Overexpression of Cu/Zn Superoxide Dismutase (Cu/Zn SOD) in Synechococcus elongatus PCC 7942 for Enhanced Azo Dye Removal through Hydrogen Peroxide Accumulation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cyanobacterium and E. coli Growth Conditions
2.2. Vector and Primers Used for Cloning of sodC
2.3. E. coli Transformation and PCR Confirmation
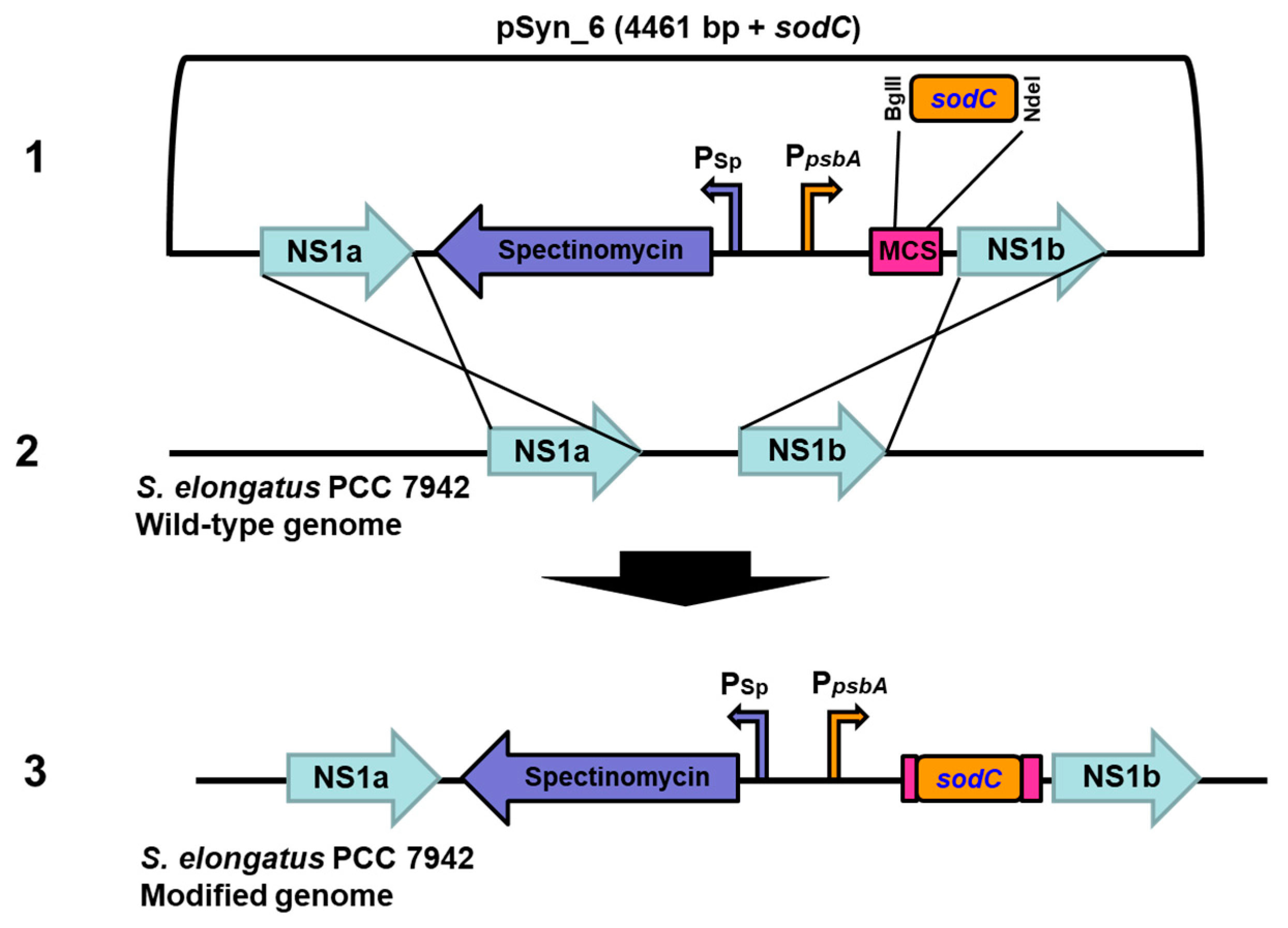
2.4. Transformation of sodC to S. elongatus PCC 7942 by Homologous Recombination
2.5. Experimental Conditions and Growth Analysis
2.6. Preparation of Enzyme Extracts and Enzyme Activity
2.7. Decolorization and Degradation Assay
2.8. Determination of H2O2 Production
2.9. Statistical Analysis
3. Results and Discussion
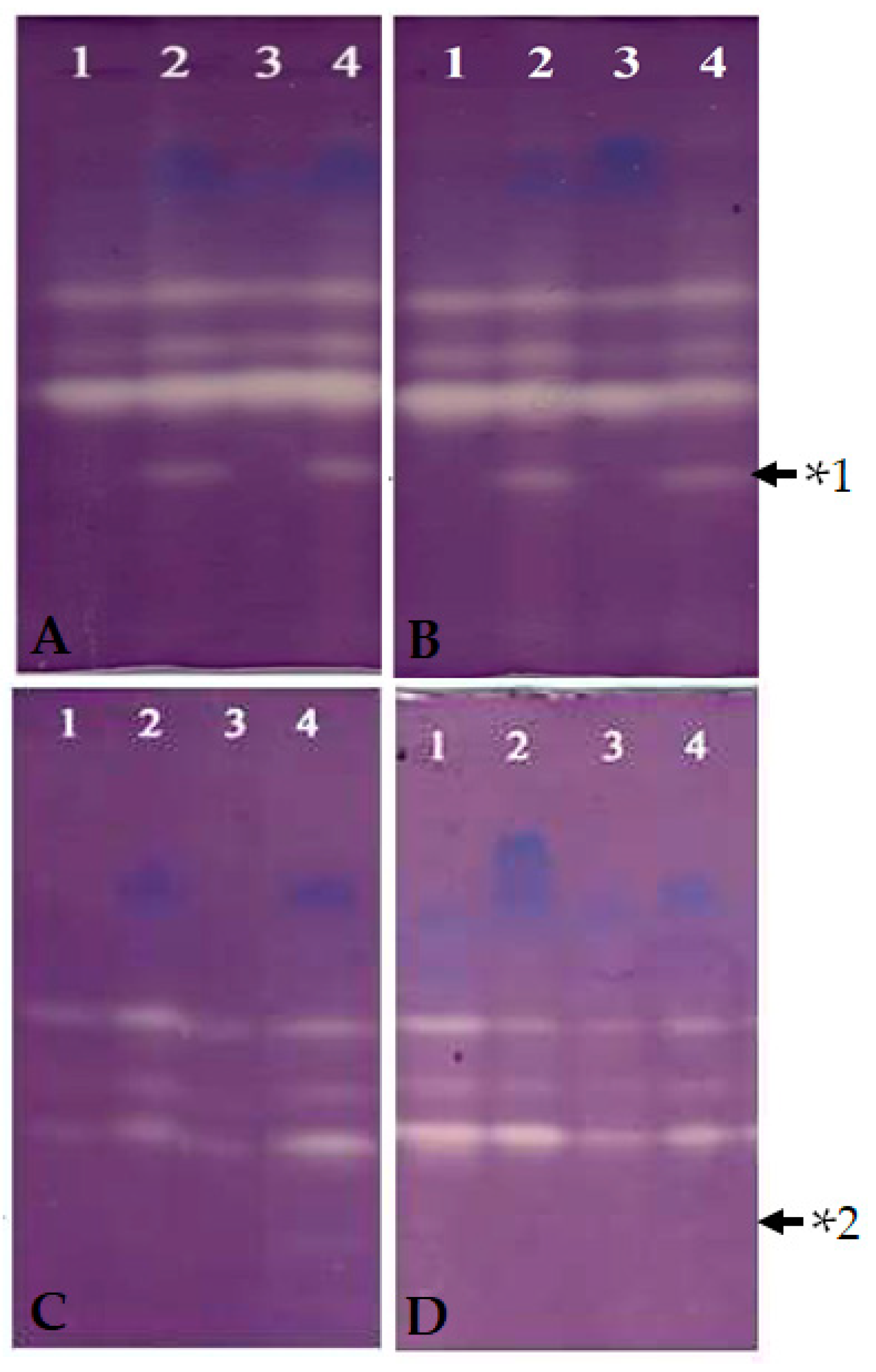
3.1. Transformation and Expression of Cu/Zn SOD in S. elongatus PCC 7942
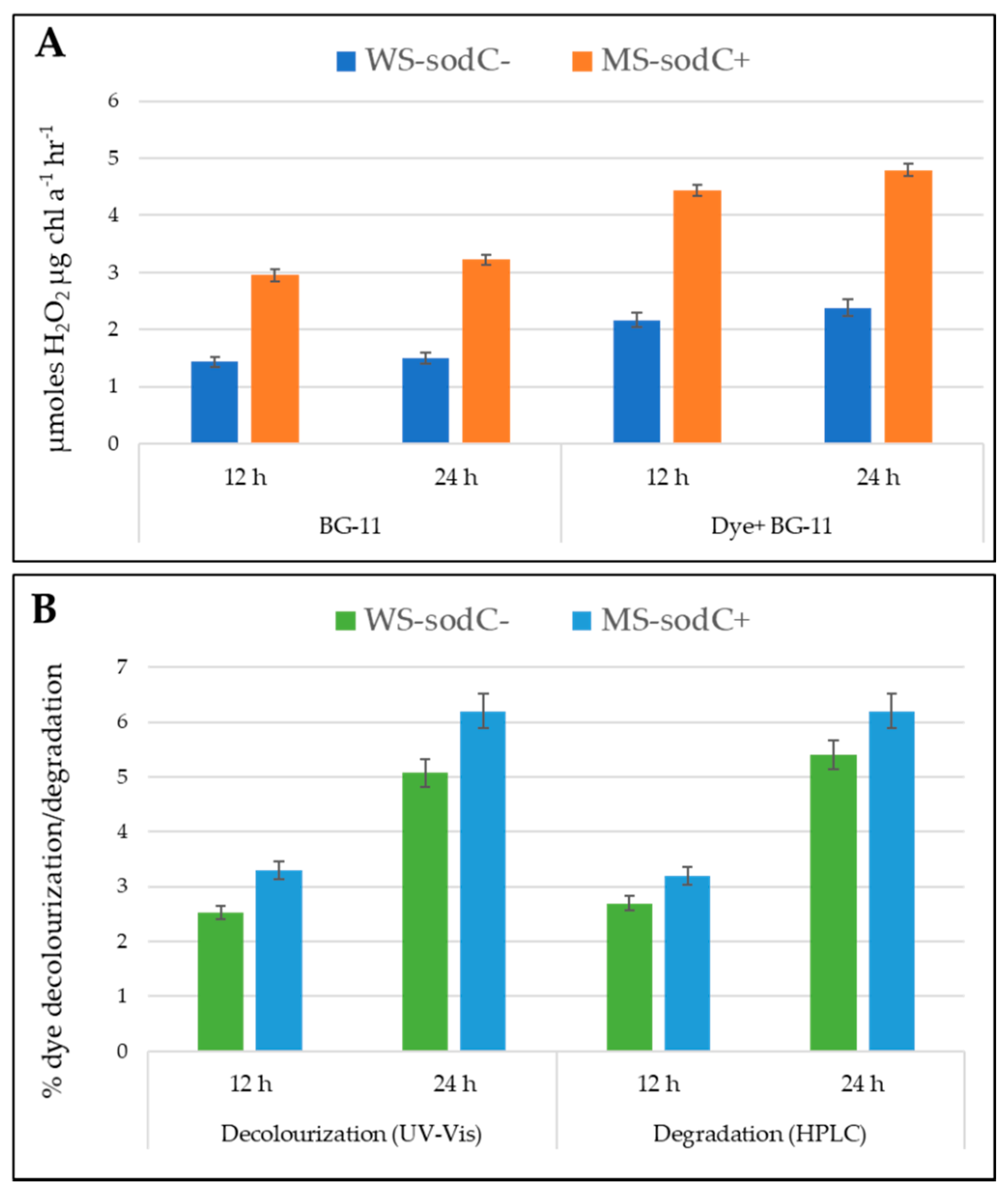
3.2. Hydrogen Peroxide Accumulation in Transgenic S. elongatus PCC 7942
3.3. Role of H2O2 in Transgenic S. elongatus PCC 7942
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dellamatrice, P.M.; Silva-Stenico, M.E.; Moraes, L.A.B.D.; Fiore, M.F.; Monteiro, R.T.R. Degradation of textile dyes by cyanobacteria. Braz. J. Microbiol. 2017, 48, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Priya, B.; Uma, L.; Ahamed, A.K.; Subramanian, G.; Prabaharan, D. Ability to use the diazo dye, CI Acid Black 1 as a nitrogen source by the marine cyanobacterium Oscillatoria curviceps BDU92191. Bioresour. Technol. 2011, 102, 7218–7223. [Google Scholar] [CrossRef]
- Dangi, A.K.; Sharma, B.; Hill, R.T.; Shukla, P. Bioremediation through microbes: Systems biology and metabolic engineering approach. Crit. Rev. Biotechnol. 