Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Pore Size and Porosity
2.3. Isolation and Culture of DPCs
2.4. Characterization of DPCs with Immunofluorescence (IF) Staining
2.5. Characterization Cell Growth Morphology with Cell Microfilaments Staining
2.6. Preparation of Material Extract and Its Biocompatibility Evaluation
2.7. Cell Behavior on the Scaffold
2.7.1. Cell Seeding on the Scaffold
2.7.2. Characterization the Behavior of Cells in the Scaffold
2.8. Regeneration of Wound Tissue in Experimental SD Rats
2.8.1. Construction of a Full-Thickness Skin Wound Model on the Back of SD Rats
2.8.2. H&E Staining of Wound Area in SD Rats
2.9. Statistical Analysis
3. Results and Discussion
3.1. Microstructure Analysis of Scaffolds
3.2. Identification of Extracted DPCs
3.3. Evaluation of The Biocompatibility of Materials
3.3.1. Toxicity of Material Extracts to DPCs
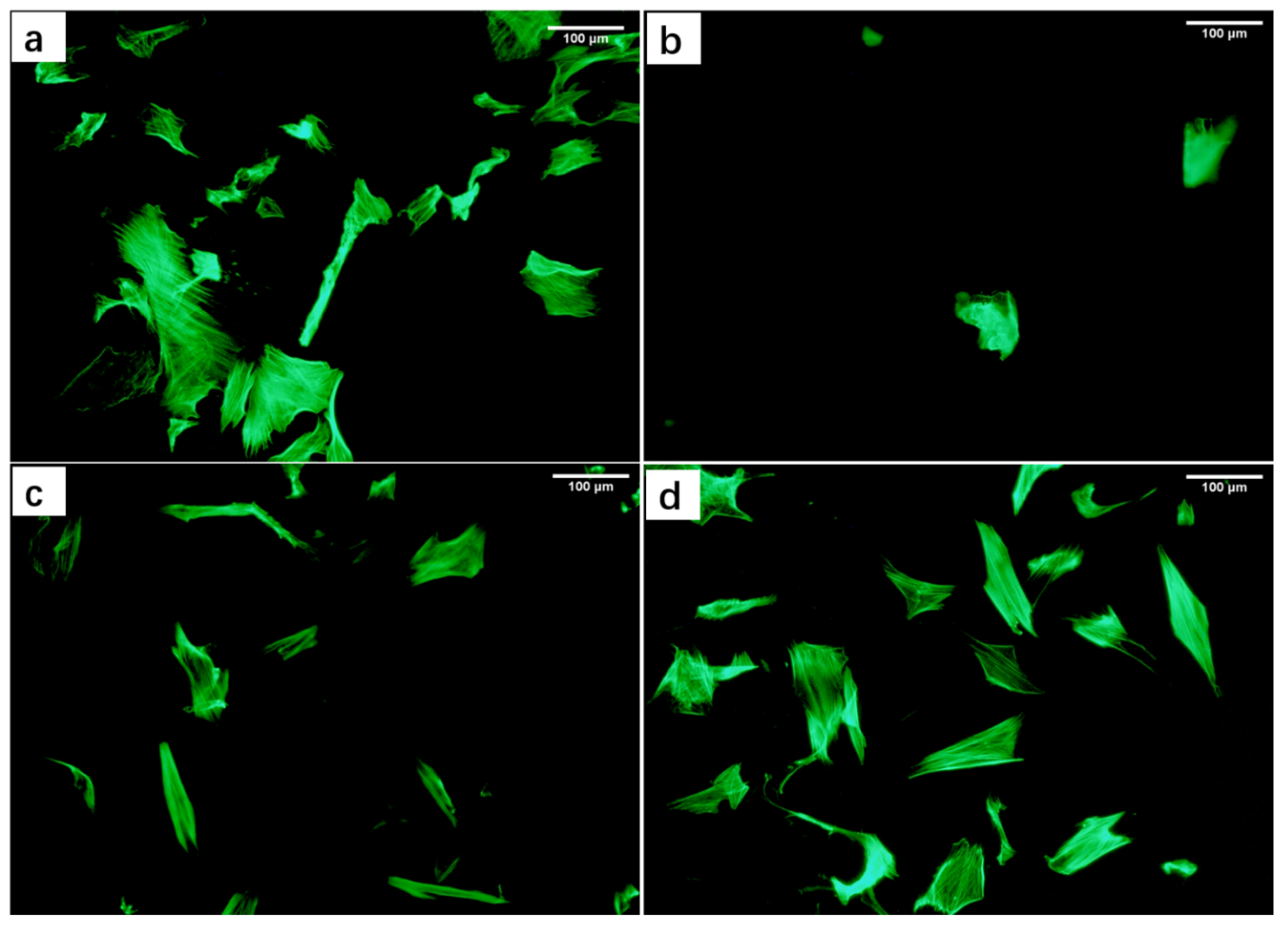
3.3.2. Effects of Material Extracts on the Morphology of DPCs
3.4. Evaluation of Culture between Scaffolds and DPCs
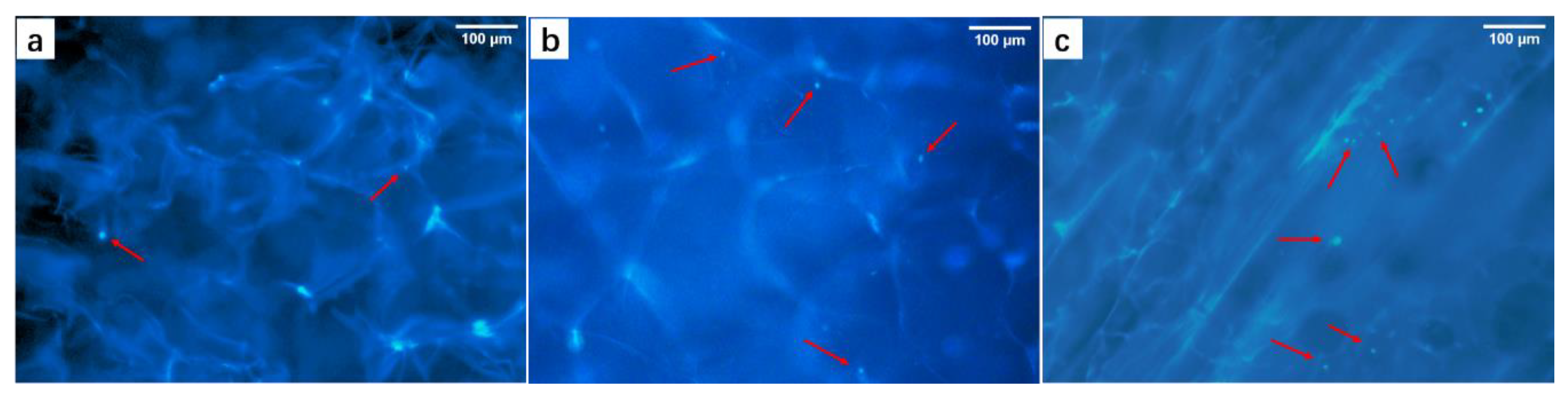
3.4.1. Nuclei Staining in M/C-Culture System
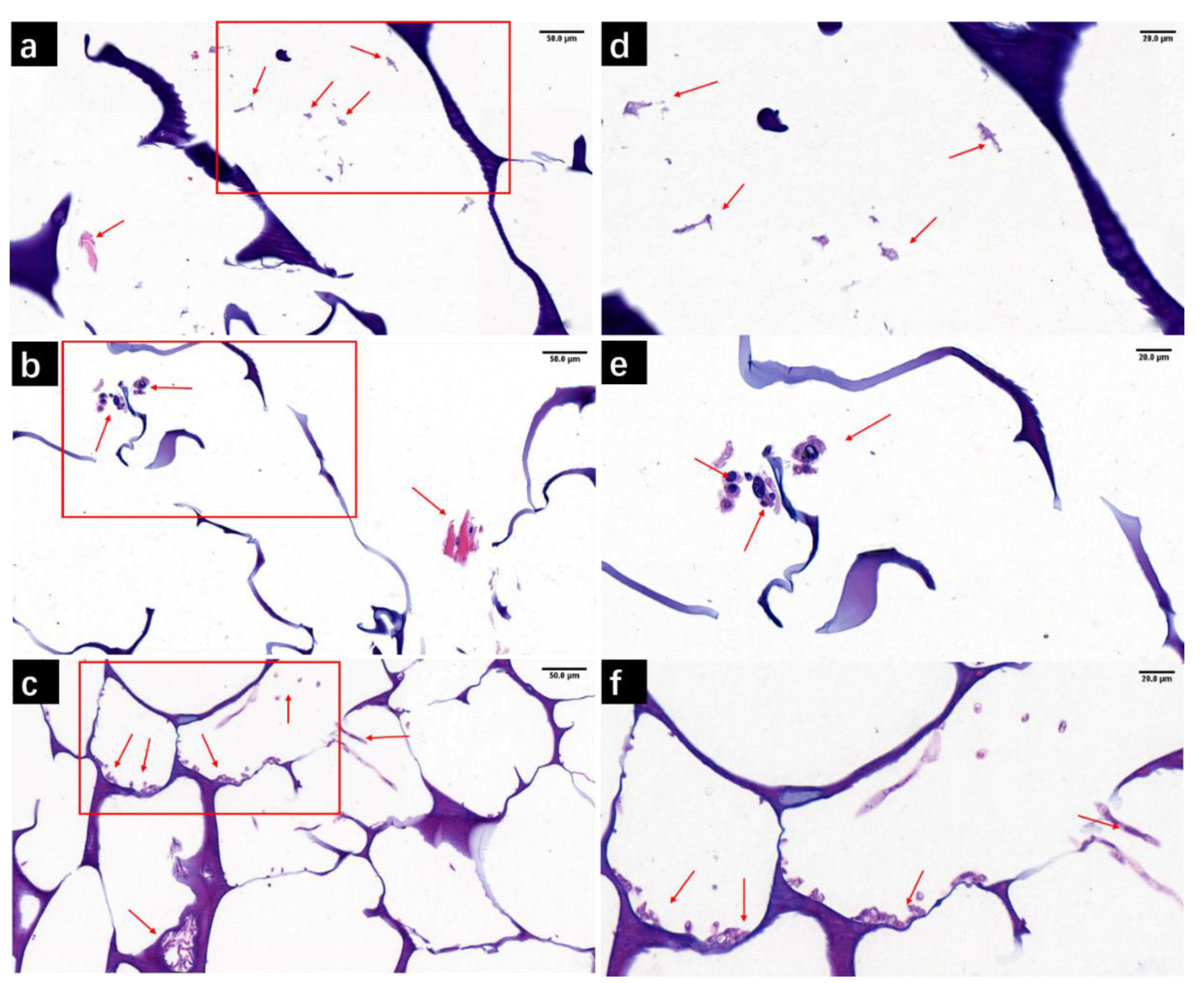
3.4.2. H&E Staining of M/C-Culture System
3.5. Analysis of Experimental Regenerated Tissues in SD Rats
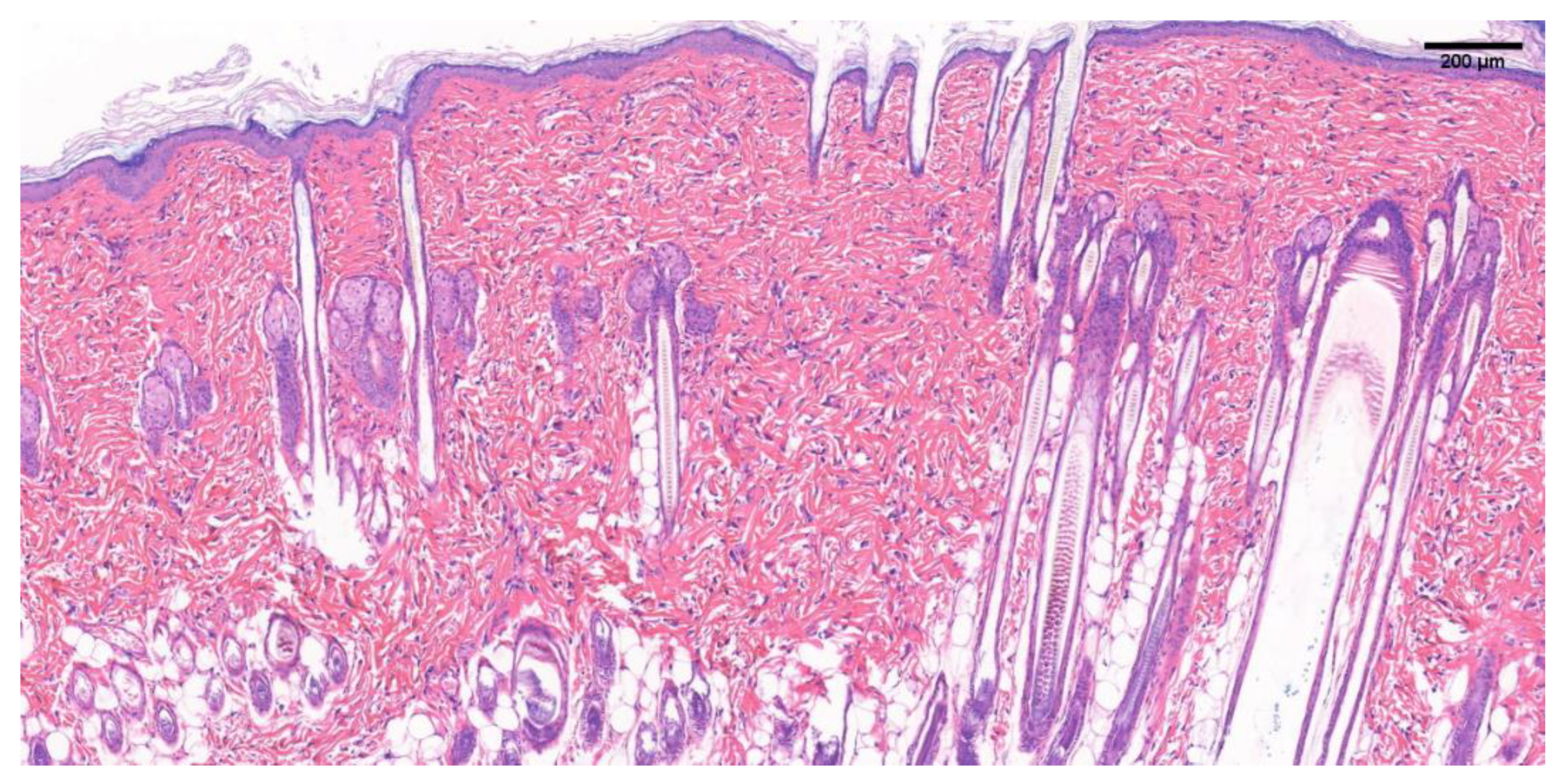
3.5.1. SD Rat Normal Skin Tissue Section
