Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
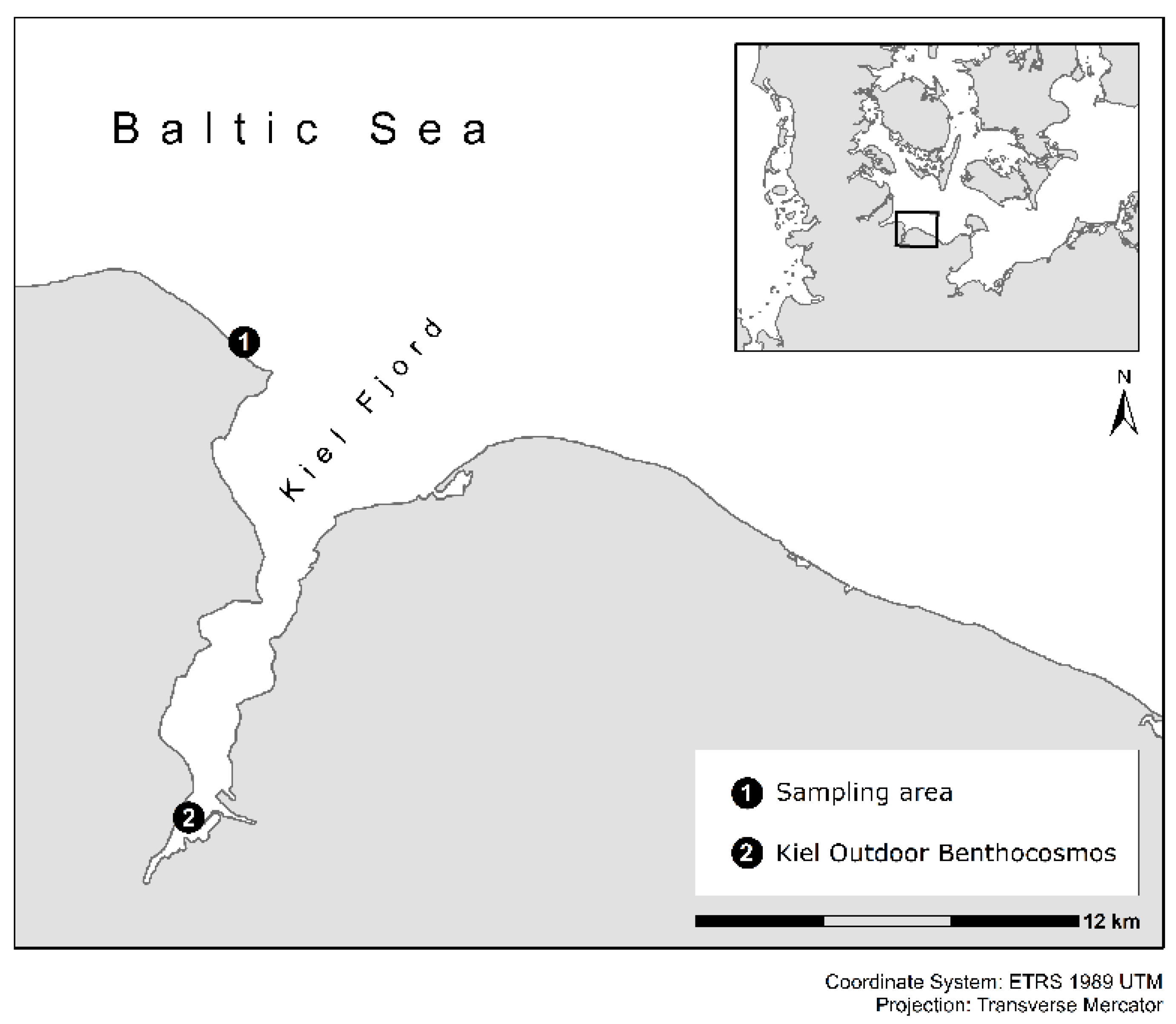
2.1. Experimental Setup and Conditions in the Kiel Outdoor Benthocosms
2.2. Treatments
2.3. Fucus vesiculosus Sampling and Response Variables
2.4. Assay for Detection of F. vesiculosus’ Antioxidative Potential
2.5. Lipid Peroxidation, Superoxide Dismutase (SOD), and Protein Assay
2.6. Statistical Analyses
3. Results
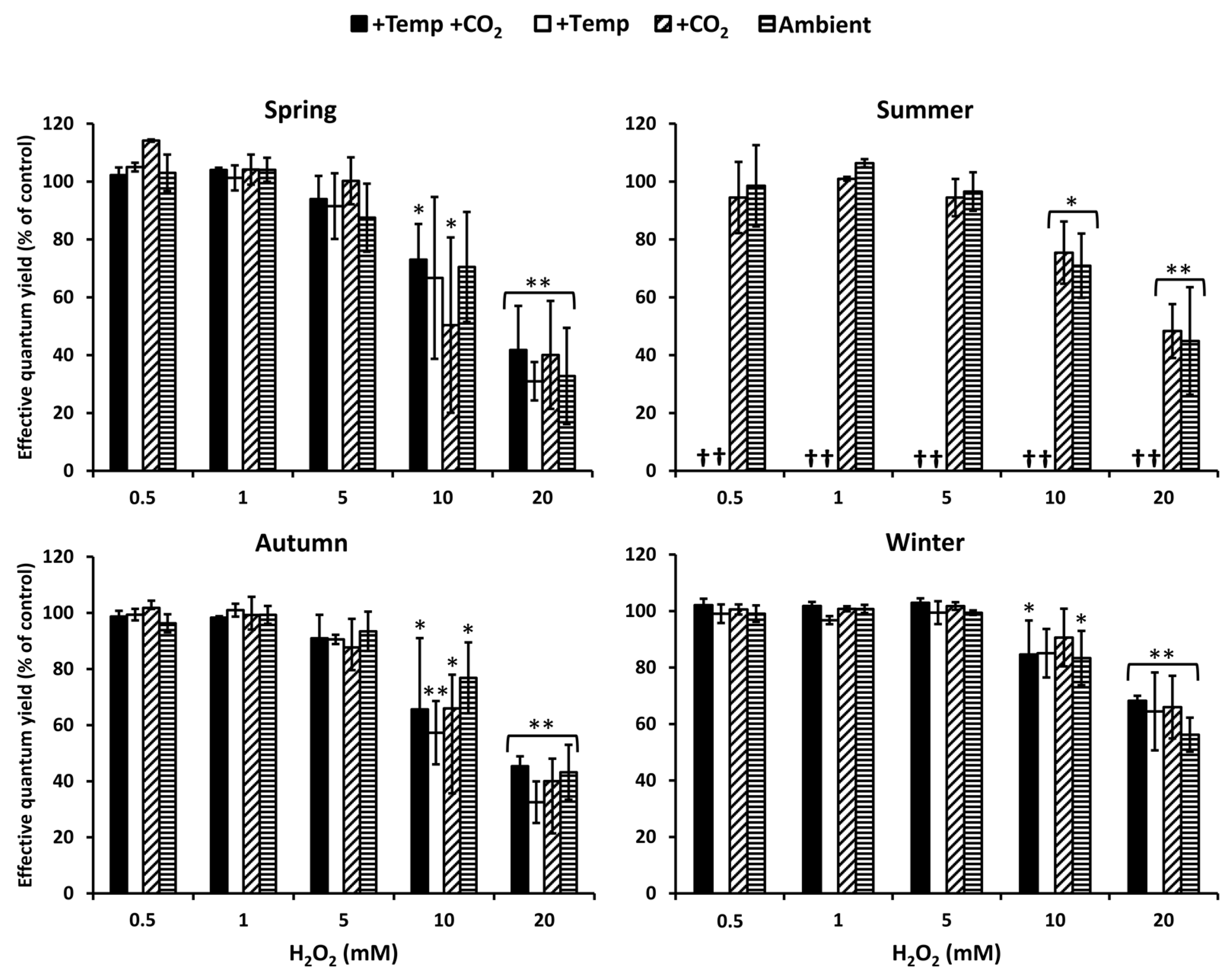
3.1. Antioxidative Potential
3.2. Lipid Peroxidation
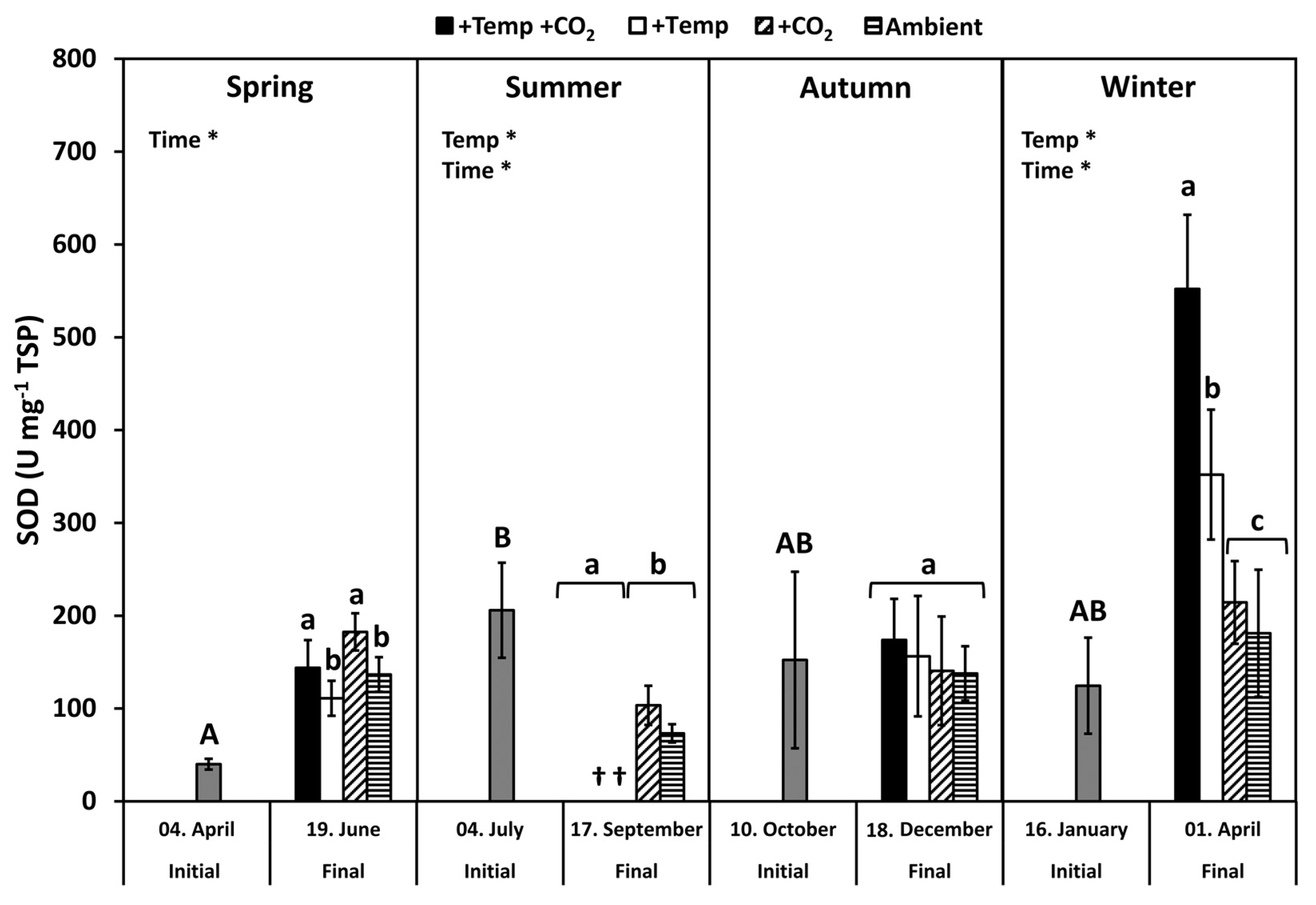
3.3. Superoxide Dismutase
4. Discussion
4.1. Seasonal Response Patterns against Oxidative Stress
4.2. Antioxidative Properties under Ocean Warming and Acidification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate Change Impact on Seaweed Meadow Distribution in the North Atlantic Rocky Intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [Green Version]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; Bettignies, T.D.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-Driven Regime Shift of a Temperate Marine Ecosystem. Science 2016, 149, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Smale, D.A. Impacts of Ocean Warming on Kelp Forest Ecosystems. New Phytol. 2020, 225, 1447–1454. [Google Scholar] [CrossRef] [Green Version]
- Dring, M. Stress Resistance and Disease Resistance in Seaweeds: The Role of Reactive Oxygen Metabolism. Adv. Bot. Res. 2005, 43, 175–207. [Google Scholar] [CrossRef]
- Potin, P. Oxidative Burst and Related Responses in Biotic Interactions of Algae. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 245–272. ISBN 978-3-540-74181-7. [Google Scholar]
- Bischof, K.; Rautenberger, R. Seaweed Responses to Environmental Stress: Reactive Oxygen and Antioxidative Strategies. In Seaweed Biology; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 109–132. ISBN 978-3-642-28451-9. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; ISBN 978-0-19-871747-8. [Google Scholar]
- Asada, K. Mechanisms for Scavenging Reactive Molecules Generated in Chloroplasts under Light Stress. In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Post, A., Baker, N.R., Bowyer, J.R., Eds.; BIOS Scientific Publishers: Oxford, UK, 1994; pp. 128–140. [Google Scholar]
- Asada, K. Production and Action of Active Oxygen Species in Photosynthetic Tissues. In Causes of Photooxidative Stress and Amelioration of Defence Systems in Plants; Foyer, C., Mullineaux, P.M., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 77–104. [Google Scholar]
- Collén, J.; Davison, I.R. Seasonality and Thermal Acclimation of Reactive Oxygen Metabolism in Fucus vesiculosus (Phaeophyceae). J. Phycol. 2001, 37, 474–481. [Google Scholar] [CrossRef]
- Aguilera, J.; Dummermuth, A.; Karsten, U.; Schriek, R.; Wiencke, C. Enzymatic Defences against Photooxidative Stress Induced by Ultraviolet Radiation in Arctic Marine Macroalgae. Polar Biol. 2002, 25, 432–441. [Google Scholar] [CrossRef] [Green Version]
- Dummermuth, A.L.; Karsten, U.; Fisch, K.M.; König, G.M.; Wiencke, C. Responses of Marine Macroalgae to Hydrogen-Peroxide Stress. J. Exp. Mar. Biol. Ecol. 2003, 289, 103–121. [Google Scholar] [CrossRef] [Green Version]