2019, 39, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E. The early history of atmospheric oxygen: Homage to Robert M. Garrels. Annu. Rev. Earth Planet. Sci. 2005, 33, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Latifi, A.; Ruiz, M.; Zhang, C.C. Oxidative stress in cyanobacteria. FEMS Microbiol. Rev. 2009, 33, 258–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2005, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Badger, M.R. Photosynthetic oxygen exchange. Ann. Rev. Plant Physiol. 1985, 36, 27–53. [Google Scholar] [CrossRef]
- Priya, B.; Premanandh, J.; Dhanalakshmi, R.T.; Seethalakshmi, T.; Uma, L.; Prabaharan, D.; Subramanian, G. Comparative analysis of cyanobacterial superoxide dismutases to discriminate canonical forms. BMC Genom. 2007, 8, 435. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Guo, C.; Li, C.; Xiao, K. Cloning, characterization and expression analysis of two superoxide dismutase (SOD) genes in wheat (Triticum aestivum L.). Front. Agric. China 2008, 2, 141–149. [Google Scholar] [CrossRef]
- Takeshima, Y.; Takatsugu, N.; Sugiura, M.; Hagiwara, H. High-level expression of human superoxide dismutase in the cyanobacterium Anacystis nidulans 6301. Proc. Natl. Acad. Sci. USA 1994, 91, 9685–9689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signalling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell. Biol. 2014, 5, 411–421. [Google Scholar] [CrossRef]
- Samuilov, V.D.; Bezryadnov, D.B.; Gusev, M.V.; Kitashov, A.V.; Fedorenko, T.A. Hydrogen peroxide inhibits photosynthetic electron transport in cells of cyanobacteria. Biochemistry 2001, 66, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Drábková, M.; Matthijs, H.C.P.; Admiraal, W.; Maršálek, B. Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 2007, 45, 363–369. [Google Scholar] [CrossRef]
- Miller, A.G.; Hunter, K.J.; O’Leary, S.J.; Hart, L.J. The Photoreduction of H2O2 by Synechococcus sp. PCC 7942 and UTEX 625. Plant Physiol. 2009, 123, 625–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef]