3.5.2. H&E Staining of Newborn Tissue Sections of Damaged Wounds in SD Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Wei, X.; Liu, L.; Marti, G.P.; Ghanamah, M.S.; Arshad, M.J.; Strom, L.; Spence, R.; Jeng, J.; Milner, S.; et al. Association of Increasing Burn Severity in Mice with Delayed Mobilization of Circulating Angiogenic Cells. Arch. Surg. 2010, 145, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Greenhalgh, D.; Palmieri, T. Review of Burn Injury Research for the Year 2009. J. Burn Care Res. 2010, 31, 836–848. [Google Scholar] [CrossRef]
- Peck, M.D.; Brigham, P.; Patterson, D. Invited critique: National study of emergency department visits for burn injuries, 1993 to 2004. J. Burn. Care Res. 2007, 28, 691–693. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Crawford, A.; Mundy, J.; Correlo, V.; Sol, P.; Bhattacharya, M.; Hatton, P.; Reis, R.; Neves, N. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracellular matrix formation. Acta Biomater. 2010, 6, 1149–1157. [Google Scholar] [CrossRef]
- Collier, J.H.; Rudra, J.S.; Gasiorowski, J.Z.; Jung, J.P. Multi-component extracellular matrices based on peptide self-assembly. Chem. Soc. Rev. 2010, 39, 3413–3424. [Google Scholar] [CrossRef] [PubMed]
- Burd, A.; Ahmed, K.; Lam, S.; Ayyappan, T.; Huang, L. Stem cell strategies in burns care. Burns 2007, 33, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.H. The Secret Life of the Hair Follicle. Trends Genet. 1992, 8, 55–61. [Google Scholar] [CrossRef]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; A Sawicki, J.