinoma cells [28,29], though Chen et al. (2018) reported a lack of effect of these surface integrins on EBV entry in HEK293 cells [24]. Interestingly, the expression of αvβ5 integrin was significantly increased in oral and laryngeal SCCs when compared to normal counterparts [30,31]. In addition, αvβ6 integrin was evidenced in almost all HNCs, including oral, oropharyngeal, hypopharyngeal, laryngeal, and nasopharyngeal carcinomas, but not in normal oral and tongue mucosa [32]. The expression of αvβ8 integrin was also found to be increased in laryngeal carcinoma compared with normal tissues [31]. A comparison between HPV and EBV tropism is shown in Table 1.

Table 1.
Comparison between HPV and EBV epithelial cell entry.
3. Epidemiology of HPV/EBV Coinfection in HNSCCs
As in epidemiological studies, the cellular location of viruses is generally not considered, and the term “copresence” is assumed when HPV and EBV are both detected in tumors. However, copresence in tumors is not synonymous with coinfection, which occurs when both HPV and EBV infects the same epithelial cell. Molecular methodologies, such as a polymerase chain reaction (PCR), cannot determine the coinfection or coexpression of viral products, although this test is frequently used in epidemiological studies. HPV/EBV copresence has been evidenced in 31.2% of HNCs, which includes larynx, lip, and nasopharynx malignancies [9]. Similarly, Drop et al. found HPV/EBV copresence in 34.1% of oral cavity, oropharyngeal, and laryngeal tumors [6], and EBV infection was significantly associated with HPV16, 18, 45, and 58 presence in HNSCCs [10]. Remarkably, the expression of both LMP1 and E6 oncoproteins in HNCs was associated with an increased cell invasiveness and advanced tumor stage [9,10]. HPV/EBV copresence was also associated with alcohol abuse and an increased histological grade of HNCs, although it was more frequent in early-stage disease [6]. In the following subsections, we will analyze the HPV/EBV copresence in HNCs from different anatomical sites.
3.1. Oral Cavity
More than 90% of oral tumors are squamous cell carcinomas (OSCCs), which arise from the mucosal epithelium lining the lips, anterior tongue, gingivae, floor of mouth, palate, and other areas of the mouth [38]. In 2020, there were an estimated 377,713 new cases and 177,757 deaths from lip and oral cavity tumors worldwide [2]. Tobacco smoking and alcohol consumption are the two most important risk factors associated with oral cancer development. Additionally, the involvement of some viral infections has also been suggested in oral carcinogenesis (reviewed in [39]). HPV infection in OSCCs ranges between 6% and 58% worldwide [40,41,42]. A meta-analysis including 62 studies and 4852 OSCCs found that 38.1% of cancers contained HPV DNA [43]. It was also discovered that HPV infection increased the probability of OSCC development in the Chinese population (OR 12.7, 95% CI: 8.0–20.0) by more than 12 times [44]. However, a recent meta-analysis published by Melo et al. (2021) found E6/E7 mRNA expression in only 4.4% of OSCCs [45]. HPV16 and 18 are the most frequent HPV genotypes detected in these tumors [45,46]. In addition, the presence of HPV16 has been related to an increased histological grade of oral tumors [47]. Meanwhile, EBV infection has been detected in 25.9% to 82.5% of OSCCs [48,49,50]. EBNA2 and LMP1 were also detected in 50.2% and 10.7% of OSCCs, respectively [51]. In particular, the expression of LMP1 was increased in OSCCs when compared to normal mucosa and oral leukoplakia [52]. She et al. (2017) conducted a meta-analysis that included 13 studies published between 1995 and 2016. In this report, OSCC development was shown to be 5 times more likely to occur in the presence of EBV (OR 5.03, 95% CI: 1.80–14.01) [53]. Another meta-analysis also found an association between EBV infection and OSCC. In fact, this study reported that the probability of OSCC development was 2.5 (OR 2.5, 95% CI: 1.2–5.36) times increased in EBV-positive cases [54]. Regarding HPV/EBV coinfection, this was evidenced in 6.5–37.5% of OSCCs [6,10,55,56]. In one study, which included 155 OSCCs from eight countries, HPV/EBV was detected in 21% of cases, making it the most frequent coinfection in these tumors [55]. The coexpression of HR-HPV E6 and LMP1 was also demonstrated in OSCCs by immunohistochemistry (IHC) [10]. In particular, HPV16 and EBV copresence was detected in 5.6% of cases [57]. Moreover, EBV infection was associated with HR-HPV16, 18, 31, 35, 45, 51, and 58 [10]. Conversely, an absence of EBV/HPV copresence has also been reported in oral cancers [58,59].
3.2. Nasopharynx
Nasopharyngeal carcinoma (NPC) is a HNC with an estimated 133,354 new cases and 80,008 deaths in 2020 [2]. NPC usually exhibits a heterogeneous racial and geographic distribution. Its frequency is high in Southern China, intermediate in Southeast Asia and Northern Africa, and low in most areas of the word, with 15–20.5 and <1 per 100,000 habitants, respectively [60]. According to the World Health Organization (WHO) pathologic classification, NPCs are divided into keratinizing squamous cell carcinoma (WHO type I) and non-keratinizing carcinoma (WHO types II and III) [61]. In endemic areas, the keratinizing subtype is relatively rare, whereas the non-keratinizing subtype can reach more than 99% of total cases [62,63]. Diverse factors, such as salted fish consumption, smoking, alcohol consumption, air pollution exposure (e.g., NO2), and genetic susceptibility, are among the risk factors related to NPC development [64,65,66]. EBV is a well-established etiological agent of undifferentiated (non-keratinizing) NPC [67,68,69], which is detected in almost 100% of cases [70,71]. Overall, 57.6% to 100% of NPCs are EBV-positive [72,73,74,75,76]. A meta-analysis reported that EBV prevalence was higher in WHO type II/III NPC compared to WHO type I tumors (83.2% vs. 21.3%) [77]. Importantly, increased levels of plasma EBV DNA are related to a more aggressive biological behavior of this cancer [78,79,80,81]. However, an association was reported between the EBV viral load and a better prognosis in NPC patients [82]. The expression of LMP1, a latent EBV protein, was associated with the TNM stage and lymph node metastasis in NPC patients [61]. In a meta-analysis conducted by Zhao et al. (2012), the occurrence of metastasis in NPC was 1.98 times increased in the presence of LMP1 (OR 1.98, 95% CI: 1.38–2.83) [83]. On the other hand, Bam HI rightward Frame 1 (BARF1), which is considered an exclusively epithelial oncoprotein, is detected in 69–100% of NPCs [84]. Regarding HPV infection, the pooled prevalence worldwide was 21%, and it increased 4.77-fold in tumor samples compared to control cases [85]. HPV16 and HPV18 are the most common genotypes detected in HPV-positive NPCs [77]. Of note, an increased prevalence of HR-HPV was evidenced in WHO type I NPC when compared with WHO type II/III cases (39.9% vs. 23.3%) [77]. Moreover, patients with HPV-positive tumors displayed a decreased overall survival, progression-free survival, and locoregional control when compared to exclusively EBV positive patients [86]. In the meta-analysis conducted by Tham et al. (2020), an increased frequency of HPV/EBV copresence was found in WHO type I NPCs when compared with WHO type II/III tumors (7.6% vs. 1.0%) [77]. In addition, HPV/EBV copresence was evidenced mainly in NPC-endemic regions, ranging from 15% to 47.7% of the tumors [8,87,88]. Of note, about 80% of HPV16 or HPV18-positive NPCs were also positive for EBV DNA [87]. The overexpression of p16, a surrogate biomarker of oncogenic HPV infection, was associated with an improved progression-free survival and locoregional control in EBV-positive NPCs [89]. However, in non-endemic areas, HPV/EBV coinfection is less common. Indeed, some authors reported that HPV and EBV infections are mutually exclusive in populations with a low NPC incidence [86,90].
3.3. Oropharynx
An estimated 98,412 new cases and 48,143 cancer deaths worldwide were attributable to oropharyngeal tumors in 2020 [2]. Most oropharyngeal tumors are squamous cell carcinomas (OPSCC), which arise from epithelial cells lining the oropharynx [91]. Etiologically, two different types of OPSCCs have been described: HPV-driven and HPV-negative carcinomas, characterized by a distinct biological and clinical behavior [92]. In fact, patients with HPV-driven OPSCCs usually show a better prognosis compared to those with HPV-negative tumors [93,94]. Furthermore, patients with HPV16-driven OPSCC showed an increased overall survival rate when compared to patients with non-HPV16-positive carcinomas [95]. HPV-negative OPSCCs are mainly related to other risk factors, such as tobacco smoke and alcohol consumption [96]. The prevalence of HPV in OPSCC displays a geographic heterogeneity. For instance, a systematic review showed HPV positivity ranging from 18% to 65% in OPSCC patients from European countries [97]. In addition, Mariz et al. (2020) found a 44.8% pooled prevalence of HPV-driven OPSCC, with higher percentages found in New Zealand (74.5%), Sweden (70.0%), and Denmark (61.7%) when compared with the Netherlands (30.3%), Germany (25.0%), and Brazil (11.1%) [98]. Regarding the oropharynx subsites, HPV infection is more frequent in OPSCCs from the base of the tongue (40%) and tonsils (56%) when compared with other sites, such as the soft palate (12%) and posterior wall (19%) [99]. HPV16 is the most frequent genotype detected in OPSCC, reaching 88% of HPV-positive cases [100]. However, other authors reported significant differences in the prevalence of HPV16-positive OPSCCs in the United States (59.3%) and Europe (31.1%) in comparison with Brazil (4.1%) [101]. Other genotypes, such as HPV18, HPV33, HPV35, and HPV58, have also been detected, although only in a small percentage of cases [102]. Interestingly, EBV infection was evidenced in 53.3–86% of OPSCCs [56,103], suggesting its contribution to carcinogenesis. In normal oropharyngeal tissues, an absence of HPV/EBV coinfection was evidenced [7]. Conversely, the copresence of HPV and EBV was detected in 20% and 25% of tonsillar and base of tongue SCCs, respectively, while the absence of HPV/EBV coinfection in soft palate tumors was reported [7]. Similarly, Drop et al. identified HPV/EBV copresence in 28.6% of oropharyngeal tumors [6], whereas other authors only found HPV/EBV infections in 8–9% [56]. Additionally, Broccolo et al. reported HPV infection in 25% of OPSCCs, whereas EBV infection was found in 45% of tumors. However, coinfection was only evidenced in 4% of OPSCCs [58].
3.4. HPV and EBV Infection in Other HNSCCs
HNCs also include tumors arising from the hypopharynx, larynx, paranasal sinuses, nasal cavity, and salivary glands. However, data regarding HPV/EBV coinfection in these neoplasms are scarce. HPV/EBV coinfection was reported in 25% and 33.3% of hypopharyngeal and paranasal sinus carcinomas, respectively [104]. In addition, the copresence of HPV and EBV was evidenced in 12.8% [105] and 21.4% [6] of laryngeal carcinomas. Of interest, a three-fold increase was reported in the invasiveness properties of HPV+/EBV+ NOK and FaDu hypopharyngeal cells in the presence of lysophosphatidic acid (LPA) compared to HPV-/EBV- and HPV-/EBV+ cells [7]. Table 2 and Table 3 show meta-analysis data for HPV and EBV detection in HNSCCs.

Table 2.
HPV prevalence in HNSCC according to meta-analysis data.

Table 3.
EBV prevalence in HNSCCs.
4. Possible Mechanisms of HR-HPV/EBV Cooperation
4.1. HPV Facilitates EBV Entry in Epithelial Cells
The cluster of differentiation 21 (CD21), also known as the complement receptor type 2 (CR2), is considered to be the major attachment receptor for EBV in B cells [114]. However, CD21 is variably detected in epithelial cells. Interestingly, an increased CD21 expression was detected in the tonsil epithelium, comparable to what was detected in peripheral B cells, Burkitt lymphoma cells [115], and OSCCs [116]. Furthermore, a CD21 mRNA increase is associated with the grade of oral epithelial dysplasia, and correlates with the presence of EBER1 transcripts [116]. Additionally, epithelial cells ectopically expressing CD21 become susceptible and permissive to EBV infection [117,118]. In fact, both human keratinocytes and skin cancer cells were susceptible to EBV infection after CD21 gene transfection, whereas un-transfected cells remained EBV-negative [117]. CD21 gene-transfected epithelial cells also increase the expression of EBERs and EBNA1, along with a limited LMP1 expression [117,118], which resembles the EBV latency program detected in NPCs [119,120]. Low levels of CD21 expression are sufficient for leading to EBV infection in human epithelial cells [121]. Taken together, these findings suggest a role of CD21 in EBV entry into epithelial cells. Conversely, an absence of CD21 expression was evidenced in oral tongue and mucosa, soft palate, and the uvula [115], and a decreased CD21 expression was found in NPCs when compared to normal nasopharynx [122]. Interestingly, CD21 levels were associated with HPV and EBV infection in tonsillar and base of tongue carcinomas [7]. A high level of CD21 was found in HPV+/EBV+ NPCs, when compared to EBV-/HPV- tumors (p = 0.0194), although no difference was observed between HPV+/EBV+ NPCs and those harboring EBV or HPV single infections [7]. Furthermore, increased levels of EphA2, the epithelial EBV receptor, was evidenced in CIN and cervical carcinomas when compared to normal cervical epithelium, and also correlated with CDK6 protein expression [123]. In addition, a 15.28-fold increase in EphA2 receptor expression was observed in non-tumor cervical cells transfected with the HPV18 E2 gene [124]. Similarly, transfection with the HPV18 E6 gene resulted in a 3.2-fold increase in EphA2 gene expression in esophageal cancer cells [125]. These facts suggest that HPV could potentially facilitate EBV entry into head and neck epithelial cells by promoting increased levels of proteins involved in this process. Interestingly, the fact that persistent viruses promote the expression of molecules involved in the entry of another virus is not new. In fact, it was recently reported that EBV infection promotes increased levels of the angiotensin-converting enzyme 2 (ACE2) receptor, facilitating SARS-CoV-2 entry to epithelial cells [126]. However, further functional studies are warranted in order to validate this possibility.