- Bischof, K.; Rautenberger, R.; Brey, L.; Pérez-Lloréns, J.L. Physiological Acclimation to Gradients of Solar Irradiance within Mats of the Filamentous Green Macroalga Chaetomorpha linum from Southern Spain. Mar. Ecol. Prog. Ser. 2006, 306, 165–175. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela, A. The Biological Significance of Malondialdehyde Determination in the Assessment of Tissue Oxidative Stress. Life Sci. 1991, 48, 301–309. [Google Scholar] [CrossRef]
- Cruces, E.; Huovinen, P.; Gómez, I. Phlorotannin and Antioxidant Responses upon Short-Term Exposure to UV Radiation and Elevated Temperature in Three South Pacific Kelps. Photochem. Photobiol. 2012, 88, 58–66. [Google Scholar] [CrossRef]
- Wei, Z.; Long, C.; Yang, F.; Long, L.; Huo, Y.; Ding, D.; Mo, J. Increased Irradiance Availability Mitigates the Physiological Performance of Species of the Calcifying Green Macroalga Halimeda in Response to Ocean Acidification. Algal Res. 2020, 48, 101906. [Google Scholar] [CrossRef]
- Zika, R.G.; Moffett, J.W.; Petasne, R.G.; Cooper, W.J.; Saltzman, E.S. Spatial and Temporal Variations of Hydrogen Peroxide in Gulf of Mexico Waters. Geochim. Cosmochim. Acta 1985, 49, 1173–1184. [Google Scholar] [CrossRef] [Green Version]
- Avery, G.B.; Cooper, W.J.; Kieber, R.J.; Willey, J.D. Hydrogen Peroxide at the Bermuda Atlantic Time Series Station: Temporal Variability of Seawater Hydrogen Peroxide. Mar. Chem. 2005, 97, 236–244. [Google Scholar] [CrossRef]
- Rusak, S.A.; Peake, B.M.; Richard, L.E.; Nodder, S.D.; Cooper, W.J. Distributions of Hydrogen Peroxide and Superoxide in Seawater East of New Zealand. Mar. Chem. 2011, 127, 155–169. [Google Scholar] [CrossRef]
- Szymczak, R.; Waite, T. Generation and Decay of Hydrogen Peroxide in Estuarine Waters. Mar. Freshw. Res. 1988, 39, 289–299. [Google Scholar] [CrossRef]
- Yuan, J.; Shiller, A.M. The Distribution of Hydrogen Peroxide in the Southern and Central Atlantic Ocean. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 2947–2970. [Google Scholar] [CrossRef]
- Twigg, I.M.; Baltar, F.; Hall, J.R.; Hepburn, C.D. Revealing Hydrogen Peroxide as an External Stressor in Macrophyte-Dominated Coastal Ecosystems. Oecologia 2020, 193, 583–591. [Google Scholar] [CrossRef]
- Cooper, W.J.; Zika, R.G.; Petasne, R.G.; Plane, J.M.C. Photochemical Formation of H2O2 in Natural Waters Exposed to Sunlight. Environ. Sci. Technol. 1988, 22, 1156–1160. [Google Scholar] [CrossRef]
- Mopper, K.; Zhou, X. Hydroxyl Radical Photoproduction in the Sea and Its Potential Impact on Marine Processes. Science 1990, 250, 661–664. [Google Scholar] [CrossRef]
- O’Sullivan, D.W.; Neale, P.J.; Coffin, R.B.; Boyd, T.J.; Osburn, C.L. Photochemical Production of Hydrogen Peroxide and Methylhydroperoxide in Coastal Waters. Mar. Chem. 2005, 97, 14–33. [Google Scholar] [CrossRef]
- Garg, S.; Rose, A.L.; Waite, T.D. Photochemical Production of Superoxide and Hydrogen Peroxide from Natural Organic Matter. Geochim. Cosmochim. Acta 2011, 75, 4310–4320. [Google Scholar] [CrossRef]
- Abele-Oeschger, D.; Tüg, H.; Röttgers, R. Dynamics of UV-Driven Hydrogen Peroxide Formation on an Intertidal Sandflat. Limnol. Oceanogr. 1997, 42, 1406–1415. [Google Scholar] [CrossRef]
- Abele, D.; Ferreyra, G.A.; Schloss, I. H2O2 Accumulation from Photochemical Production and Atmospheric Wet Deposition in Antarctic Coastal and Off-Shore Waters of Potter Cove, King George Island, South Shetland Islands. Antarct. Sci. 1999, 11, 131–139. [Google Scholar] [CrossRef]