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Wang, X.; Tredget, E.E.; Wu, Y. Dynamic Signals for Hair Follicle Development and Regeneration. Stem Cells Dev. 2012, 21, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hunt, D.P.J.; Morris, P.N.; Sterling, J.; Anderson, J.A.; Joannides, A.; Jahoda, C.; Compston, A.; Chandran, S. A Highly Enriched Niche of Precursor Cells with Neuronal and Glial Potential Within the Hair Follicle Dermal Papilla of Adult Skin. Stem Cells 2008, 26, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of minoxidil on proliferation and apoptosis in dermal papilla cells of human hair follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, W.; Abbasi, S.; Hagner, A.; Raharjo, E.; Kumar, R.; Hotta, A.; Magness, S.; Metzger, D.; Biernaskie, J. Hair Follicle Dermal Stem Cells Regenerate the Dermal Sheath, Repopulate the Dermal Papilla, and Modulate Hair Type. Dev. Cell 2014, 31, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.; Guerrero-Juarez, C.F.; Plikus, M.V. Hair Follicle Signaling Networks: A Dermal Papilla–Centric Approach. J. Investig. Dermatol. 2013, 133, 2306–2308. [Google Scholar] [CrossRef]
- Driskell, R.R.; Clavel, C.; Rendl, M.; Watt, F.M. Hair follicle dermal papilla cells at a glance. J. Cell Sci. 2011, 124, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, W.; Choi, D.; Son, B.; Park, T. Nonanal Stimulates Growth Factors via Cyclic Adenosine Monophosphate (cAMP) Signaling in Human Hair Follicle Dermal Papilla Cells. Int. J. Mol. Sci. 2020, 21, 8054. [Google Scholar] [CrossRef]