- Bertani, G. Studies on lysogenesis I.: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.K.; Swaminathan, P.; Raghavan, C.; Uma, L.; Subramanian, G. Ligninolytic and antioxidative enzymes of a marine cyanobacterium Oscillatoria willei BDU 130511 during Poly R-478 decolourization. Bioresour. Technol. 2010, 101, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Chadd, H.E.; Newman, J.; Mann, N.H.; Carr, N.G. Identification of iron superoxide dismutase and a copper/zinc superoxide dismutase enzyme activity within the marine cyanobacterium Synechococcus sp. WH 7803. FEMS Microbiol. Lett. 1996, 138, 161–165. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Green, M.J.; Hill, H.A.O. Chemistry of dioxygen. Methods Enzymol. 1984, 105, 3–22. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeter. Biodegr. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Protection Strategies for Sustainable Development; Springer: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Ananthashankar, R.; Alhattab, M.V.V.R.; Ramakrishnan, V.V. Production, characterization and treatment of textile effluents: A critical review. J. Chem. Eng. Process Technol. 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Joutey, N.T.; Bahafid, W.; Sayel, H.; El Ghachtouli, N. Biodegradation: Involved Microorganisms and Genetically Engineered Microorganisms. In Biodegradation-Life of Science; Chamy, R., Rosenkranz, F., Eds.; InTech Open: Rijeka, Croatia, 2013; pp. 290–308. [Google Scholar] [CrossRef] [Green Version]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Uma, L.; Subramanian, G. Nitrogen stress induced changes in the marine cyanobacterium Oscillatoria willei BDU 130511. FEMS Microbiol. Ecol. 2003, 45, 263–272. [Google Scholar] [CrossRef]