4.2. HPV Infection Could Promote EBV Latency Establishment and Lytic Cycle Activation in Head and Neck Epithelial Cells
Cultures of normal epithelial cells in which the EBV lytic cycle is preferentially established appear unable to sustain latent EBV infection [51,127,128]. For instance, Temple et al. (2014) demonstrated that EBV establishes a predominantly productive infection in organotypic cultures of primary oral keratinocytes, characterized by the expression of latent cycle proteins (e.g., EBNA1, LMP1, EBNA2) and lytic cycle proteins (e.g., gB, gp350). Nevertheless, cells expressing exclusively latent proteins were not detected [23]. It is well-known that EBV lytic infection occurs in normal differentiated oral cells from immunosuppressed patients, causing oral hairy leukoplakia (OHL) [129]. Although the presence of EBV latent infection was reported in morphologically normal mucosa, these samples corresponded to tongue and gingival fibrous overgrowths, which are characterized by abnormal enlargement of tissues [51]. Accordingly, it was suggested that normal epithelial cells from the periodontium could be latently infected by EBV [130], but patients considered as “healthy donors” displayed mild periodontal disease (clinical attachment level 1 ≤ x < 3 mm) [131,132]. Additionally, the absence of EBER1 mRNA expression was observed in the normal epithelium adjacent to oral dysplasia [116]. Indeed, an EBV latent infection can be established in nasopharyngeal cells displaying previous genetic alterations, such as hTERT, cyclin D1, or Bmi-1 overexpression, as well as p16 inactivation [133,134,135]. These molecular changes are commonly detected in dysplastic epithelial cells [136,137], which are also more susceptible to EBV infection [116]. Interestingly, the pooled prevalence of HPV infection was 27.2% in oral epithelial dysplasia [138], whereas other authors found a 50% positivity in oral cavity and oropharyngeal dysplasia [139]. Guidry and Scott (2018) reported a significant reduction in EBV DNA replication in both HPV-positive and E7-immortalized human keratinocytes when compared with the EBV DNA replication observed in primary keratinocytes cultured in organotypic rafts [140], suggesting the contribution of HPV to the establishment of EBV latency. Later, a significant decrease in the expression of both EBV immediate early (BZLF1 and BRLF1) and early (BALF5 and BMRF1) genes was identified in HPV-positive tonsillar epithelial rafts when compared with HPV-negative rafts. In addition, a significant increase in the expression of EBER1 (a noncoding RNA highly expressed in latently EBV-infected cells) was evidenced in HPV-positive rafts compared with HPV-negative rafts [141]. On the other hand, the differentiation-dependent cellular transcription factors KLF4 and BLIMP1 can activate BZLF1 and BRLF1 promoters, inducing a differentiation-dependent EBV lytic infection [142]. However, HPV16 E6 and E7 proteins reduce the expression levels of some late differentiation markers, such as FLG, LOR, and WNT5A (which are KLF4 transcriptional targets), blocking EBV replication in human foreskin keratinocytes [141]. Previous studies demonstrated the capacity of TGF-ꞵ/Smad signaling to activate the EBV latent-lytic switch, promoting BZLF1 gene expression [143,144]. Regarding this issue, HPV16 E7 can downregulate TGF-βRII expression in a transgenic mouse model [145]. HPV16 E5 also downregulates the expression of TGF-βRII, which disrupts TGFβ1/Smad signaling in human epithelial cells [146]. Additionally, HPV18 can stabilize the maintenance of EBV genomes in monolayer cultures of oral epithelial cells [147]. Interestingly, HPV integration, a very important event during HPV-driven carcinogenesis, has been related to EBV infection. In fact, EBV presence was associated with a seven-fold (OR 7.11, 95% CI 1.70–29.67) increased probability of the occurrence of HPV16 genome integration [148]. Moreover, EBV infection was found to increase the probability of HPV16 genome integration in the host genome by five-fold (OR 5, 95% CI: 1.15–21.8) [149]. Taken together, these findings suggest the possibility that HR-HPV infection and HR-HPV genome integration can promote EBV latency establishment, which is a very important initial event during EBV-driven carcinogenesis. Meanwhile, an increased percentage of oral cells expressing the Zta protein was evidenced in EBV/HPV18-coinfected raft cultures when compared with HPV18-negative raft cultures [147]. This fact suggests the capacity of HPV18 to promote EBV lytic reactivation in the suprabasal layers of raft cultures, which was also associated with E6 and E7 expression [147].
4.3. Immune Evasion Orchestrated by HPV Facilitates the EBV Second Infection
Both HPV and EBV demonstrate a broad range of mechanisms to evade both the innate and adaptive antiviral host response for virus replication (reviewed in [150,151]). Interestingly, some mechanisms of immune evasion in HPV infection are also used by EBV. This fact leads us to hypothesize that primary HPV infection in head and neck epithelial cells could create a more favorable environment for EBV secondary infection. In the following sections, we will describe the mechanisms by which both HPV and EBV evade the immune response to their infection. In general, that response involves the following steps: first, toll-like receptors (TLRs) recognize pathogen-associated molecular patterns (PAMPs) to initiate the antiviral innate immune response [152,153]; next, the nuclear factor kappa light chain enhancer of activated B cells (NF-κB) and interferon regulatory factors (IRFs) are activated, inducing the expression of IFNs and proinflammatory cytokines.
4.3.1. Modulation of Toll-Like Receptors (TLRs)
An increased expression of TLR3, TLR7, and TLR8 has been associated with HPV16 clearance/control in cervical specimens [154]. However, HPV16 can inhibit TLR2, TLR3, TLR7, TLR8, and TLR9 expression, thus contributing to viral persistence in the cervical mucosa [155]. In HPV-positive OPSCCs, TLR5 and TLR9 expression was reduced compared to HPV-negative tumors [156]. In addition, the expression of HPV16 E6 and E7 oncoproteins was found to be related to TLR9 downregulation at both mRNA and protein levels, whereas HPV6 E6 and E7 proteins were unable to repress the activity of the TLR9 promoter [157]. This finding was also associated with the capacity of HPV16 E7 to induce the recruitment of an inhibitory NF-κBp50–p65 complex in the TLR9 promoter, suggesting that TLR9 is involved in cervical carcinogenesis [158]. EBV also disrupts the TLRs sensing in order to modulate the host´s innate immune response. For instance, BGLF5, an alkaline exonuclease expressed during the EBV lytic phase, decreases the expression of TLR2 and TLR9 [159,160]. The large tegument protein BPLF1 also suppresses the TLR2-mediated activation of NF-κB signaling, as well as IL-18 production [161]. Furthermore, EBV can downregulate the expression of TLR9 through LMP1-mediated NF-κB activation [162]. However, LMP1 induces TLR3 expression, leading to an increased NF-κB p65 signaling activation in NPC cells [163]. Interestingly, the expression of nuclear TLR2 and TLR5 was significantly decreased in EBV-positive NPC tumors when compared with HPV-positive and EBV/HPV-negative groups [164]. Since TLR2, TLR3, TLR7, and TLR9 are able to sense EBV infection by monocytes and plasmacytoid dendritic cells, consequently activating the host antiviral response [152,153,165,166], it could be hypothesized that TLR downregulation promoted by HPV infection may disrupt the innate immune recognition of EBV, thus facilitating this infection. In this regard, a polymorphism in the TLR9 1635 locus, related to the HIV acquisition, was also associated with an increased risk of EBV infection [167]. Finally, single nucleotide polymorphisms (SNPs) in TLR4 and TLR9 genes were associated with an increased risk of infectious mononucleosis (IM), an acute disease typically caused by EBV infection [168].
4.3.2. Regulation of IRF Signaling and IFNs Production
IFNs constitute one of the first lines of host defense against virus infections. The production of IFN-α and IFN-β is activated mainly by the IRFs 3 and 7 (reviewed in: [169]). The repression of IRF and Type I IFN production is a mechanism used by HR-HPV to disrupt an effective antiviral response. Specifically, HPV16 E6 binds to IRF3, inhibiting the expression of IFN-β [170]. Additionally, E6 can decrease the expression of IFN-α [171]. A role was also reported for HPV16 E5 in the suppression of stromal Type I IFNs signaling, which involved the function of IRF3 and IRF7 [172]. The capacity of HR-HPVs to inhibit the expression of IFN-stimulated genes (ISG), such as IFIT1, MX1, STAT1, RIG-I, and MDA5, consequently impairing the production of IFN-β and IFN-λ, has previously been reported [173]. Additionally, HPV16 E6 can reduce IFN-κ at mRNA and protein levels in human keratinocytes [173,174,175]. The production of IFN-κ is also decreased by HPV16 and HPV18 E2 through STING downregulation [176]. Similarly, the immediate-early EBV BZLF1 and BRLF1 proteins reduce the activation of IRF3 and IRF7, decreasing the synthesis of IFN-α and IFN-ꞵ [177,178]. BZLF1 also reduces the activation of the IRF1 and STAT1 phosphorylation mediated by IFN-γ [179]. LF2 (BILF4) inhibits IRF7, leading to the suppression of IFN-α production [180], whereas BGLF4 interferes with IRF3 activity, reducing the synthesis of IFN-β [181]. The microRNA (miR)-BART16-5p downregulates the expression of the CREB-binding protein (CBP), a transcriptional coactivator in IFN signaling, abrogating the production of ISGs (IFIT1 and ISG15) in response to IFN-α stimulation [182]. Finally, the miR-BART6-3p suppresses the IFN-β production, targeting the RIG-I-like receptor [183].
4.3.3. Interference with Other Innate Effector Molecules
The host innate response to viral infection is composed of the production and release of pro-inflammatory cytokines, which are also hijacked by viral proteins. For instance, HPV E2, E6, and E7 oncoproteins induce the production of interleukin-10 (IL-10), which, in turn, activates the Janus kinase-1/tyrosine kinase-2/signal transducer and activator of transcription 3 (JAK1/Tyk2/STAT3). The stimulation of the STAT3 pathway inhibits macrophage activation and the production of some inflammatory cytokines, such as TNF-α, IL-6, and IL-1β [184]. Other pro-inflammatory cytokines, such as IL-2 and IL-23, are downregulated in HPV-positive cervical lesions [185]. HPV16 E6 induces the downregulation of the pleiotropic IL-18 [186], which also induces the production of IFN-γ [187]. Reduced levels of IL-1β and IL-18 are associated with an increased risk of developing cervical cancer [188]. HPV E6 reduces the mRNA and protein levels of GM-CSF and TNF-α, interfering with the NF-κB signaling pathway [189]. Similarly, the EBV BCRF1 gene encodes for viral interleukin-10 (vIL-10), a homolog of human IL-10. vIL-10 inhibits IFN-γ, TNF-α, and GM-CSF production by monocytes [190]. BARF1 acts as a soluble receptor for the macrophage colony-stimulating factor (M-CSF), reducing the differentiation and activation of macrophages and inhibiting both the synthesis and release of IFN-α [191,192]. Moreover, BARF1 induces the downregulation of other human cytokines, such as IL-1α and IL-8 [193]. The expression of IL-1α on the cell membrane was found to be related to an anti-tumor immune response [194]. The EBV miR-BHRF1-2-5p targets IL-1 receptor 1 (IL1R1), blocking the activation of NF-κB mediated by IL-1β [195]. HPV16 E6 reduces the level of IL-1β by the proteasomal degradation of pro-IL-1β [196]. However, both tumor-inhibiting and tumor-promoting effects have been reported for IL-1β. This cytokine activates the Th1 CD4+ T cells, which, in turn, secrete IFN-γ and TNF-α inflammatory cytokines [197,198].