- Winterbourn, C.C. The Biological Chemistry of Hydrogen Peroxide. In Methods in Enzymology; Abelson, J.N., Simon, M.I., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 528, pp. 3–25. ISBN 978-0-12-405881-1. [Google Scholar]
- Badger, M. The Roles of Carbonic Anhydrases in Photosynthetic CO2 Concentrating Mechanisms. Photosynth. Res. 2003, 77, 83–94. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Keys, A.J.; Madgwick, P.J.; Carmo-Silva, A.E.; Andralojc, P.J. Rubisco Regulation: A Role for Inhibitors. J. Exp. Bot. 2008, 59, 1569–1580. [Google Scholar] [CrossRef] [Green Version]
- Tcherkez, G. The Mechanism of Rubisco-Catalysed Oxygenation. Plant. Cell Environ. 2016, 39, 983–997. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen Peroxide- and Glutathione-Associated Mechanisms of Acclimatory Stress Tolerance and Signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Collén, J.; Davison, I.R. Reactive Oxygen Production and Damage in Intertidal Fucus Spp. (Phaeophyceae). J. Phycol. 1999, 35, 54–61. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Neto, A.M.P.; Tonon, A.P.; Pinto, E.; Cardozo, K.H.M.; Brigagão, M.R.P.L.; Barros, M.P.; Torres, M.A.; Magalhães, P.; Cg, S. Circadian Protection against Oxidative Stress in Marine Algae. Hypnos 2004, 1, 142–157. [Google Scholar]
- Takahashi, M.-A.; Asada, K. Superoxide Anion Permeability of Phospholipid Membranes and Chloroplast Thylakoids. Arch. Biochem. Biophys. 1983, 226, 558–566. [Google Scholar] [CrossRef]
- Ross, C.; Alstyne, K.L.V. Intraspecific Variation in Stress-Induced Hydrogen Peroxide Scavenging by the Ulvoid Macroalga Ulva lactuca. J. Phycol. 2007, 43, 466–474. [Google Scholar] [CrossRef]
- Dittami, S.M.; Scornet, D.; Petit, J.-L.; Ségurens, B.; Da Silva, C.; Corre, E.; Dondrup, M.; Glatting, K.-H.; König, R.; Sterck, L.; et al. Global Expression Analysis of the Brown Alga Ectocarpus siliculosus (Phaeophyceae) Reveals Large-Scale Reprogramming of the Transcriptome in Response to Abiotic Stress. Genome Biol. 2009, 10, R66. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, W.; Sun, X.; Liang, Z.; Wang, F. RNA-Seq Revealed Complex Response to Heat Stress on Transcriptomic Level in Saccharina japonica (Laminariales, Phaeophyta). J. Appl. Phycol. 2014, 26, 1585–1596. [Google Scholar] [CrossRef]
- Li, H.; Monteiro, C.; Heinrich, S.; Bartsch, I.; Valentin, K.; Harms, L.; Glöckner, G.; Corre, E.; Bischof, K. Responses of the Kelp Saccharina latissima (Phaeophyceae) to the Warming Arctic: From Physiology to Transcriptomics. Physiol. Plant. 2020, 168, 5–26. [Google Scholar] [CrossRef]
- Collén, J.; Guisle-Marsollier, I.; Léger, J.J.; Boyen, C. Response of the Transcriptome of the Intertidal Red Seaweed Chondrus crispus to Controlled and Natural Stresses. New Phytol. 2007, 176, 45–55. [Google Scholar] [CrossRef]
- Pearson, G.A.; Hoarau, G.; Lago-Leston, A.; Coyer, J.A.; Kube, M.; Reinhardt, R.; Henckel, K.; Serrão, E.A.; Corre, E.; Olsen, J.L. An Expressed Sequence Tag Analysis of the Intertidal Brown Seaweeds Fucus serratus (L.) and F. vesiculosus (L.) (Heterokontophyta, Phaeophyceae) in Response to Abiotic Stressors. Mar. Biotechnol. 2010, 12, 195–213. [Google Scholar] [CrossRef]
- Heinrich, S.; Valentin, K.; Frickenhaus, S.; John, U.; Wiencke, C. Transcriptomic Analysis of Acclimation to Temperature and Light Stress in Saccharina latissima (Phaeophyceae). PLoS ONE 2012, 7, e44342. [Google Scholar] [CrossRef] [Green Version]