- Subramanian, G.; Uma, L. Potential Applications of Cyanobacteria in Environmental Biotechnology Photosynthetic Microorganisms in Environmental Biotechnology; Springer: Hong Kong, China, 2001; pp. 41–49. ISBN 9624301360/9789624301366. [Google Scholar]
- Pattamapitoon, T.; Sirirote, P.; Pakkong, P.; Chunkao, K. Nature of solar radiation as encouraged to produce an increment of dissolved oxygen and hydrogen peroxide in oxidation ponds for community wastewater treatment at HM The King’s LERD project site in Phetchaburi Province, Thailand. Mod. Appl. Sci. 2013, 7, 1–26. [Google Scholar] [CrossRef]
- Patterson, C.P.; Myers, J. Photosynthetic production of hydrogen peroxide by Anacystis nidulans. Plant Physiol. 1973, 51, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncel, M.; Navarro, J.A.; De la Rosa, M.A. Coupling of Solar Energy to Hydrogen Peroxide Production in the Cyanobacterium Anacystis nidulans. Appl. Environ. Microbiol. 1989, 55, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, S.E., Jr.; Patterson, C.P.; Myers, J. The production of hydrogen peroxide by blue-green algae: A survey. J. Phycol. 1973, 9, 427–430. [Google Scholar] [CrossRef]
- Robinson, J.M.; Smith, M.G.; Gibbs, M. Influence of hydrogen peroxide upon carbon dioxide photoassimilation in the spinach chloroplasts. I. Hydrogen peroxide generated by broken chloroplasts in an “intact” chloroplast preparation is a causal agent of the Warburg effect. Plant Physiol. 1980, 65, 755–759. [Google Scholar] [CrossRef]
- Priya, B.; Sivaprasanth, R.K.; Jensi, V.D.; Uma, L.; Subramanian, G.; Prabaharan, D. Characterization of manganese superoxide dismutase from a marine cyanobacterium Leptolyngbya valderiana BDU 20041. Saline Syst. 2010, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Berwal, M.K.; Ram, C. Superoxide Dismutase: A stable biochemical marker for abiotic stress tolerance in higher plants. In Abiotic and Biotic Stress in Plants; De Oliveira, A.B., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, L.; Dong, F.; Gao, J.; Galbraith, D.W.; Song, C.P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef] [Green Version]