4.3.4. Disruption of NF-κB Signaling Pathway
It has been reported that HPV16 E7 decreased the activity of the IKK complex (α and β), which induces the IκBα phosphorylation and degradation of the NF-κB inhibitor [199]. Furthermore, HPV16 E6 interferes with the NF-κB RelA/p65-dependent transcriptional activity, cooperating with the disruption of TNFα-induced NF-κB signaling [199]. HPV16 E6 and E7 increase the p105 (NFKB1) and p100 (NFKB2) levels, thus inhibiting the transcriptional activity of NF-κB complexes [200]. E6 also inhibits the activation of NF-κB induced by the CBP/p300 coactivator [201], although both HPV E6 and E7 bind to CBP/p300 (a p65 transcriptional coactivator) and p300/CBP-associated factor (P/CAF), disrupting IL-18 transcription [202]. Additionally, EBV genes are involved in disrupting NF-κB signaling. For instance, BZLF1 suppresses the NF-κB pathway, inducing p65 nuclear translocation but disrupting its transcriptional function [203]. Additionally, BPLF1 deubiquitinates the TNF receptor-associated factor 6 (TRAF6), inhibiting NF-κB signaling during lytic infection [161]. EBNA1 inhibits the phosphorylation of IKKα/β, disrupting the canonical NF-κB pathway [204]. EBV BGLF4 downregulates NF-κB transactivation by disrupting the interaction of the NF-κB coactivator UXT and p65 [205]. BGLF2 downregulates TNFα-induced NF-κB activity, blocking the nuclear translocation of p65 [206]. Taken together, these facts suggest that both HPV and EBV have the capacity to disrupt the antiviral immune response in the head and neck region, which potentially facilitates secondary EBV infection. Interestingly, both HPV and EBV-encoded proteins are also able to activate the NF-κB signaling pathway, promoting cancer progression [207,208]. Indeed, NF-κB is a pivotal link between chronic inflammation and cancer [209]. Conversely, it was suggested that NF-κB has tumor suppressor properties after HPV infection, and that its downregulation by E6/E7 viral proteins contributes to cervical cell immortalization [210].
4.3.5. Downregulation of Major Histocompatibility Complex (MHC) by HPV Oncoproteins
It has been reported that the HR-HPV E5 oncoprotein can disrupt antigen presentation by downregulating MHC/HLA class I membrane expression with accumulation in the Golgi’s apparatus [211,212]. This effect results in a reduced recognition of HPV-infected cells by CD8+ T cells [212]. Additionally, the HR-HPV E7 oncoprotein has been shown to repress the MHC class I heavy chain promoter [213], contributing to escaping from immune surveillance.
4.4. Other Mechanisms Involved in EBV/HPV-Associated HNCs
It has been proposed that HR-HPV and EBV interactions could contribute to cancer EMT and cancer progression, including HNC [214,215]. However, mechanistic studies that are focused on the role of EBV/HPV co-infection and HNC progression are scarce. In this section, we describe additional pathways and mechanisms involved in a potential cooperation between both viruses for HNC development.
4.4.1. Cooperation between HR-HPV E6 and EBV LMP1
Al-Thawadi et al. found an association between the LMP1/E6 co-expression and upregulation of the inhibitors of DNA binding 1 (Id-1) in cervical carcinomas [216]. Indeed, LMP1 has been reported to induce Id-1 by inactivating the Foxo3a function in nasopharyngeal cells [217]. Id-1 can activate the NF-κB/survivin and phosphoinositide 3-Kinase/Akt signaling pathways, promoting HNC cell growth [218]. Additionally, LMP1 alone, or in combination with HPV16 E6, was able to induce the expression of γH2AX, which is considered a DNA damage biomarker. Moreover, a HPV16 E6 and LMP1 copresence reduces the expression of p53, RB, p27, and ChK1, suggesting a synergism between E6/LMP1 proteins and DDR [219]. Similarly, under genotoxic conditions, a reduction in DDR proteins, such as ATM, ATR, Chk1, and Chk2, as well as an increased resistance to apoptosis, was evidenced in HPV16 E6+/EBV LMP1+ cells compared to those expressing only the HPV16 E6 protein [220]. In fact, the expression of both HPV16 E6 and EBV LMP1 was able to increase the proliferation rates of MEFs cells compared to those cells expressing the HPV16 E6 oncoprotein alone, which was associated with NF-κB activation and p53 suppression [219,220]. In addition, increased soft agar colonies, as well as tumor formation in nude mice, were evidenced when HPV16 E6+/EBV LMP1+ cells were compared to mock cells. This finding was associated with increased levels of AKT and adhesion molecules (MMP2, CAT-1, and paxillin) in HPV16 E6 +/ EBV LMP1+ cells [220]. However, no effect in the proliferation rates was evidenced when MEFs expressing EBNA1 or EBNA1/E6 were analyzed [219]. The expression of HR-HPV E6 and EBV LMP1 oncoproteins was associated with grade 3 infiltrating ductal carcinoma, as well as with the occurrence of one or more lymph node metastasis when compared with HPV-, EBV-, or HPV-/EBV- tumors [221].
4.4.2. Gene Promoter Methylation Promoted by HR-HPV/EBV Infection
An increased frequency of TP53 promoter methylation was evidenced in HR-HPV/EBV-infected OPSCCs compared to HR-HPV or EBV infection alone [222]. Additionally, HPV/EBV coinfection was associated with the E-cadherin gene (CDH1) and RB1 promoter methylation in cervical lesions [223]. However, HR-HPV/EBV coinfection was not associated with the methylation status of the death-associated protein kinase (DAPK) gene in HSILs [224]. DAPK is a family of serine/threonine kinases with tumor suppressor functions that operate by regulating apoptosis and autophagy, which are frequently downregulated in cancer by gene promoter methylation [225].
4.4.3. Reduced Activity of Detoxifying Enzymes Promoted by HR-HPV/EBV Infection
In OPSCC, the activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD) antioxidant enzymes was significantly decreased in patients with HPV/EBV coinfection when compared with HPV or EBV infection alone. A low GPx and SOD activity was observed in HPV/EBV-coinfected patients with poorly differentiated tumors and an advanced stage of disease [226].
4.4.4. Gene Polymorphisms and HR-HPV/EBV Coinfection
Sharma et al. (2019) studied the association of the TLR 9 (−1486 T/C) genotype, and wild (TT), hetero (CT), and homozygous (CC) allele, with HPV/EBV coinfection in pre-malignant and OSCC samples [227]. Interestingly, in HPV+/EBV+ patients, the occurrence of the TLR 9 (−1486 T/C) hetero and homozygous (CT + CC) genotype was associated with a 22 (OR 22.5, 95% CI: 1.608 to 314.77) and 30 (OR 30.0, 95% CI: 2.188–411.25) times increased probability of pre-malignant and OSCC development, respectively [227]. An association was also identified between TLR 4 (+896A/G) polymorphism and the development of oral premalignant lesions in HPV/EBV-coinfected patients [227]. TLR polymorphisms induce modification in both cytokines and chemokines patterns, thus increasing the probability of NPC development [228,229]. Furthermore, evidence suggested that high levels of EBER1 may contribute to HPV-mediated oncogenesis by modulating both IFN-related genes and pro-inflammatory cytokine genes [230]. A hypothetical model of HR-HPV/EBV cooperation is shown in Figure 1.
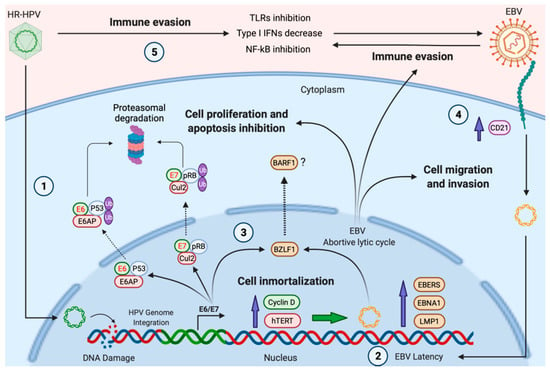
Figure 1.
A hypothetical model of HPV and EBV cooperation for the development of HNCs. (1), HR-HPV infection and viral genome integration promote DNA damage in head and neck epithelial cells; (2), previous DNA alterations, as well as the increased expression of cyclin D1 and hTERT induced by HR-HPV, can promote the establishment of EBV latency; (3), HR-HPV E6/E7 oncoproteins induce the expression of BZLF1, favoring the expression of EBV lytic genes with oncogenic properties, such as BARF1 (abortive lytic cycle); (4), HR-HPV infection induces CD21 (CR2), which, in turn, facilitates EBV cell entry; (5), the expression of HR-HPV oncoproteins inhibits protagonists of the anti-viral immune response, inducing immune evasion. Green shapes with red text represent HR-HPV oncoproteins. Brown shapes symbolize EBV proteins. Created by BioRender.com (accessed on 5 November 2021).
5. Conclusions and Remarks
Single HR-HPV or EBV infections are insufficient for carcinogenesis, suggesting the involvement of additional factors. Both HR-HPV and EBV have been detected in HNCs, most notably in oropharyngeal tumors. The HPV/EBV copresence in HNCs is highly variable worldwide, ranging from 6.5% to 37.5% of OSCCs, 15% to 47.7% of NPCs in endemic regions, and 53.3% to 86% of OPSCCs. Potential mechanisms for HPV/EBV cooperation remain unclear, although some possibilities have been suggested. First, HPV could facilitate EBV entry by promoting an increased expression of integrins and CD21. Second, HPV could facilitate EBV latency establishment by promoting genetic alterations due to HR-HPV genome integration into the host genome. Third, local immune evasion promoted by HPV infection could facilitate secondary EBV infection in epithelial cells. Additionally, HPV and EBV viral oncoproteins can synergistically cooperate for head and neck carcinogenesis. Additional studies are warranted in order to evaluate the mechanisms involved in the cooperation between HPV and EBV for head and neck carcinogenesis.