- Sennett, R.; Rendl, M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef]
- Jahoda, C.A.; Oliver, R.F.; Reynolds, A.J.; Forrester, J.C.; Horne, K.A. Human Hair Follicle Regeneration Following Amputation and Grafting into the Nude Mouse. J. Investig. Dermatol. 1996, 107, 804–807. [Google Scholar] [CrossRef]
- Kalabusheva, E.; Terskikh, V.; Vorotelyak, E. Hair Germ Model In Vitro via Human Postnatal Keratinocyte-Dermal Papilla Interactions: Impact of Hyaluronic Acid. Stem Cells Int. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. Cold Spring Harb. Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef]
- Yang, C.-C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2010, 57, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mali, N.M.; Kim, Y.-H.; Park, J.M.; Kim, D.; Heo, W.; Le Dao, B.; Lim, J.O.; Oh, J.W. Characterization of Human Dermal Papilla Cells in Alginate Spheres. Appl. Sci. 2018, 8, 1993. [Google Scholar] [CrossRef]
- Tan, J.J.Y.; Tee, J.K.; Chou, K.O.; Pan, J.; Ho, H.K.; Ho, P.C.L.; Kang, L.; AuYong, S.; Ho, C.L.P. Impact of substrate stiffness on dermal papilla aggregates in microgels. Biomater. Sci. 2018, 6, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.; King, N. Diverse evolutionary paths to cell adhesion. Trends Cell Biol. 2010, 20, 734–742. [Google Scholar] [CrossRef]