- Kautsky, H.; Kautsky, L.; Kautsky, N.; Kautsky, U.; Lindblad, C. Studies on the Fucus vesiculosus Community in the Baltic Sea. In Phycological Studies of Nordic Coastal Waters; Wallentinus, I., Snoeijs, P., Eds.; Svenska växtgeografiska sällskapet: Uppsala, Sweden, 1992; pp. 33–48. [Google Scholar]
- Rönnbäck, P.; Kautsky, N.; Pihl, L.; Troell, M.; Söderqvist, T.; Wennhage, H. Ecosystem Goods and Services from Swedish Coastal Habitats: Identification, Valuation, and Implications of Ecosystem Shifts. Ambio 2007, 36, 534–544. [Google Scholar] [CrossRef]
- Wahl, M.; Shahnaz, L.; Dobretsov, S.; Saha, M.; Symanowski, F.; David, K.; Lachnit, T.; Vasel, M.; Weinberger, F. Ecology of Antifouling Resistance in the Bladder Wrack Fucus vesiculosus: Patterns of Microfouling and Antimicrobial Protection. Mar. Ecol. Prog. Ser. 2010, 411, 33–48. [Google Scholar] [CrossRef]
- Wahl, M.; Jormalainen, V.; Eriksson, B.K.; Coyer, J.A.; Molis, M.; Schubert, H.; Dethier, M.; Karez, R.; Kruse, I.; Lenz, M.; et al. Stress Ecology in Fucus: Abiotic, Biotic and Genetic Interactions. In Advances in Marine Biology; Lesser, M., Ed.; Elsevier Ltd.: Oxford, UK, 2011; Volume 59, pp. 37–105. ISBN 978-0-12-385536-7. [Google Scholar]
- Kautsky, H.; van der Maarel, E. Multivariate Approaches to the Variation in Phytobenthic Communities and Environmental Vectors in the Baltic Sea. Mar. Ecol. Prog. Ser. 1990, 60, 169–184. [Google Scholar] [CrossRef]
- Jormalainen, V.; Wikström, S.A.; Honkanen, T. Fouling Mediates Grazing: Intertwining of Resistances to Multiple Enemies in the Brown Alga Fucus vesiculosus. Oecologia 2008, 155, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Werner, F.J.; Graiff, A.; Matthiessen, B. Temperature Effects on Seaweed-Sustaining Top-down Control Vary with Season. Oecologia 2016, 180, 889–901. [Google Scholar] [CrossRef] [PubMed]
- Torn, K.; Krause-Jensen, D.; Martin, G. Present and Past Depth Distribution of Bladderwrack (Fucus vesiculosus) in the Baltic Sea. Aquat. Bot. 2006, 84, 53–62. [Google Scholar] [CrossRef]
- Takolander, A.; Cabeza, M.; Leskinen, E. Climate Change Can Cause Complex Responses in Baltic Sea Macroalgae: A Systematic Review. J. Sea Res. 2017, 123, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Elken, J.; Lehmann, A.; Myrberg, K. Recent Change-Marine Circulation and Stratification. In Second Assessment of Climate Change for the Baltic Sea Basin; The BACC II Author Team, Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 131–144. ISBN 978-3-319-16005-4. [Google Scholar]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A Hierarchical Approach to Defining Marine Heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef] [Green Version]
- HELCOM. Climate Change in the Baltic Sea Area: HELCOM Thematic Assessment in 2013. In Baltic Sea Environment Proceedings; Helsinki Commission: Helsinki, Finland, 2013; Volume 137, ISBN 0357-2994. [Google Scholar]
- Müller, J.D.; Schneider, B.; Rehder, G. Long-Term Alkalinity Trends in the Baltic Sea and Their Implications for CO2-Induced Acidification. Limnol. Oceanogr. 2016, 61, 1984–2002. [Google Scholar] [CrossRef] [Green Version]
- Omstedt, A.; Edman, M.K.; Claremar, B.; Frodin, P.; Gustafsson, E.; Humborg, C.; Hägg, H.; Mörth, M.; Rutgersson, A.; Schurgers, G.; et al. Future Changes in the Baltic Sea Acid-Base (PH) and Oxygen Balances. Tellus 2012, 64, 19586. [Google Scholar] [CrossRef]