Author Contributions
Conceptualization, R.B. and F.A.; writing—original draft preparation, R.B.; writing—review and editing, R.B., F.A., D.C.-B. and A.H.C.; supervision, F.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID-FONDECYT Postdoctoral Grant 3190723 (R.B.), and CONICYT-FONDAP 15130011 (A.H.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
To Brianna Johnson for extensive manuscript revision and comments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, M.S.; Siddiqui, S.A.; Perween, R. Epidemiological profile of head and neck cancer patients in Western Uttar Pradesh and analysis of distributions of risk factors in relation to site of tumor. J. Cancer Res. Ther. 2017, 13, 430–435. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef]
- Chien, Y.C.; Chen, J.Y.; Liu, M.Y.; Yang, H.I.; Hsu, M.M.; Chen, C.J.; Yang, C.S. Serologic markers of Epstein-Barr virus infection and nasopharyngeal carcinoma in Taiwanese men. N. Engl. J. Med. 2001, 345, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Batur, P. Human papillomavirus in 2019: An update on cervical cancer prevention and screening guidelines. Clevel. Clin. J. Med. 2019, 86, 173–178. [Google Scholar] [CrossRef]
- Drop, B.; Strycharz-Dudziak, M.; Kliszczewska, E.; Polz-Dacewicz, M. Coinfection with Epstein-Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma BK Virus (BKPyV) in Laryngeal, Oropharyngeal and Oral Cavity Cancer. Int. J. Mol. Sci. 2017, 18, 2752. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Ekshyyan, O.; Moore-Medlin, T.; Rong, X.; Nathan, S.; Gu, X.; Abreo, F.; Rosenthal, E.L.; Shi, M.; Guidry, J.T.; et al. Association between human papilloma virus/Epstein-Barr virus coinfection and oral carcinogenesis. J. Oral Pathol. Med. 2015, 44, 28–36. [Google Scholar] [CrossRef]
- Laantri, N.; Attaleb, M.; Kandil, M.; Naji, F.; Mouttaki, T.; Dardari, R.; Belghmi, K.; Benchakroun, N.; El Mzibri, M.; Khyatti, M. Human papillomavirus detection in moroccan patients with nasopharyngeal carcinoma. Infect. Agents Cancer 2011, 6, 3. [Google Scholar] [CrossRef]
- Gupta, I.; Ghabreau, L.; Al-Thawadi, H.; Yasmeen, A.; Vranic, S.; Al Moustafa, A.E.; Malki, M.I. Co-incidence of Human Papillomaviruses and Epstein-Barr Virus Is Associated With High to Intermediate Tumor Grade in Human Head and Neck Cancer in Syria. Front. Oncol. 2020, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Al-Thawadi, H.; Gupta, I.; Jabeen, A.; Skenderi, F.; Aboulkassim, T.; Yasmeen, A.; Malki, M.I.; Batist, G.; Vranic, S.; Al Moustafa, A.E. Co-presence of human papillomaviruses and Epstein-Barr virus is linked with advanced tumor stage: A tissue microarray study in head and neck cancer patients. Cancer Cell Int. 2020, 20, 361. [Google Scholar] [CrossRef]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Möhl, B.S.; Chen, J.; Sathiyamoorthy, K.; Jardetzky, T.S.; Longnecker, R. Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus. Mol. Cells 2016, 39, 286–291. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Rev. Microbiol. 2021, 1–14. [Google Scholar] [CrossRef]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Sterkand, R.T.; Ozbun, M.A. Interaction of human papillomavirus type 16 particles with heparan sulfate and syndecan-1 molecules in the keratinocyte extracellular matrix plays an active role in infection. J. Gen. Virol. 2015, 96, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Ozbun, M.A.; Campos, S.K. The long and winding road: Human papillomavirus entry and subcellular trafficking. Curr. Opin. Virol. 2021, 50, 76–86. [Google Scholar] [CrossRef]
- Werner, J.; Decarlo, C.A.; Escott, N.; Zehbe, I.; Ulanova, M. Expression of integrins and Toll-like receptors in cervical cancer: Effect of infectious agents. Innate Immun. 2011, 18, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.; Filippova, M.; Filippov, V.; Bashkirova, S.; Zhang, G.; Reeves, M.E.; Duerksen-Hughes, P. Overexpression of HPV16 E6* Alters β-Integrin and Mitochondrial Dysfunction Pathways in Cervical Cancer Cells. Cancer Genom. Proteom. 2016, 13, 259–273. [Google Scholar]
- Eckhardt, M.; Zhang, W.; Gross, A.M.; Von Dollen, J.; Johnson, J.R.; Franks-Skiba, K.E.; Swaney, D.L.; Johnson, T.L.; Jang, G.M.; Shah, P.S.; et al. Multiple Routes to Oncogenesis Are Promoted by the Human Papillomavirus-Host Protein Network. Cancer Discov. 2018, 8, 1474–1489. [Google Scholar] [CrossRef]
- Romero-Medina, M.C.; Venuti, A.; Melita, G.; Robitaille, A.; Ceraolo, M.G.; Pacini, L.; Sirand, C.; Viarisio, D.; Taverniti, V.; Gupta, P.; et al. Human papillomavirus type 38 alters wild-type p53 activity to promote cell proliferation via the downregulation of integrin alpha 1 expression. PLoS Pathog. 2020, 16, e1008792. [Google Scholar] [CrossRef]
- Hutt-Fletcher, L.M. The Long and Complicated Relationship between Epstein-Barr Virus and Epithelial Cells. J. Virol. 2017, 91, e01677-16. [Google Scholar] [CrossRef]
- Hatton, O.L.; Harris-Arnold, A.; Schaffert, S.; Krams, S.M.; Martinez, O.M. The interplay between Epstein-Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014, 58, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Temple, R.M.; Zhu, J.; Budgeon, L.; Christensen, N.D.; Meyers, C.; Sample, C.E. Efficient replication of Epstein-Barr virus in stratified epithelium in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16544–16549. [Google Scholar] [CrossRef]
- Chen, J.; Sathiyamoorthy, K.; Zhang, X.; Schaller, S.; Perez White, B.E.; Jardetzky, T.S.; Longnecker, R. Ephrin receptor A2 is a functional entry receptor for Epstein-Barr virus. Nat. Microbiol. 2018, 3, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Wang, H.B.; Zhang, A.; Chen, M.L.; Fang, Z.X.; Dong, X.D.; Li, S.B.; Du, Y.; Xiong, D.; et al. Ephrin receptor A2 is an epithelial cell receptor for Epstein-Barr virus entry. Nat. Microbiol. 2018, 3, 1–8. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Qiu, Y.; Huang, D.; Zhang, S.; Xie, L.; Qi, L.; Yu, C.; Zhou, X.; Hu, G.; et al. Clinical significance of EphA2 expression in squamous-cell carcinoma of the head and neck. J. Cancer Res. Clin. Oncol. 2011, 137, 761–769. [Google Scholar] [CrossRef]
- Huang, Y.C.; Lin, S.J.; Lin, K.M.; Chou, Y.C.; Lin, C.W.; Yu, S.C.; Chen, C.L.; Shen, T.L.; Chen, C.K.; Lu, J.; et al. Regulation of EBV LMP1-triggered EphA4 downregulation in EBV-associated B lymphoma and its impact on patients’ survival. Blood 2016, 128, 1578–1589. [Google Scholar] [CrossRef]
- Chesnokova, L.S.; Nishimura, S.L.; Hutt-Fletcher, L.M. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc. Natl. Acad. Sci. USA 2009, 106, 20464–20469. [Google Scholar] [CrossRef]
- Chesnokova, L.S.; Hutt-Fletcher, L.M. Fusion of Epstein-Barr virus with epithelial cells can be triggered by αvβ5 in addition to αvβ6 and αvβ8, and integrin binding triggers a conformational change in glycoproteins gHgL. J. Virol. 2011, 85, 13214–13223. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, E.M.; Wildner, G.P.; Kruse-Boitschenko, U.; Hoffmeister, B.; Goodman, S.L.; Raguse, J.D. Immunohistochemical analysis of integrins αvβ3, αvβ5 and α5β1, and their ligands, fibrinogen, fibronectin, osteopontin and vitronectin, in frozen sections of human oral head and neck squamous cell carcinomas. Exp. Ther. Med. 2011, 2, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Liu, Y.; Kan, X.; Li, Y.; Liu, M.; Lu, J.G. Elevated expression of integrin αv and β5 subunit in laryngeal squamous-cell carcinoma associated with lymphatic metastasis and angiogenesis. Pathol. Res. Pract. 2013, 209, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, J.R.; Chang, Y.; Chen, Y.L.; Hsieh, S.H.; Hsu, K.F.; Wang, C.F.; Tsai, S.T.; Jin, Y.T. Cyclic alphavbeta6-targeting peptide selected from biopanning with clinical potential for head and neck squamous cell carcinoma. Head Neck 2010, 32, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30, F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, Y.; Yachie, A. Cell type specific infection of Epstein-Barr virus (EBV) in EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Crit. Rev. Oncol. Hematol. 2002, 44, 283–294. [Google Scholar] [CrossRef]
- Horvath, C.A.; Boulet, G.A.; Renoux, V.M.; Delvenne, P.O.; Bogers, J.P. Mechanisms of cell entry by human papillomaviruses: An overview. Virol. J. 2010, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Longnecker, R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol. Rev. 2019, 43, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, P.; Gottschalk, E.Y.; Meneses, P.I. HPV entry into cells. Mutat. Res. Rev. Mutat. Res. 2017, 772, 13–22. [Google Scholar] [CrossRef]
- Speight, P.M.; Farthing, P.M. The pathology of oral cancer. Br. Dent. J. 2018, 225, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Krüger, M.; Pabst, A.M.; Walter, C.; Sagheb, K.; Günther, C.; Blatt, S.; Weise, K.; Al-Nawas, B.; Ziebart, T. The prevalence of human papilloma virus (HPV) infections in oral squamous cell carcinomas: A retrospective analysis of 88 patients and literature overview. J. Craniomaxillofac. Surg. 2014, 42, 1506–1514. [Google Scholar] [CrossRef]
- Petito, G.; Carneiro, M.A.; Santos, S.H.; Silva, A.M.; Alencar, R.C.; Gontijo, A.P.; Saddi, V.A. Human papillomavirus in oral cavity and oropharynx carcinomas in the central region of Brazil. Braz. J. Otorhinolaryngol. 2017, 83, 38–44. [Google Scholar] [CrossRef]
- Chakrobarty, B.; Roy, J.G.; Majumdar, S.; Uppala, D. Relationship among tobacco habits, human papilloma virus (HPV) infection, p53 polymorphism/mutation and the risk of oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2014, 18, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Termine, N.; Panzarella, V.; Falaschini, S.; Russo, A.; Matranga, D.; Lo Muzio, L.; Campisi, G. HPV in oral squamous cell carcinoma vs head and neck squamous cell carcinoma biopsies: A meta-analysis (1988–2007). Ann. Oncol. 2008, 19, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Ling, Y.; Dong, C.; Zhou, X.; Wang, F. The relationship between oral squamous cell carcinoma and human papillomavirus: A meta-analysis of a Chinese population (1994–2011). PLoS ONE 2012, 7, e36294. [Google Scholar] [CrossRef]
- Melo, B.A.C.; Vilar, L.G.; de Oliveira, N.R.; de Lima, P.O.; Pinheiro, M.B.; Domingueti, C.P.; Pereira, M.C. Human papillomavirus infection and oral squamous cell carcinoma—A systematic review. Braz. J. Otorhinolaryngol. 2021, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Reyes, M.; Rojas-Alcayaga, G.; Pennacchiotti, G.; Carrillo, D.; Muñoz, J.P.; Peña, N.; Montes, R.; Lobos, N.; Aguayo, F. Human papillomavirus infection in oral squamous cell carcinomas from Chilean patients. Exp. Mol. Pathol. 2015, 99, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Pandya, S.; Singh, M.; Mehrotra, R. Identification of high-risk human papillomavirus-16 and -18 infections by multiplex PCR and their expression in oral submucous fibrosis and oal squamous cell carcinoma. Head Neck Oncol. 2013, 5, 1–10. [Google Scholar]
- Yen, C.Y.; Lu, M.C.; Tzeng, C.C.; Huang, J.Y.; Chang, H.W.; Chen, R.S.; Liu, S.Y.; Liu, S.T.; Shieh, B.; Li, C. Detection of EBV infection and gene expression in oral cancer from patients in Taiwan by microarray analysis. J. Biomed. Biotechnol. 2009, 2009, 904589. [Google Scholar] [CrossRef]