- Zhang, A.; Jiang, M.; Zhang, J.; Tan, M.; Hu, X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 2006, 141, 475–487. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Han, X.; Song, X.; Zhang, Y.; Jiang, J.; Han, Q.; Liu, M.; Qiao, G.; Zhuo, R. Overexpressing the Sedum alfredii Cu/Zn superoxide dismutase increased resistance to oxidative stress in transgenic Arabidopsis. Front. Plant Sci. 2017, 8, 1010. [Google Scholar] [CrossRef] [Green Version]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta (BBA)-Biomembr. 2006, 8, 994–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.M.; Zou, Y.N.; Wu, Q.S. Alleviation of drought stress by mycorrhizas is related to increased root H2O2 efflux in trifoliate orange. Sci. Rep. 2017, 7, 42335. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Biological production, detection, and fate of hydrogen peroxide. Antiox. Redox Sign. 2018, 29, 541–559. [Google Scholar] [CrossRef] [PubMed]
- Tabaï, A.; Bechiri, O.; Abbessi, M. Study of the degradation of a toxic dye by the catalytic system (H1.5Fe1.5P2W12Mo6O61, 22H2O)/H2O2. Euro-Mediterr. J. Environ. Integr. 2017, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, G.D.; Saranya, R. Influence of H2O2 Activation with Doped Catalyst for Treating Recalcitrant Textile Dyes. J. Thermodyn. Catal. 2018, 9, 2. [Google Scholar] [CrossRef]
- Gholami-Borujeni, F.; Mahvi, A.H.; Nasseri, S.; Faramarzi, M.A.; Nabizadeh, R.; Alimohammadi, M. Enzymatic treatment and detoxification of acid orange 7 from textile wastewater. App. Biochem. Biotechnol. 2011, 165, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, J.; Ohura, R.; Iwasaki, T.; Takeuchi, Y.; Kashiwada, A.; Nango, M. Decoloration of Azo Dyes by Hydrogen Peroxide Catalyzed by Water-Soluble Manganese Porphyrins. Textile Res. J. 1999, 69, 956–960. [Google Scholar] [CrossRef]