- BACC II Author Team. Second Assessment of Climate Change for the Baltic Sea Basin; Bolle, H.-J., Menenti, M., Rasool, S.I., Eds.; Springer International Publishing AG Switzerland: Gesthacht, Switzerland, 2015; ISBN 978-3-319-16005-4. [Google Scholar]
- Schneider, B.; Eilola, K.; Lukkari, K.; Müller-Karulis, B.; Neumann, T. Environmental Impacts—Marine Biogeochemistry. In Second Assessment of Climate Change for the Baltic Sea Basin; The BACC II Author Team, Ed.; Springer International Publishing: Berlin, Germany, 2015. [Google Scholar]
- Wahl, M.; Buchholz, B.; Winde, V.; Golomb, D.; Guy-Haim, T.; Müller, J.; Rilov, G.; Scotti, M.; Böttcher, M.E. A Mesocosm Concept for the Simulation of Near-Natural Shallow Underwater Climates: The Kiel Outdoor Benthocosms (KOB). Limnol. Oceanogr. Methods 2015, 13, 651–663. [Google Scholar] [CrossRef] [Green Version]
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean. CO2 Measurements. In PICES Special Publication 3; PICES: Victoria, BC, Canada, 2007; ISBN 1-897176-07-4. [Google Scholar]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS Excel Program. Developed for CO2 System Calculations; Oak Ridge National Laboratory: Oak Ridge, TX, USA, 2006. [Google Scholar] [CrossRef]
- Graiff, A.; Bartsch, I.; Ruth, W.; Wahl, M.; Karsten, U. Season Exerts Differential Effects of Ocean Acidification and Warming on Growth and Carbon Metabolism of the Seaweed Fucus vesiculosus in the Western Baltic Sea. Front. Mar. Sci. 2015, 2, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Hanelt, D. Capability of Dynamic Photoinhibition in Arctic Macroalgae Is Related to Their Depth Distribution. Mar. Biol. 1998, 131, 361–369. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the Thiobarbituric Acid-Reactive-Substances Assay for Estimating Lipid Peroxidation in Plant Tissues Containing Anthocyanin and Other Interfering Compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Graiff, A.; Liesner, D.; Karsten, U.; Bartsch, I. Temperature Tolerance of Western Baltic Sea Fucus vesiculosus—Growth, Photosynthesis and Survival. J. Exp. Mar. Biol. Ecol. 2015, 471, 8–16. [Google Scholar] [CrossRef]
- Gaylord, B.; Rosman, J.H.; Reed, D.C.; Koseff, J.R.; Fram, J.; MacIntyre, S.; Arkema, K.; McDonald, C.; Brzezinski, M.A.; Largier, J.L.; et al. Spatial Patterns of Flow and Their Modification within and around a Giant Kelp Forest. Limnol. Oceanogr. 2007, 52, 1838–1852. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Hepburn, C.D.; Pilditch, C.A.; Hurd, C.L. Concentration Boundary Layers around Complex Assemblages of Macroalgae: Implications for the Effects of Ocean Acidification on Understory Coralline Algae. Limnol. Oceanogr. 2013, 58, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Hurd, C.L. Slow-Flow Habitats as Refugia for Coastal Calcifiers from Ocean Acidification. J. Phycol. 2015, 51, 599–605. [Google Scholar] [CrossRef]
- Wahl, M.; Schneider Covachã, S.; Saderne, V.; Hiebenthal, C.; Müller, J.D.; Pansch, C.; Sawall, Y. Macroalgae May Mitigate Ocean Acidification Effects on Mussel Calcification by Increasing pH and its Fluctuations. Limnol. Oceanogr. 2018, 63, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Murie, K.A.; Bourdeau, P.E. Fragmented Kelp Forest Canopies Retain Their Ability to Alter Local Seawater Chemistry. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Collén, J.; Del Rio, M.J.; García-Reina, G.; Pedersén, M. Photosynthetic Production of Hydrogen Peroxide by Ulva rigida C. Ag. (Chlorophyta). Planta 1995, 196, 225–230. [Google Scholar] [CrossRef]