- Acharya, S.; Ekalaksananan, T.; Vatanasapt, P.; Loyha, K.; Phusingha, P.; Promthet, S.; Kongyingyoes, B.; Pientong, C. Association of Epstein-Barr virus infection with oral squamous cell carcinoma in a case-control study. J. Oral Pathol. Med. 2014, 44, 252–257. [Google Scholar] [CrossRef]
- Heawchaiyaphum, C.; Iizasa, H.; Ekalaksananan, T.; Burassakarn, A.; Kiyono, T.; Kanehiro, Y.; Yoshiyama, H.; Pientong, C. Epstein-Barr Virus Infection of Oral Squamous Cells. Microorganisms 2020, 8, 419. [Google Scholar] [CrossRef]
- Kikuchi, K.; Noguchi, Y.; de Rivera, M.W.G.-N.; Hoshino, M.; Sakashita, H.; Yamada, T.; Inoue, H.; Miyazaki, Y.; Nozaki, T.; González-López, B.S.; et al. Detection of Epstein-Barr virus genome and latent infection gene expression in normal epithelia, epithelial dysplasia, and squamous cell carcinoma of the oral cavity. Tumor Biol. 2016, 37, 3389–3404. [Google Scholar] [CrossRef]
- Rahman, R.; Poomsawat, S.; Juengsomjit, R.; Buajeeb, W. Overexpression of Epstein-Barr virus-encoded latent membrane protein-1 (LMP-1) in oral squamous cell carcinoma. BMC Oral Health 2019, 19, 142. [Google Scholar] [CrossRef] [PubMed]
- She, Y.; Nong, X.; Zhang, M.; Wang, M. Epstein-Barr virus infection and oral squamous cell carcinoma risk: A meta-analysis. PLoS ONE 2017, 12, e0186860. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.A.P.; Teodoro, I.P.P.; de Galiza, L.E.; Filho, P.H.B.M.; Marques, F.M.; Pinheiro Junior, R.F.F.; Macedo, G.E.C.; Facundo, H.T.; da Silva, C.G.L.; Lima, M.V.A. Association between Epstein-Barr Virus and Oral Carcinoma: A Systematic Review with Meta-Analysis. Crit. Rev. Oncog. 2019, 24, 349–368. [Google Scholar] [CrossRef]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; Ibrahim, S.O.; Larsson, P.A.; Sand, L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res. 2012, 32, 571–580. [Google Scholar] [PubMed]
- Polz-Gruszka, D.; Morshed, K.; Jarzyński, A.; Polz-Dacewicz, M. Prevalence of Polyoma BK Virus (BKPyV), Epstein-Barr Virus (EBV) and Human Papilloma Virus (HPV) in Oropharyngeal Cancer. Pol. J. Microbiol. 2015, 64, 323–328. [Google Scholar] [CrossRef]
- Vanshika, S.; Preeti, A.; Sumaira, Q.; Vijay, K.; Shikha, T.; Shivanjali, R.; Shankar, S.U.; Mati, G.M. Incidence OF HPV and EBV in oral cancer and their clinico-pathological correlation—A pilot study of 108 cases. J. Oral Biol. Craniofac. Res. 2021, 11, 180–184. [Google Scholar] [CrossRef]
- Broccolo, F.; Ciccarese, G.; Rossi, A.; Anselmi, L.; Drago, F.; Toniolo, A. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non- keratinizing squamous cell carcinoma of the oropharynx. Infect. Agents Cancer 2018, 13, 32. [Google Scholar] [CrossRef]
- Naqvi, S.U.; Khan, S.; Ahmed, A.; Lail, A.; Gul, S.; Ahmed, S. Prevalence of EBV, CMV, and HPV in oral squamous cell carcinoma patients in the Pakistani population. J. Med. Virol. 2020, 92, 3880–3883. [Google Scholar] [CrossRef]
- Tang, L.L.; Chen, W.Q.; Xue, W.Q.; He, Y.Q.; Zheng, R.S.; Zeng, Y.X.; Jia, W.H. Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett. 2016, 374, 22–30. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, W.; Jin, M.; Zhang, J.; Li, S.; Tong, F.; Zhou, Y. Differential expression of EBV proteins LMP1 and BHFR1 in EBV-associated gastric and nasopharyngeal cancer tissues. Mol. Med. Rep. 2016, 13, 4151–4158. [Google Scholar] [CrossRef]
- Pan, J.J.; Ng, W.T.; Zong, J.F.; Chan, L.L.; O’Sullivan, B.; Lin, S.J.; Sze, H.C.; Chen, Y.B.; Choi, H.C.; Guo, Q.J.; et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016, 122, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Chen, Y.P.; Mao, Y.P.; Wang, Z.X.; Guo, R.; Chen, L.; Tian, L.; Lin, A.H.; Li, L.; Sun, Y.; et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J. Natl. Compr. Cancer Netw. 2017, 15, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Okekpa, S.I.; Mydin, R.B.S.M.N.; Mangantig, E.; Azmi, N.S.A.; Zahari, S.N.S.; Kaur, G.; Musa, Y. Nasopharyngeal Carcinoma (NPC) Risk Factors: A Systematic Review and Meta-Analysis of the Association with Lifestyle, Diets, Socioeconomic and Sociodemographic in Asian Region. Asian Pac. J. Cancer Prev. 2019, 20, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Bei, J.X.; Zuo, X.Y.; Liu, W.S.; Guo, Y.M.; Zeng, Y.X. Genetic susceptibility to the endemic form of NPC. Chin. Clin. Oncol. 2016, 5, 15. [Google Scholar] [CrossRef]
- Fan, H.C.; Chen, C.Y.; Hsu, Y.C.; Chou, R.H.; Teng, C.J.; Chiu, C.H.; Hsu, C.Y.; Muo, C.H.; Chang, M.Y.; Chang, K.H. Increased risk of incident nasopharyngeal carcinoma with exposure to air pollution. PLoS ONE 2018, 13, e0204568. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Yu, D.; Liu, Y.; Xue, K.; Zhao, X. Role of Epstein-Barr Virus in the Development of Nasopharyngeal Carcinoma. Open Med. 2017, 12, 171–176. [Google Scholar] [CrossRef]
- Fan, C.; Tang, Y.; Wang, J.; Xiong, F.; Guo, C.; Wang, Y.; Xiang, B.; Zhou, M.; Li, X.; Wu, X.; et al. The emerging role of Epstein-Barr virus encoded microRNAs in nasopharyngeal carcinoma. J. Cancer 2018, 9, 2852–2864. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef]
- Asante, D.B.; Asmah, R.H.; Adjei, A.A.; Kyei, F.; Simpong, D.L.; Brown, C.A.; Gyasi, R.K. Detection of Human Papillomavirus Genotypes and Epstein-Barr Virus in Nasopharyngeal Carcinomas at the Korle-Bu Teaching Hospital, Ghana. Sci. World J. 2017, 2017, 2721367. [Google Scholar] [CrossRef]
- Breda, E.; Catarino, R.J.; Azevedo, I.; Lobão, M.; Monteiro, E.; Medeiros, R. Epstein-Barr virus detection in nasopharyngeal carcinoma: Implications in a low-risk area. Braz. J. Otorhinolaryngol. 2010, 76, 310–315. [Google Scholar] [CrossRef]
- Dogan, S.; Hedberg, M.L.; Ferris, R.L.; Rath, T.J.; Assaad, A.M.; Chiosea, S.I. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a low-incidence population. Head Neck 2014, 36, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Svajdler, M.; Kaspirkova, J.; Mezencev, R.; Laco, J.; Torday, T.; Dubinsky, P.; Straka, L.; Ondic, O.; Michal, M.; Skalova, A. Human papillomavirus and Epstein-Barr virus in nasopharyngeal carcinoma in a non-endemic eastern european population. Neoplasma 2016, 63, 107–114. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruuskanen, M.; Irjala, H.; Minn, H.; Vahlberg, T.; Randen-Brady, R.; Hagström, J.; Syrjänen, S.; Leivo, I. Epstein-Barr virus and human papillomaviruses as favorable prognostic factors in nasopharyngeal carcinoma: A nationwide study in Finland. Head Neck 2019, 41, 349–357. [Google Scholar] [CrossRef]
- Rumayor Piña, A.; Dos Santos, H.T.; Carlos, R.; Altemani, A.; de Almeida, O.P. Epstein-Barr Virus in Nasopharyngeal Carcinoma of Guatemalan and Brazilian Patients. Int. J. Surg. Pathol. 2017, 25, 304–309. [Google Scholar] [CrossRef]
- Tatlı Doğan, H.; Kılıçarslan, A.; Doğan, M.; Süngü, N.; Güler Tezel, G.; Güler, G. Retrospective analysis of oncogenic human papilloma virus and Epstein-Barr virus prevalence in Turkish nasopharyngeal cancer patients. Pathol. Res. Pract. 2016, 212, 1021–1026. [Google Scholar] [CrossRef]
- Tham, T.; Machado, R.; Russo, D.P.; Herman, S.W.; Teegala, S.; Costantino, P. Viral markers in nasopharyngeal carcinoma: A systematic review and meta-analysis on the detection of p16INK4a, human papillomavirus (HPV), and Ebstein-Barr virus (EBV). Am. J. Otolaryngol. 2021, 42, 102762. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, C.; Song, Y.; Li, Q.; Huang, J.; Ma, X. Epstein-Barr virus DNA level as a novel prognostic factor in nasopharyngeal carcinoma: A meta-analysis. Medicine 2016, 95, e5130. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Chen, L.; Zhang, Y.; Guo, R.; Li, W.F.; Mao, Y.P.; Tan, L.L.; Sun, Y.; Zhang, F.; Liu, L.Z.; et al. Survival analysis of patients with advanced-stage nasopharyngeal carcinoma according to the Epstein-Barr virus status. Oncotarget 2016, 7, 24208–24216. [Google Scholar] [CrossRef]
- Liu, T.B.; Zheng, Z.H.; Pan, J.; Pan, L.L.; Chen, L.H. Prognostic role of plasma Epstein-Barr virus DNA load for nasopharyngeal carcinoma: A meta-analysis. Clin. Investig. Med. 2017, 40, E1–E12. [Google Scholar] [CrossRef]
- Du, Y.Y.; Luo, D.H.; Sun, X.S.; Tang, L.Q.; Mai, H.Q.; Chen, Q.Y.; Zhong, J.H.; Mai, D.M.; Zhang, W.R.; Chen, W.H.; et al. Combining pretreatment plasma Epstein-Barr virus DNA level and cervical node necrosis improves prognostic stratification in patients with nasopharyngeal carcinoma: A cohort study. Cancer Med. 2019, 8, 6841–6852. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.S.; Forslund, O.; Andersson, F.C.; Lindstedt, M.; Greiff, L. Intralesional EBV-DNA load as marker of prognosis for nasopharyngeal cancer. Sci. Rep. 2019, 9, 15432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Y.; Zeng, S.; Hu, X. LMP1 expression is positively associated with metastasis of nasopharyngeal carcinoma: Evidence from a meta-analysis. J. Clin. Pathol. 2012, 65, 41–45. [Google Scholar] [CrossRef]
- Hu, C.; Wei, W.; Chen, X.; Woodman, C.B.; Yao, Y.; Nicholls, J.M.; Joab, I.; Sihota, S.K.; Shao, J.Y.; Derkaoui, K.D.; et al. A global view of the oncogenic landscape in nasopharyngeal carcinoma: An integrated analysis at the genetic and expression levels. PLoS ONE 2012, 7, e41055. [Google Scholar] [CrossRef]
- Luo, F.-F.; Li, Z.-Y.; Huang, S.-N.; Chen, G.; Xie, T.-T.; LI, Y.-Q.; Xing, W.-W.; Li, W.-Y.; Lu, Y.-K.; Ding, H. Prevalence of human papillomavirus in patients with nasopharyngeal carcinoma: A meta-analysis. Int. J. Clin. Exp. Med. 2017, 10, 9837–9847. [Google Scholar]
- Stenmark, M.H.; McHugh, J.B.; Schipper, M.; Walline, H.M.; Komarck, C.; Feng, F.Y.; Worden, F.P.; Wolf, G.T.; Chepeha, D.B.; Prince, M.E.; et al. Nonendemic HPV-positive nasopharyngeal carcinoma: Association with poor prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 580–588. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, K.H.; Chu, P.Y.; Hsu, C.C.; Kuo, W.R. Detection of human papilloma virus and Epstein-Barr virus DNA in nasopharyngeal carcinoma by polymerase chain reaction. Kaohsiung J. Med. Sci. 1999, 15, 256–262. [Google Scholar]
- Mirzamani, N.; Salehian, P.; Farhadi, M.; Tehran, E.A. Detection of EBV and HPV in nasopharyngeal carcinoma by in situ hybridization. Exp. Mol. Pathol. 2006, 81, 231–234. [Google Scholar] [CrossRef]
- Jiang, W.; Chamberlain, P.D.; Garden, A.S.; Kim, B.Y.; Ma, D.; Lo, E.J.; Bell, D.; Gunn, G.B.; Fuller, C.D.; Rosenthal, D.I.; et al. Prognostic value of p16 expression in Epstein-Barr virus-positive nasopharyngeal carcinomas. Head Neck 2016, 38, E1459–E1466. [Google Scholar] [CrossRef]
- Simon, J.; Schroeder, L.; Ingarfield, K.; Diehl, S.; Werner, J.; Brenner, N.; Liu, Z.; Pawlita, M.; Pring, M.; Butt, J.; et al. Epstein-Barr virus and human papillomavirus serum antibodies define the viral status of nasopharyngeal carcinoma in a low endemic country. Int. J. Cancer 2020, 147, 461–471. [Google Scholar] [CrossRef]
- Osborne, R.F.; Brown, J.J. Carcinoma of the oral pharynx: An analysis of subsite treatment heterogeneity. Surg. Oncol. Clin. N. Am. 2004, 13, 71–80. [Google Scholar] [CrossRef]
- Pan, C.; Issaeva, N.; Yarbrough, W.G. HPV-driven oropharyngeal cancer: Current knowledge of molecular biology and mechanisms of carcinogenesis. Cancers Head Neck 2018, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, A.W.; Galloway, T.J.; Bhayani, M.K.; Liu, J.C. Effect of HPV Status on Survival of Oropharynx Cancer with Distant Metastasis. Otolaryngol. Head Neck Surg. 2020, 163, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Wendt, M.; Hammarstedt-Nordenvall, L.; Zupancic, M.; Friesland, S.; Landin, D.; Munck-Wikland, E.; Dalianis, T.; Näsman, A.; Marklund, L. Long-Term Survival and Recurrence in Oropharyngeal Squamous Cell Carcinoma in Relation to Subsites, HPV, and p16-Status. Cancers 2021, 13, 2553. [Google Scholar] [CrossRef] [PubMed]