- Bennett, J.; Miah, Y.A.; Varsani, D.S.; Salvadori, E.; Sheriff, T.S. Selective oxidative degradation of azo dyes by hydrogen peroxide catalysed by manganese(II) ions. RSC Adv. 2016, 6, 103372–103381. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohandass, S.; Ragavan, M.; Gnanasekaran, D.; Lakshmanan, U.; Dharmar, P.; Saha, S.K. Overexpression of Cu/Zn Superoxide Dismutase (Cu/Zn SOD) in Synechococcus elongatus PCC 7942 for Enhanced Azo Dye Removal through Hydrogen Peroxide Accumulation. Biology 2021, 10, 1313. https://doi.org/10.3390/biology10121313
Mohandass S, Ragavan M, Gnanasekaran D, Lakshmanan U, Dharmar P, Saha SK. Overexpression of Cu/Zn Superoxide Dismutase (Cu/Zn SOD) in Synechococcus elongatus PCC 7942 for Enhanced Azo Dye Removal through Hydrogen Peroxide Accumulation. Biology. 2021; 10(12):1313. https://doi.org/10.3390/biology10121313
Chicago/Turabian StyleMohandass, ShylajaNaciyar, Mangalalakshmi Ragavan, Dineshbabu Gnanasekaran, Uma Lakshmanan, Prabaharan Dharmar, and Sushanta Kumar Saha. 2021. "Overexpression of Cu/Zn Superoxide Dismutase (Cu/Zn SOD) in Synechococcus elongatus PCC 7942 for Enhanced Azo Dye Removal through Hydrogen Peroxide Accumulation" Biology 10, no. 12: 1313. https://doi.org/10.3390/biology10121313
APA StyleMohandass, S., Ragavan, M., Gnanasekaran, D., Lakshmanan, U., Dharmar, P., & Saha, S. K. (2021). Overexpression of Cu/Zn Superoxide Dismutase (Cu/Zn SOD) in Synechococcus elongatus PCC 7942 for Enhanced Azo Dye Removal through Hydrogen Peroxide Accumulation. Biology, 10(12), 1313. https://doi.org/10.3390/biology10121313