- Collén, J.; Pedersén, M. Production, Scavenging and Toxicity of Hydrogen Peroxide in the Green Seaweed Ulva rigida. Eur. J. Phycol. 1996, 31, 265–271. [Google Scholar] [CrossRef]
- Truchot, J.-P.; Duhamel-Jouve, A. Oxygen and Carbon Dioxide in the Marine Intertidal Environment: Diurnal and Tidal Changes in Rockpools. Respirin Physiol. 1980, 39, 241–254. [Google Scholar] [CrossRef]
- Jormalainen, V.; Honkanen, T. Macroalgal Chemical Defenses and Their Roles in Structuring Temperate Marine Communities. In Algal Chemical Ecology; Amsler, C.D., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2008; pp. 57–89. ISBN 978-3-540-74180-0. [Google Scholar]
- Kubanek, J.; Lester, S.; Fenical, W.; Hay, M. Ambiguous Role of Phlorotannins as Chemical Defenses in the Brown Alga Fucus vesiculosus. Mar. Ecol. Prog. Ser. 2004, 277, 79–93. [Google Scholar] [CrossRef]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant Marine Algae Phlorotannins and Radioprotection: A Review of Experimental Evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef]
- Ahn, G.-N.; Kim, K.-N.; Cha, S.-H.; Song, C.-B.; Lee, J.; Heo, M.-S.; Yeo, I.-K.; Lee, N.-H.; Jee, Y.-H.; Kim, J.-S.; et al. Antioxidant Activities of Phlorotannins Purified from Ecklonia cava on Free Radical Scavenging Using ESR and H2O2-Mediated DNA Damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Springer, K.; Lütz, C.; Lütz-Meindl, U.; Wendt, A.; Bischof, K. Hyposaline Conditions Affect UV Susceptibility in the Arctic Kelp Alaria esculenta (Phaeophyceae). Phycologia 2017, 56, 675–685. [Google Scholar] [CrossRef]
- Costa, M.M.; Barrote, I.; Silva, J.; Olivé, I.; Alexandre, A.; Albano, S.; Santos, R.O.P. Epiphytes Modulate Posidonia Oceanica Photosynthetic Production, Energetic Balance, Antioxidant Mechanisms and Oxidative Damage. Front. Mar. Sci. 2015, 2, 111. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Barrote, I.; Costa, M.M.; Albano, S.; Santos, R. Physiological Responses of Zostera marina and Cymodocea nodosa to Light-Limitation Stress. PLoS ONE 2013, 8, e81058. [Google Scholar] [CrossRef]
- Dummermuth, A. Antioxidative Properties of Marine Macroalgae from the Arctic. Ph.D. Thesis, Bremen University, Bremen, Germany, 2003. Volume 458. [Google Scholar]
- Collén, J.; Davison, I.R. Stress Tolerance and Reactive Oxygen Metabolism in the Intertidal Red Seaweeds Mastocarpus stellatus and Chondrus crispus. Plant. Cell Environ. 1999, 22, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Collén, J.; Davison, I.R. Reactive Oxygen Metabolism in Intertidal Fucus spp. (Phaeophyceae). J. Phycol. 1999, 35, 62–69. [Google Scholar] [CrossRef]
- Mallick, N.; Mohn, F.H. Reactive Oxygen Species: Response of Algal Cells. J. Plant. Physiol. 2000, 157, 183–193. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative Damage and Antioxidative System in Algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Carlson, L. Seasonal Variation in Growth, Reproduction and Nitrogen Content of Fucus vesiculosus L. in the Öresund, Southern Sweden. Bot. Mar. 1991, 34, 447–453. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Borum, J. Nutrient Control of Algal Growth in Estuarine Waters. Nutrient Limitation and the Importance of Nitrogen Requirements and Nitrogen Storage among Phytoplankton and Species of Macroalgae. Mar. Ecol. Prog. Ser. 1996, 142, 261–272. [Google Scholar] [CrossRef] [Green Version]