- Mazul, A.L.; Rodriguez-Ormaza, N.; Taylor, J.M.; Desai, D.D.; Brennan, P.; Anantharaman, D.; Gheit, T.; Tommasino, M.; Abedi-Ardekani, B.; Olshan, A.F.; et al. Prognostic significance of non-HPV16 genotypes in oropharyngeal squamous cell carcinoma. Oral Oncol. 2016, 61, 98–103. [Google Scholar] [CrossRef]
- Smith, E.M.; Rubenstein, L.M.; Haugen, T.H.; Hamsikova, E.; Turek, L.P. Tobacco and alcohol use increases the risk of both HPV-associated and HPV-independent head and neck cancers. Cancer Causes Control. 2010, 21, 1369–1378. [Google Scholar] [CrossRef]
- Stjernstrøm, K.D.; Jensen, J.S.; Jakobsen, K.K.; Grønhøj, C.; von Buchwald, C. Current status of human papillomavirus positivity in oropharyngeal squamous cell carcinoma in Europe: A systematic review. Acta Oto-Laryngol. 2019, 139, 1112–1116. [Google Scholar] [CrossRef]
- Mariz, B.A.L.A.; Kowalski, L.P.; William, W.N.; de Castro, G.; Chaves, A.L.F.; Santos, M.; de Oliveira, T.B.; Araújo, A.L.D.; Normando, A.G.C.; Ribeiro, A.C.P.; et al. Global prevalence of human papillomavirus-driven oropharyngeal squamous cell carcinoma following the ASCO guidelines: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2020, 156, 103116. [Google Scholar] [CrossRef]
- Haeggblom, L.; Ramqvist, T.; Tommasino, M.; Dalianis, T.; Näsman, A. Time to change perspectives on HPV in oropharyngeal cancer. A systematic review of HPV prevalence per oropharyngeal sub-site the last 3 years. Papillomavirus Res. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Donà, M.G.; Spriano, G.; Pichi, B.; Rollo, F.; Laquintana, V.; Covello, R.; Pellini, R.; Giuliani, M.; Pescarmona, E.; Benevolo, M. Human papillomavirus infection and p16 overexpression in oropharyngeal squamous cell carcinoma: A case series from 2010 to 2014. Future Microbiol. 2015, 10, 1283–1291. [Google Scholar] [CrossRef]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.X.; de Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.; Kruk-Zagajewska, A.; Wal, M.; Jopek, A.; Wierzbicka, M.; Kuch, A. Epstein-Barr virus and human papillomavirus infections and oropharyngeal squamous cell carcinomas. Clin. Exp. Med. 2002, 2, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Tyan, Y.S.; Liu, S.T.; Ong, W.R.; Chen, M.L.; Shu, C.H.; Chang, Y.S. Detection of Epstein-Barr virus and human papillomavirus in head and neck tumors. J. Clin. Microbiol. 1993, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Guillen, J.M.; Palacios-Saucedo, G.C.; Rivera-Morales, L.G.; Alonzo-Morado, M.V.; Burciaga-Bernal, S.B.; Montufar-Martinez, M.; Ortiz-Lopez, R.; Gonzalez-Villasana, V.; Martinez-Torres, A.C.; Serna-Hernandez, J.C.; et al. Infection and coinfection by human papillomavirus, Epstein-Barr virus and Merkel cell polyomavirus in patients with squamous cell carcinoma of the larynx: A retrospective study. PeerJ 2018, 6, e5834. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Johnstone, B.M. Human papillomavirus as a risk factor for oral squamous cell carcinoma: A meta-analysis, 1982–1997. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 622–635. [Google Scholar] [CrossRef]
- Dayyani, F.; Etzel, C.J.; Liu, M.; Ho, C.H.; Lippman, S.M.; Tsao, A.S. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol. 2010, 2, 15. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef]
- Kreimer, A.R.; Clifford, G.M.; Boyle, P.; Franceschi, S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol. Biomark. Prev. 2005, 14, 467–475. [Google Scholar] [CrossRef]
- Shaikh, M.H.; McMillan, N.A.; Johnson, N.W. HPV-associated head and neck cancers in the Asia Pacific: A critical literature review & meta-analysis. Cancer Epidemiol. 2015, 39, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, L.; Li, H.; Gao, J.; Yang, Y.; Zhou, F.; Gao, C.; Li, M.; Jin, Q. Human papillomavirus infection and laryngeal cancer risk: A systematic review and meta-analysis. J. Infect. Dis. 2013, 207, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Altekin, I.; Taş, A.; Yalcin, O.; Guven, S.G.; Aslan, Z.; Adali, M.K.; Karasalihoğlu, A.R. Frequency of Epstein-Barr virus and human papilloma virus in patients with nasopharyngeal carcinoma. Eur. Arch. Otorhinolaryngol. 2020, 277, 2041–2047. [Google Scholar] [CrossRef]
- Deng, Z.; Uehara, T.; Maeda, H.; Hasegawa, M.; Matayoshi, S.; Kiyuna, A.; Agena, S.; Pan, X.; Zhang, C.; Yamashita, Y.; et al. Epstein-Barr virus and human papillomavirus infections and genotype distribution in head and neck cancers. PLoS ONE 2014, 9, e113702. [Google Scholar] [CrossRef]
- Hutt-Fletcher, L.M. Epstein-Barr virus entry. J. Virol. 2007, 81, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Gu, X.; Nathan, C.O.; Hutt-Fletcher, L. Laser-capture microdissection of oropharyngeal epithelium indicates restriction of Epstein-Barr virus receptor/CD21 mRNA to tonsil epithelial cells. J. Oral Pathol. Med. 2008, 37, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Gu, X.; Moore-Medlin, T.N.; Nathan, C.A.; Hutt-Fletcher, L.M. Oral dysplasia and squamous cell carcinoma: Correlation between increased expression of CD21, Epstein-Barr virus and CK19. Oral Oncol. 2012, 48, 836–841. [Google Scholar] [CrossRef][Green Version]
- Li, Q.X.; Young, L.S.; Niedobitek, G.; Dawson, C.W.; Birkenbach, M.; Wang, F.; Rickinson, A.B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature 1992, 356, 347–350. [Google Scholar] [CrossRef]
- Knox, P.G.; Li, Q.X.; Rickinson, A.B.; Young, L.S. In vitro production of stable Epstein-Barr virus-positive epithelial cell clones which resemble the virus:cell interaction observed in nasopharyngeal carcinoma. Virology 1996, 215, 40–50. [Google Scholar] [CrossRef][Green Version]
- Brooks, L.; Yao, Q.Y.; Rickinson, A.B.; Young, L.S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: Coexpression of EBNA1, LMP1, and LMP2 transcripts. J. Virol. 1992, 66, 2689–2697. [Google Scholar] [CrossRef]
- Murono, S.; Yoshizaki, T.; Park, C.S.; Furukawa, M. Association of Epstein-Barr virus infection with p53 protein accumulation but not bcl-2 protein in nasopharyngeal carcinoma. Histopathology 1999, 34, 432–438. [Google Scholar] [CrossRef]
- Fingeroth, J.D.; Diamond, M.E.; Sage, D.R.; Hayman, J.; Yates, J.L. CD21-Dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 1999, 73, 2115–2125. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Mei, X.; Hu, H. Transcriptomic Analyses Reveal Gene Expression Profiles and Networks in Nasopharyngeal Carcinoma. Biomed Res. Int. 2021, 2021, 8890176. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Z.; He, Y.; He, Z.; Ban, Z.; Zhu, Y.; Ding, L.; Yang, C.; Jeong, J.H.; Yuan, W.; et al. EphA2 promotes tumorigenicity of cervical cancer by up-regulating CDK6. J. Cell. Mol. Med. 2021, 25, 2967–2975. [Google Scholar] [CrossRef]
- Fuentes-González, A.M.; Muñoz-Bello, J.O.; Manzo-Merino, J.; Contreras-Paredes, A.; Pedroza-Torres, A.; Fernández-Retana, J.; Pérez-Plasencia, C.; Lizano, M. Intratype variants of the E2 protein from human papillomavirus type 18 induce different gene expression profiles associated with apoptosis and cell proliferation. Arch. Virol. 2019, 164, 1815–1827. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Ganguly, N. Transcriptomic analyses of genes differentially expressed by high-risk and low-risk human papilloma virus E6 oncoproteins. Virusdisease 2015, 26, 105–116. [Google Scholar] [CrossRef]
- Verma, D.; Church, T.M.; Swaminathan, S. Epstein-Barr Virus Lytic Replication Induces ACE2 Expression and Enhances SARS-CoV-2 Pseudotyped Virus Entry in Epithelial Cells. J. Virol. 2021, 95, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.M.; Deng, W.; Yip, Y.L.; Zeng, M.S.; Lo, K.W.; Tsao, S.W. Epstein-Barr virus infection and persistence in nasopharyngeal epithelial cells. Chin. J. Cancer 2014, 33, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sixbey, J.W.; Vesterinen, E.H.; Nedrud, J.G.; Raab-Traub, N.; Walton, L.A.; Pagano, J.S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature 1983, 306, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Frangou, P.; Middeldorp, J.; Niedobitek, G. Epstein-Barr virus replication in tongue epithelial cells. J. Gen. Virol. 2002, 83, 2995–2998. [Google Scholar] [CrossRef]
- Vincent-Bugnas, S.; Vitale, S.; Mouline, C.C.; Khaali, W.; Charbit, Y.; Mahler, P.; Prêcheur, I.; Hofman, P.; Maryanski, J.L.; Doglio, A. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS ONE 2013, 8, e80336. [Google Scholar] [CrossRef]
- Rheu, G.B.; Ji, S.; Ryu, J.J.; Lee, J.B.; Shin, C.; Lee, J.Y.; Huh, J.B.; Shin, S.W. Risk assessment for clinical attachment loss of periodontal tissue in Korean adults. J. Adv. Prosthodont. 2011, 3, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Tsang, C.M.; Zhang, G.; Seto, E.; Takada, K.; Deng, W.; Yip, Y.L.; Man, C.; Hau, P.M.; Chen, H.; Cao, Y.; et al. Epstein-Barr virus infection in immortalized nasopharyngeal epithelial cells: Regulation of infection and phenotypic characterization. Int. J. Cancer 2010, 127, 1570–1583. [Google Scholar] [CrossRef]
- Tsang, C.M.; Yip, Y.L.; Lo, K.W.; Deng, W.; To, K.F.; Hau, P.M.; Lau, V.M.; Takada, K.; Lui, V.W.; Lung, M.L.; et al. Cyclin D1 overexpression supports stable EBV infection in nasopharyngeal epithelial cells. Proc. Natl. Acad. Sci. USA 2012, 109, E3473–E3482. [Google Scholar] [CrossRef]
- Yip, Y.L.; Tsang, C.M.; Deng, W.; Cheung, P.Y.; Jin, Y.; Cheung, A.L.; Lung, M.L.; Tsao, S.W. Expression of Epstein-Barr virus-encoded LMP1 and hTERT extends the life span and immortalizes primary cultures of nasopharyngeal epithelial cells. J. Med. Virol. 2010, 82, 1711–1723. [Google Scholar] [CrossRef]
- Raghunandan, B.N.; Sanjai, K.; Kumaraswamy, J.; Papaiah, L.; Pandey, B.; Jyothi, B.M. Expression of human telomerase reverse transcriptase protein in oral epithelial dysplasia and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2016, 20, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Moharil, R.B.; Khandekar, S.; Dive, A.; Bodhade, A. Cyclin D1 in oral premalignant lesions and oral squamous cell carcinoma: An immunohistochemical study. J. Oral Maxillofac. Pathol. 2020, 24, 397. [Google Scholar] [CrossRef] [PubMed]
- de la Cour, C.D.; Sperling, C.D.; Belmonte, F.; Syrjänen, S.; Verdoodt, F.; Kjaer, S.K. Prevalence of human papillomavirus in oral epithelial dysplasia: Systematic review and meta-analysis. Head Neck 2020, 42, 2975–2984. [Google Scholar] [CrossRef]
- Sarkar, S.; Alam, N.; Chakraborty, J.; Biswas, J.; Mandal, S.S.; Roychoudhury, S.; Panda, C.K. Human papilloma virus (HPV) infection leads to the development of head and neck lesions but offers better prognosis in malignant Indian patients. Med. Microbiol. Immunol. 2017, 206, 267–276. [Google Scholar] [CrossRef]
- Guidry, J.T.; Birdwell, C.E.; Scott, R.S. Epstein-Barr virus in the pathogenesis of oral cancers. Oral Dis. 2018, 24, 497–508. [Google Scholar] [CrossRef]
- Guidry, J.T.; Myers, J.E.; Bienkowska-Haba, M.; Songock, W.K.; Ma, X.; Shi, M.; Nathan, C.O.; Bodily, J.M.; Sapp, M.J.; Scott, R.S. Inhibition of Epstein-Barr Virus Replication in Human Papillomavirus-Immortalized Keratinocytes. J. Virol. 2019, 93, e01216-18. [Google Scholar] [CrossRef]