- Lehvo, A.; Bäck, S.; Kiirikki, M. Growth of Fucus vesiculosus L. (Phaeophyta) in the Northern Baltic Proper: Energy and Nitrogen Storage in Seasonal Environment. Bot. Mar. 2001, 44, 345–350. [Google Scholar] [CrossRef]
- Lohrmann, N.L.; Logan, B.A.; Johnson, A.S. Seasonal Acclimatization of Antioxidants and Photosynthesis in Chondrus crispus and Mastocarpus stellatus, Two Co-Occurring Red Algae with Differing Stress Tolerances. Biol. Bull. 2004, 207, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Asada, K.; Takahashi, M.-A. Production and Scavenging of Active Oxygen in Photosynthesis. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Bartsch, I.; Wiencke, C.; Laepple, T. Global Seaweed Biogeography under a Changing Climate: The Prospected Effects of Temperature. In Seaweed Biology; Wiencke, C., Bischof, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 383–406. ISBN 978-3-642-28451-9. [Google Scholar]
- Graiff, A.; Dankworth, M.; Wahl, M.; Karsten, U.; Bartsch, I. Seasonal Variations of Fucus vesiculosus Fertility under Ocean Acidification and Warming in the Western Baltic Sea. Bot. Mar. 2017, 60, 239–255. [Google Scholar] [CrossRef]
- Graiff, A.; Bartsch, I.; Glaser, K.; Karsten, U. Seasonal Photophysiological Performance of Adult Western Baltic Fucus vesiculosus (Phaeophyceae) under Ocean Warming and Acidification. Front. Mar. Sci. 2021, 8, 476. [Google Scholar] [CrossRef]
- Rohde, S.; Hiebenthal, C.; Wahl, M.; Karez, R.; Bischof, K. Decreased Depth Distribution of Fucus vesiculosus (Phaeophyceae) in the Western Baltic: Effects of Light Deficiency and Epibionts on Growth and Photosynthesis. Eur. J. Phycol. 2008, 43, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.J.; Li, S.X.; Huang, B.Q.; Zheng, F.Y.; Huang, X.G. Effect of Excessive CO2 on Physiological Functions in Coastal Diatom. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Kumar, A.; AbdElgawad, H.; Castellano, I.; Lorenti, M.; Delledonne, M.; Beemster, G.T.S.; Asard, H.; Buia, M.C.; Palumbo, A. Physiological and Biochemical Analyses Shed Light on the Response of Sargassum vulgare to Ocean Acidification at Different Time. Front. Plant. Sci. 2017, 8, 570. [Google Scholar] [CrossRef]
- Iñiguez, C.; Heinrich, S.; Harms, L.; Gordillo, F.J.L. Increased Temperature and CO2 Alleviate Photoinhibition in Desmarestia anceps: From Transcriptomics to Carbon Utilization. J. Exp. Bot. 2017, 68, 3971–3984. [Google Scholar] [CrossRef] [Green Version]
- Thomsen, J.; Gutowska, M.A.; Saphörster, J.; Heinemann, A.; Trübenbach, K.; Fietzke, J.; Hiebenthal, C.; Eisenhauer, A.; Körtzinger, A.; Wahl, M.; et al. Calcifying Invertebrates Succeed in a Naturally CO2-Rich Coastal Habitat but Are Threatened by High Levels of Future Acidification. Biogeosciences 2010, 7, 3879–3891. [Google Scholar] [CrossRef] [Green Version]
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.A.; Bange, H.W.; Hansen, H.P.; Körtzinger, A. Future Ocean Acidification Will Be Amplified by Hypoxia in Coastal Habitats. Mar. Biol. 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- Saderne, V. The Ecological Effect of CO2 on the Brown Algae Fucus serratus and Its Epibionts: From the Habitat to the Organismic Scale. Ph.D. Thesis, Christian-Albrechts-University, Kiel, Germany, 2012. [Google Scholar]