- Nawandar, D.M.; Wang, A.; Makielski, K.; Lee, D.; Ma, S.; Barlow, E.; Reusch, J.; Jiang, R.; Wille, C.K.; Greenspan, D.; et al. Differentiation-Dependent KLF4 Expression Promotes Lytic Epstein-Barr Virus Infection in Epithelial Cells. PLoS Pathog. 2015, 11, e1005195. [Google Scholar] [CrossRef]
- Liang, C.L.; Chen, J.L.; Hsu, Y.P.; Ou, J.T.; Chang, Y.S. Epstein-Barr virus BZLF1 gene is activated by transforming growth factor-beta through cooperativity of Smads and c-Jun/c-Fos proteins. J. Biol. Chem. 2002, 277, 23345–23357. [Google Scholar] [CrossRef] [PubMed]
- Iempridee, T.; Das, S.; Xu, I.; Mertz, J.E. Transforming growth factor beta-induced reactivation of Epstein-Barr virus involves multiple Smad-binding elements cooperatively activating expression of the latent-lytic switch BZLF1 gene. J. Virol. 2011, 85, 7836–7848. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Chavez, J.; Hernandez-Pando, R.; Lambert, P.F.; Gariglio, P. Down-regulation of transforming growth factor-beta type II receptor (TGF-betaRII) protein and mRNA expression in cervical cancer. Mol. Cancer 2008, 7, 3. [Google Scholar] [CrossRef]
- French, D.; Belleudi, F.; Mauro, M.V.; Mazzetta, F.; Raffa, S.; Fabiano, V.; Frega, A.; Torrisi, M.R. Expression of HPV16 E5 down-modulates the TGFbeta signaling pathway. Mol. Cancer 2013, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Makielski, K.R.; Lee, D.; Lorenz, L.D.; Nawandar, D.M.; Chiu, Y.F.; Kenney, S.C.; Lambert, P.F. Human papillomavirus promotes Epstein-Barr virus maintenance and lytic reactivation in immortalized oral keratinocytes. Virology 2016, 495, 52–62. [Google Scholar] [CrossRef]
- Szostek, S.; Zawilinska, B.; Kopec, J.; Kosz-Vnenchak, M. Herpesviruses as possible cofactors in HPV-16-related oncogenesis. Acta Biochim. Pol. 2009, 56, 337–342. [Google Scholar] [CrossRef]
- Kahla, S.; Oueslati, S.; Achour, M.; Kochbati, L.; Chanoufi, M.B.; Maalej, M.; Oueslati, R. Correlation between ebv co-infection and HPV16 genome integrity in Tunisian cervical cancer patients. Braz. J. Microbiol. 2012, 43, 744–753. [Google Scholar] [CrossRef]
- Ressing, M.E.; van Gent, M.; Gram, A.M.; Hooykaas, M.J.; Piersma, S.J.; Wiertz, E.J. Immune Evasion by Epstein-Barr Virus. Curr. Top. Microbiol. Immunol. 2015, 391, 355–381. [Google Scholar] [CrossRef]
- Lo Cigno, I.; Calati, F.; Albertini, S.; Gariglio, M. Subversion of Host Innate Immunity by Human Papillomavirus Oncoproteins. Pathogens 2020, 9, 292. [Google Scholar] [CrossRef]
- Gaudreault, E.; Fiola, S.; Olivier, M.; Gosselin, J. Epstein-Barr virus induces MCP-1 secretion by human monocytes via TLR2. J. Virol. 2007, 81, 8016–8024. [Google Scholar] [CrossRef] [PubMed]
- Iwakiri, D.; Zhou, L.; Samanta, M.; Matsumoto, M.; Ebihara, T.; Seya, T.; Imai, S.; Fujieda, M.; Kawa, K.; Takada, K. Epstein-Barr virus (EBV)-encoded small RNA is released from EBV-infected cells and activates signaling from Toll-like receptor 3. J. Exp. Med. 2009, 206, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.E.; Ma, Y.; Farhat, S.; Moscicki, A.B. Expression of nucleic acid-sensing Toll-like receptors predicts HPV16 clearance associated with an E6-directed cell-mediated response. Int. J. Cancer 2015, 136, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Daud, I.I.; Scott, M.E.; Ma, Y.; Shiboski, S.; Farhat, S.; Moscicki, A.B. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int. J. Cancer 2011, 128, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Jouhi, L.; Mohamed, H.; Mäkitie, A.; Remes, S.M.; Haglund, C.; Atula, T.; Hagström, J. Toll-like receptor 5 and 7 expression may impact prognosis of HPV-positive oropharyngeal squamous cell carcinoma patients. Cancer Immunol. Immunother. 2017, 66, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.A.; Bates, E.; Takeshita, F.; Biliato, A.; Accardi, R.; Bouvard, V.; Mansour, M.; Vincent, I.; Gissmann, L.; Iftner, T.; et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 2007, 178, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Hasan, U.A.; Zannetti, C.; Parroche, P.; Goutagny, N.; Malfroy, M.; Roblot, G.; Carreira, C.; Hussain, I.; Müller, M.; Taylor-Papadimitriou, J.; et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef]
- van Gent, M.; Gram, A.M.; Boer, I.G.J.; Geerdink, R.J.; Lindenbergh, M.F.S.; Lebbink, R.J.; Wiertz, E.J.; Ressing, M.E. Silencing the shutoff protein of Epstein-Barr virus in productively infected B cells points to (innate) targets for immune evasion. J. Gen. Virol. 2015, 96, 858–865. [Google Scholar] [CrossRef]
- van Gent, M.; Griffin, B.D.; Berkhoff, E.G.; van Leeuwen, D.; Boer, I.G.; Buisson, M.; Hartgers, F.C.; Burmeister, W.P.; Wiertz, E.J.; Ressing, M.E. EBV lytic-phase protein BGLF5 contributes to TLR9 downregulation during productive infection. J. Immunol. 2011, 186, 1694–1702. [Google Scholar] [CrossRef]
- van Gent, M.; Braem, S.G.; de Jong, A.; Delagic, N.; Peeters, J.G.; Boer, I.G.; Moynagh, P.N.; Kremmer, E.; Wiertz, E.J.; Ovaa, H.; et al. Epstein-Barr virus large tegument protein BPLF1 contributes to innate immune evasion through interference with toll-like receptor signaling. PLoS Pathog. 2014, 10, e1003960. [Google Scholar] [CrossRef] [PubMed]
- Fathallah, I.; Parroche, P.; Gruffat, H.; Zannetti, C.; Johansson, H.; Yue, J.; Manet, E.; Tommasino, M.; Sylla, B.S.; Hasan, U.A. EBV latent membrane protein 1 is a negative regulator of TLR9. J. Immunol. 2010, 185, 6439–6447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Hang, X.; Xie, L. Toll-like receptor 3 (TLR3) functions as a pivotal target in latent membrane protein 1 (LMP1)-mediated nasopharyngeal carcinoma cell proliferation. Int. J. Clin. Exp. Pathol. 2020, 13, 153–162. [Google Scholar]
- Ruuskanen, M.; Leivo, I.; Minn, H.; Vahlberg, T.; Haglund, C.; Hagström, J.; Irjala, H. Expression of toll-like receptors in non-endemic nasopharyngeal carcinoma. BMC Cancer 2019, 19, 624. [Google Scholar] [CrossRef] [PubMed]
- Fiola, S.; Gosselin, D.; Takada, K.; Gosselin, J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 185, 3620–3631. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.E.; Roman, R.M.; Rudenga, B.J.; Holers, V.M.; Craft, J.E. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum. 2010, 62, 1693–1701. [Google Scholar] [CrossRef]
- Beima-Sofie, K.; Wamalwa, D.; Maleche-Obimbo, E.; Lingappa, J.R.; Mackelprang, R.; Gantt, S.; John-Stewart, G.; Casper, C.; Slyker, J.A. Toll-like receptor 9 polymorphism is associated with increased Epstein-Barr virus and Cytomegalovirus acquisition in HIV-exposed infants. AIDS 2018, 32, 267–270. [Google Scholar] [CrossRef]
- Jabłońska, A.; Studzińska, M.; Szenborn, L.; Wiśniewska-Ligier, M.; Karlikowska-Skwarnik, M.; Gęsicki, T.; Paradowska, E. TLR4 896A/G and TLR9 1174G/A polymorphisms are associated with the risk of infectious mononucleosis. Sci. Rep. 2020, 10, 13154. [Google Scholar] [CrossRef]
- Chiang, H.S.; Liu, H.M. The Molecular Basis of Viral Inhibition of IRF- and STAT-Dependent Immune Responses. Front. Immunol. 2018, 9, 3086. [Google Scholar] [CrossRef]
- Ronco, L.V.; Karpova, A.Y.; Vidal, M.; Howley, P.M. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 1998, 12, 2061–2072. [Google Scholar] [CrossRef]
- Nees, M.; Geoghegan, J.M.; Hyman, T.; Frank, S.; Miller, L.; Woodworth, C.D. Papillomavirus type 16 oncogenes downregulate expression of interferon-responsive genes and upregulate proliferation-associated and NF-kappaB-responsive genes in cervical keratinocytes. J. Virol. 2001, 75, 4283–4296. [Google Scholar] [CrossRef] [PubMed]
- Raikhy, G.; Woodby, B.L.; Scott, M.L.; Shin, G.; Myers, J.E.; Scott, R.S.; Bodily, J.M. Suppression of Stromal Interferon Signaling by Human Papillomavirus 16. J. Virol. 2019, 93, e00458-19. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Hurst, J.; Voges, M.; Krauss, P.; Münch, P.; Iftner, T.; Stubenrauch, F. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J. Virol. 2011, 85, 11372–11380. [Google Scholar] [CrossRef]
- DeCarlo, C.A.; Severini, A.; Edler, L.; Escott, N.G.; Lambert, P.F.; Ulanova, M.; Zehbe, I. IFN-κ, a novel type I IFN, is undetectable in HPV-positive human cervical keratinocytes. Lab. Investig. 2010, 90, 1482–1491. [Google Scholar] [CrossRef][Green Version]
- Rincon-Orozco, B.; Halec, G.; Rosenberger, S.; Muschik, D.; Nindl, I.; Bachmann, A.; Ritter, T.M.; Dondog, B.; Ly, R.; Bosch, F.X.; et al. Epigenetic silencing of interferon-kappa in human papillomavirus type 16-positive cells. Cancer Res. 2009, 69, 8718–8725. [Google Scholar] [CrossRef]
- Sunthamala, N.; Thierry, F.; Teissier, S.; Pientong, C.; Kongyingyoes, B.; Tangsiriwatthana, T.; Sangkomkamhang, U.; Ekalaksananan, T. E2 proteins of high risk human papillomaviruses down-modulate STING and IFN-κ transcription in keratinocytes. PLoS ONE 2014, 9, e91473. [Google Scholar] [CrossRef]
- Hahn, A.M.; Huye, L.E.; Ning, S.; Webster-Cyriaque, J.; Pagano, J.S. Interferon regulatory factor 7 is negatively regulated by the Epstein-Barr virus immediate-early gene, BZLF-1. J. Virol. 2005, 79, 10040–10052. [Google Scholar] [CrossRef]
- Bentz, G.L.; Liu, R.; Hahn, A.M.; Shackelford, J.; Pagano, J.S. Epstein-Barr virus BRLF1 inhibits transcription of IRF3 and IRF7 and suppresses induction of interferon-beta. Virology 2010, 402, 121–128. [Google Scholar] [CrossRef]
- Morrison, T.E.; Mauser, A.; Wong, A.; Ting, J.P.; Kenney, S.C. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 2001, 15, 787–799. [Google Scholar] [CrossRef]
- Wu, L.; Fossum, E.; Joo, C.H.; Inn, K.S.; Shin, Y.C.; Johannsen, E.; Hutt-Fletcher, L.M.; Hass, J.; Jung, J.U. Epstein-Barr virus LF2: An antagonist to type I interferon. J. Virol. 2009, 83, 1140–1146. [Google Scholar] [CrossRef]
- Wang, J.T.; Doong, S.L.; Teng, S.C.; Lee, C.P.; Tsai, C.H.; Chen, M.R. Epstein-Barr virus BGLF4 kinase suppresses the interferon regulatory factor 3 signaling pathway. J. Virol. 2009, 83, 1856–1869. [Google Scholar] [CrossRef]
- Hooykaas, M.J.G.; van Gent, M.; Soppe, J.A.; Kruse, E.; Boer, I.G.J.; van Leenen, D.; Groot Koerkamp, M.J.A.; Holstege, F.C.P.; Ressing, M.E.; Wiertz, E.J.H.J.; et al. EBV MicroRNA BART16 Suppresses Type I IFN Signaling. J. Immunol. 2017, 198, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qin, Z.; Wang, J.; Zheng, X.; Lu, J.; Zhang, X.; Wei, L.; Peng, Q.; Zheng, Y.; Ou, C.; et al. Epstein-Barr Virus miR-BART6-3p Inhibits the RIG-I Pathway. J. Innate Immun. 2017, 9, 574–586. [Google Scholar] [CrossRef]
- Berti, F.C.B.; Pereira, A.P.L.; Cebinelli, G.C.M.; Trugilo, K.P.; Brajão de Oliveira, K. The role of interleukin 10 in human papilloma virus infection and progression to cervical carcinoma. Cytokine Growth Factor Rev. 2017, 34, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Petrini, C.G.; Bastos, L.B.; Duarte, G.; Dos Santos Melli, P.P.; Alves-Filho, J.C.; Quintana, S.M. Downregulation of IL-2 and IL-23 in Cervical Biopsies of Cervical Intraepithelial Lesions: A Cross-Sectional Study. Acta Cytol. 2020, 64, 442–451. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kang, J.W.; Cho, M.; Cho, C.W.; Lee, S.; Choe, Y.K.; Kim, Y.; Choi, I.; Park, S.N.; Kim, S.; et al. Down modulation of IL-18 expression by human papillomavirus type 16 E6 oncogene via binding to IL-18. FEBS Lett. 2001, 501, 139–145. [Google Scholar] [CrossRef]