- Wahl, M.; Werner, F.J.; Buchholz, B.; Raddatz, S.; Graiff, A.; Matthiessen, B.; Karsten, U.; Hiebenthal, C.; Hamer, J.; Ito, M.; et al. Season Affects Strength and Direction of the Interactive Impacts of Ocean Warming and Biotic Stress in a Coastal Seaweed Ecosystem. Limnol. Oceanogr. 2019, 65, 807–827. [Google Scholar] [CrossRef] [Green Version]

| Source of Variation | DF | F-Value | p-Value |
|---|---|---|---|
| (a) Spring | |||
| Temperature | 1 | 1.078 | 0.33 |
| CO2 | 1 | 0.188 | 0.68 |
| Time | 1 | 0.632 | 0.45 |
| Temp × CO2 | 1 | 4.164 | 0.08 |
| Temp × Time | 1 | 5.712 | 0.04 |
| CO2 × Time | 1 | 0.536 | 0.49 |
| Temp × CO2 × Time | 1 | 0.264 | 0.62 |
| (b) Summer | |||
| Temperature | 1 | 20.979 | 0.002 |
| CO2 | 1 | 1.548 | 0.25 |
| Time | 1 | 22.603 | 0.001 |
| Temp × CO2 | 1 | 0.002 | 0.96 |
| Temp × Time | 1 | 22.663 | 0.001 |
| CO2 × Time | 1 | 0.059 | 0.81 |
| Temp × CO2 × Time | 1 | 2.944 | 0.13 |
| (c) Autumn | |||
| Temperature | 1 | 1.910 | 0.21 |
| CO2 | 1 | 0.113 | 0.75 |
| Time | 1 | 24.796 | 0.002 |
| Temp × CO2 | 1 | 0.717 | 0.43 |
| Temp × Time | 1 | 13.281 | 0.008 |
| CO2 × Time | 1 | 0.409 | 0.54 |
| Temp × CO2 × Time | 1 | 0.708 | 0.33 |
| (d) Winter | |||
| Temperature | 1 | 7.368 | 0.03 |
| CO2 | 1 | 0.016 | 0.93 |
| Time | 1 | 17.644 | 0.003 |
| Temp × CO2 | 1 | 3.047 | 0.12 |
| Temp × Time | 1 | 18.457 | 0.003 |
| CO2 × Time | 1 | 0.381 | 0.55 |
| Temp × CO2 × Time | 1 | 1.989 | 0.19 |
| Source of variation | DF | F-Value | p-Value |
|---|---|---|---|
| (a) Spring | |||
| Temperature | 1 | 1.078 | 0.33 |
| CO2 | 1 | 0.188 | 0.68 |
| Time | 1 | 5.712 | 0.04 |
| Temp × CO2 | 1 | 4.464 | 0.08 |
| Temp × Time | 1 | 0.632 | 0.45 |
| CO2 × Time | 1 | 0.536 | 0.49 |
| Temp × CO2 × Time | 1 | 0.264 | 0.62 |
| (b) Summer | |||
| Temperature | 1 | 13.536 | 0.006 |
| CO2 | 1 | 0.583 | 0.47 |
| Time | 1 | 14.162 | 0.006 |
| Temp × CO2 | 1 | 0.331 | 0.58 |
| Temp × Time | 1 | 3.284 | 0.11 |
| CO2 × Time | 1 | 1.332 | 0.28 |
| Temp × CO2 × Time | 1 | 0.914 | 0.37 |
| (c) Autumn | |||
| Temperature | 1 | 2.070 | 0.19 |
| CO2 | 1 | 0.028 | 0.87 |
| Time | 1 | 0.000 | 0.99 |
| Temp × CO2 | 1 | 0.212 | 0.66 |
| Temp × Time | 1 | 0.090 | 0.77 |
| CO2 × Time | 1 | 0.019 | 0.89 |
| Temp × CO2 × Time | 1 | 0.014 | 0.91 |
| (d) Winter | |||
| Temperature | 1 | 9.983 | 0.01 |
| CO2 | 1 | 0.012 | 0.92 |
| Time | 1 | 12.226 | 0.008 |
| Temp × CO2 | 1 | 0.044 | 0.84 |
| Temp × Time | 1 | 4.919 | 0.06 |
| CO2 × Time | 1 | 0.897 | 0.371 |
| Temp × CO2 × Time | 1 | 0.470 | 0.512 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graiff, A.; Karsten, U. Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment. Biology 2021, 10, 1330. https://doi.org/10.3390/biology10121330
Graiff A, Karsten U. Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment. Biology. 2021; 10(12):1330. https://doi.org/10.3390/biology10121330
Chicago/Turabian StyleGraiff, Angelika, and Ulf Karsten. 2021. "Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment" Biology 10, no. 12: 1330. https://doi.org/10.3390/biology10121330
APA StyleGraiff, A., & Karsten, U. (2021). Antioxidative Properties of Baltic Sea Keystone Macroalgae (Fucus vesiculosus, Phaeophyceae) under Ocean Warming and Acidification in a Seasonally Varying Environment. Biology, 10(12), 1330. https://doi.org/10.3390/biology10121330