- Yasuda, K.; Nakanishi, K.; Tsutsui, H. Interleukin-18 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 649. [Google Scholar] [CrossRef]
- Matamoros, J.A.; da Silva, M.I.F.; de Moura, P.M.M.F.; Leitão, M.D.C.G.; Coimbra, E.C. Reduced Expression of IL-1β and IL-18 Proinflammatory Interleukins Increases the Risk of Developing Cervical Cancer. Asian Pac. J. Cancer Prev. 2019, 20, 2715–2721. [Google Scholar] [CrossRef]
- Havard, L.; Delvenne, P.; Fraré, P.; Boniver, J.; Giannini, S.L. Differential production of cytokines and activation of NF-kappaB in HPV-transformed keratinocytes. Virology 2002, 298, 271–285. [Google Scholar] [CrossRef]
- Niiro, H.; Otsuka, T.; Abe, M.; Satoh, H.; Ogo, T.; Nakano, T.; Furukawa, Y.; Niho, Y. Epstein-Barr virus BCRF1 gene product (viral interleukin 10) inhibits superoxide anion production by human monocytes. Lymphokine Cytokine Res. 1992, 11, 209–214. [Google Scholar]
- Cohen, J.I.; Lekstrom, K. Epstein-Barr virus BARF1 protein is dispensable for B-cell transformation and inhibits alpha interferon secretion from mononuclear cells. J. Virol. 1999, 73, 7627–7632. [Google Scholar] [CrossRef]
- Shim, A.H.; Chang, R.A.; Chen, X.; Longnecker, R.; He, X. Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein-Barr virus BARF1. Proc. Natl. Acad. Sci. USA 2012, 109, 12962–12967. [Google Scholar] [CrossRef] [PubMed]
- Wiech, T.; Nikolopoulos, E.; Lassman, S.; Heidt, T.; Schöpflin, A.; Sarbia, M.; Werner, M.; Shimizu, Y.; Sakka, E.; Ooka, T.; et al. Cyclin D1 expression is induced by viral BARF1 and is overexpressed in EBV-associated gastric cancer. Virchows Arch. 2008, 452, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Dotan, S.; Krelin, Y.; Song, X.; Elkabets, M.; Carmi, Y.; Rider, P.; Cohen, I.; Romzova, M.; Kaplanov, I.; et al. Unique Versus Redundant Functions of IL-1α and IL-1β in the Tumor Microenvironment. Front. Immunol. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Skinner, C.M.; Ivanov, N.S.; Barr, S.A.; Chen, Y.; Skalsky, R.L. An Epstein-Barr Virus MicroRNA Blocks Interleukin-1 (IL-1) Signaling by Targeting IL-1 Receptor 1. J. Virol. 2017, 91, e00530-17. [Google Scholar] [CrossRef] [PubMed]
- Niebler, M.; Qian, X.; Höfler, D.; Kogosov, V.; Kaewprag, J.; Kaufmann, A.M.; Ly, R.; Böhmer, G.; Zawatzky, R.; Rösl, F.; et al. Post-translational control of IL-1β via the human papillomavirus type 16 E6 oncoprotein: A novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013, 9, e1003536. [Google Scholar] [CrossRef]
- Szabo, S.J.; Sullivan, B.M.; Peng, S.L.; Glimcher, L.H. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol. 2003, 21, 713–758. [Google Scholar] [CrossRef]
- Haabeth, O.A.; Lorvik, K.B.; Yagita, H.; Bogen, B.; Corthay, A. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 2016, 5, e1039763. [Google Scholar] [CrossRef]
- Spitkovsky, D.; Hehner, S.P.; Hofmann, T.G.; Möller, A.; Schmitz, M.L. The human papillomavirus oncoprotein E7 attenuates NF-kappa B activation by targeting the Ikappa B kinase complex. J. Biol. Chem. 2002, 277, 25576–25582. [Google Scholar] [CrossRef]
- Havard, L.; Rahmouni, S.; Boniver, J.; Delvenne, P. High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: Role of E6 and E7 oncoproteins. Virology 2005, 331, 357–366. [Google Scholar] [CrossRef]
- Patel, D.; Huang, S.M.; Baglia, L.A.; McCance, D.J. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 1999, 18, 5061–5072. [Google Scholar] [CrossRef]
- Huang, S.M.; McCance, D.J. Down regulation of the interleukin-8 promoter by human papillomavirus type 16 E6 and E7 through effects on CREB binding protein/p300 and P/CAF. J. Virol. 2002, 76, 8710–8721. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.E.; Kenney, S.C. BZLF1, an Epstein-Barr virus immediate-early protein, induces p65 nuclear translocation while inhibiting p65 transcriptional function. Virology 2004, 328, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.; Dawson, C.W.; Hu, C.; Shah, K.M.; Owen, T.J.; Date, K.L.; Maia, S.P.; Shao, J.; Arrand, J.R.; Young, L.S.; et al. Epstein-Barr virus-encoded EBNA1 inhibits the canonical NF-kappaB pathway in carcinoma cells by inhibiting IKK phosphorylation. Mol. Cancer 2010, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.S.; Wang, J.T.; Doong, S.L.; Lee, C.P.; Chang, C.W.; Tsai, C.H.; Yeh, S.W.; Hsieh, C.Y.; Chen, M.R. Epstein-Barr virus BGLF4 kinase downregulates NF-κB transactivation through phosphorylation of coactivator UXT. J. Virol. 2012, 86, 12176–12186. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, Y.; Xu, Z.; Zou, X.; Wang, P.; Ou, X.; Li, Y.; Peng, T.; Chen, D.; Li, M.; et al. Epstein-Barr virus tegument protein BGLF2 inhibits NF-κB activity by preventing p65 Ser536 phosphorylation. FASEB J. 2019, 33, 10563–10576. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zheng, H.; Duan, Z.; Hu, D.; Li, M.; Liu, S.; Li, Z.; Deng, X.; Wang, Z.; Tang, M.; et al. LMP1-augmented kappa intron enhancer activity contributes to upregulation expression of Ig kappa light chain via NF-kappaB and AP-1 pathways in nasopharyngeal carcinoma cells. Mol. Cancer 2009, 8, 92. [Google Scholar] [CrossRef]
- Chang, M.S.; Kim, D.H.; Roh, J.K.; Middeldorp, J.M.; Kim, Y.S.; Kim, S.; Han, S.; Kim, C.W.; Lee, B.L.; Kim, W.H.; et al. Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric carcinoma cells through regulation of NF-κB. J. Virol. 2013, 87, 10515–10523. [Google Scholar] [CrossRef]
- Karin, M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a000141. [Google Scholar] [CrossRef]
- Vandermark, E.R.; Deluca, K.A.; Gardner, C.R.; Marker, D.F.; Schreiner, C.N.; Strickland, D.A.; Wilton, K.M.; Mondal, S.; Woodworth, C.D. Human papillomavirus type 16 E6 and E 7 proteins alter NF-kB in cultured cervical epithelial cells and inhibition of NF-kB promotes cell growth and immortalization. Virology 2012, 425, 53–60. [Google Scholar] [CrossRef]
- de Freitas, A.C.; de Oliveira, T.H.A.; Barros, M.R.; Venuti, A. hrHPV E5 oncoprotein: Immune evasion and related immunotherapies. J. Exp. Clin. Cancer Res. 2017, 36, 71. [Google Scholar] [CrossRef] [PubMed]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-regulates expression of surface HLA class I and reduces recognition by CD8 T cells. Virology 2010, 407, 137–142. [Google Scholar] [CrossRef]
- Georgopoulos, N.T.; Proffitt, J.L.; Blair, G.E. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1 and LMP2 genes by the human papillomavirus (HPV) type 6b, 16 and 18 E7 oncoproteins. Oncogene 2000, 19, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Cyprian, F.S.; Al-Farsi, H.F.; Vranic, S.; Akhtar, S.; Al Moustafa, A.E. Epstein-Barr Virus and Human Papillomaviruses Interactions and Their Roles in the Initiation of Epithelial-Mesenchymal Transition and Cancer Progression. Front. Oncol. 2018, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Al Moustafa, A.E.; Chen, D.; Ghabreau, L.; Akil, N. Association between human papillomavirus and Epstein-Barr virus infections in human oral carcinogenesis. Med. Hypotheses 2009, 73, 184–186. [Google Scholar] [CrossRef]
- Al-Thawadi, H.; Ghabreau, L.; Aboulkassim, T.; Yasmeen, A.; Vranic, S.; Batist, G.; Al Moustafa, A.E. Co-Incidence of Epstein-Barr Virus and High-Risk Human Papillomaviruses in Cervical Cancer of Syrian Women. Front. Oncol. 2018, 8, 250. [Google Scholar] [CrossRef]
- Lo, A.K.; Dawson, C.W.; Lo, K.W.; Yu, Y.; Young, L.S. Upregulation of Id1 by Epstein-Barr virus-encoded LMP1 confers resistance to TGFbeta-mediated growth inhibition. Mol. Cancer 2010, 9, 155. [Google Scholar] [CrossRef]
- Lin, J.; Guan, Z.; Wang, C.; Feng, L.; Zheng, Y.; Caicedo, E.; Bearth, E.; Peng, J.R.; Gaffney, P.; Ondrey, F.G. Inhibitor of differentiation 1 contributes to head and neck squamous cell carcinoma survival via the NF-kappaB/survivin and phosphoinositide 3-kinase/Akt signaling pathways. Clin. Cancer Res. 2010, 16, 77–87. [Google Scholar] [CrossRef]
- Shimabuku, T.; Tamanaha, A.; Kitamura, B.; Tanabe, Y.; Tawata, N.; Ikehara, F.; Arakaki, K.; Kinjo, T. Dual expression of Epstein-Barr virus, latent membrane protein-1 and human papillomavirus-16 E6 transform primary mouse embryonic fibroblasts through NF-κB signaling. Int. J. Clin. Exp. Pathol. 2014, 7, 1920–1934. [Google Scholar]
- Uehara, K.; Tanabe, Y.; Hirota, S.; Higa, S.; Toyoda, Z.; Kurima, K.; Kina, S.; Nakasone, T.; Arasaki, A.; Kinjo, T. Co-expression of low-risk HPV E6/E7 and EBV LMP-1 leads to precancerous lesions by DNA damage. BMC Cancer 2021, 21, 688. [Google Scholar] [CrossRef]
- Al Moustafa, A.E.; Al-Antary, N.; Aboulkassim, T.; Akil, N.; Batist, G.; Yasmeen, A. Co-prevalence of Epstein-Barr virus and high-risk human papillomaviruses in Syrian women with breast cancer. Hum. Vaccin. Immunother. 2016, 12, 1936–1939. [Google Scholar] [CrossRef] [PubMed]
- Jarzynski, A.; Boguszewska, A.; Kliszczewska, E.; Rolniak, L.; Dworzanska, A.; Polz-Dacewicz, M. TP53 promoter methylation in EBV and HPV associated oropharyngeal carcinoma—A pilot study. Curr. Issues Pharm. Med. Sci. 2017, 30, 119–122. [Google Scholar] [CrossRef]
- McCormick, T.M.; Canedo, N.H.; Furtado, Y.L.; Silveira, F.A.; de Lima, R.J.; Rosman, A.D.; Almeida Filho, G.L.; Carvalho, M.a.G. Association between human papillomavirus and Epstein—Barr virus DNA and gene promoter methylation of RB1 and CDH1 in the cervical lesions: A transversal study. Diagn. Pathol. 2015, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Lattario, F.; Furtado, Y.L.; Fonseca, R.; Silveira, F.A.; do Val, I.C.; Almeida, G.; Carvalho, M.G. Analysis of human papillomavirus and Epstein-Barr virus infection and aberrant death-associated protein kinase methylation in high-grade squamous intraepithelial lesions. Int. J. Gynecol. Cancer 2008, 18, 785–789. [Google Scholar] [CrossRef]
- Singh, P.; Ravanan, P.; Talwar, P. Death Associated Protein Kinase 1 (DAPK1): A Regulator of Apoptosis and Autophagy. Front. Mol. Neurosci. 2016, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Strycharz-Dudziak, M.; Fołtyn, S.; Dworzański, J.; Kiełczykowska, M.; Malm, M.; Drop, B.; Polz-Dacewicz, M. Glutathione Peroxidase (GPx) and Superoxide Dismutase (SOD) in Oropharyngeal Cancer Associated with EBV and HPV Coinfection. Viruses 2020, 12, 1008. [Google Scholar] [CrossRef]
- Sharma, U.; Singhal, P.; Bandil, K.; Patle, R.; Kumar, A.; Neyaz, K.; Bose, S.; Kumar Dewan, A.; Mehrotra, R.; Sharma, V.; et al. Genetic variations of TLRs and their association with HPV/EBV, co-infection along with nicotine exposure in the development of premalignant/malignant lesions of the oral cavity in Indian population. Cancer Epidemiol. 2019, 61, 38–49. [Google Scholar] [CrossRef]
- Yang, Z.H.; Dai, Q.; Gu, Y.J.; Guo, Q.X.; Gong, L. Cytokine and chemokine modification by Toll-like receptor polymorphisms is associated with nasopharyngeal carcinoma. Cancer Sci. 2012, 103, 653–658. [Google Scholar] [CrossRef]
- Dai, Q.; Li, X.P.; Chai, L.; Long, H.A.; Yang, Z.H. Polymorphisms of Toll-like receptor 9 are associated with nasopharyngeal carcinoma susceptibility. Tumor Biol. 2014, 35, 3247–3253. [Google Scholar] [CrossRef]
- Aromseree, S.; Middeldorp, J.M.; Pientong, C.; van Eijndhoven, M.; Ramayanti, O.; Lougheed, S.M.; Pegtel, D.M.; Steenbergen, R.D.; Ekalaksananan, T. High Levels of EBV-Encoded RNA 1 (EBER1) Trigger Interferon and Inflammation-Related Genes in Keratinocytes Expressing HPV16 E6/E7. PLoS ONE 2017, 12, e0169290. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).