- Thorne, J.T.; Segal, T.R.; Chang, S.; Jorge, S.; Segars, J.H.; Leppert, P.C. Dynamic Reciprocity between Cells and Their Microenvironment in Reproduction. Biol. Reprod. 2015, 92, 1–10. [Google Scholar] [CrossRef]
- Chermnykh, E.; Kalabusheva, E.; Vorotelyak, E. Extracellular Matrix as a Regulator of Epidermal Stem Cell Fate. Int. J. Mol. Sci. 2018, 19, 1003. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Liu, T.-L.; Miao, J.-C.; Sheng, W.-H.; Xie, Y.-F.; Huang, Q.; Shan, Y.-B.; Yang, J.-C. Cytocompatibility of regenerated silk fibroin film: A medical biomaterial applicable to wound healing. J. Zhejiang Univ. Sci. B 2010, 11, 10–16. [Google Scholar] [CrossRef]
- Marelli, B.; Alessandrino, A.; Farè, S.; Freddi, G.; Mantovani, D.; Tanzi, M.C. Compliant electrospun silk fibroin tubes for small vessel bypass grafting. Acta Biomater. 2010, 6, 4019–4026. [Google Scholar] [CrossRef]
- Arai, T.; Freddi, G.; Innocenti, R.; Tsukada, M. Biodegradation ofBombyx mori silk fibroin fibers and films. J. Appl. Polym. Sci. 2003, 91, 2383–2390. [Google Scholar] [CrossRef]
- Caykara, T.; Demirci, S.; Eroğlu, M.S.; Güven, O. Poly(Ethylene Oxide) and Its Blends with Sodium Alginate. Polymer 2005, 46, 10750–10757. [Google Scholar] [CrossRef]
- Sahoo, D.R.; Biswal, T. Alginate and its application to tissue engineering. SN Appl. Sci. 2021, 3, 1–19. [Google Scholar] [CrossRef]
- Fergal, O.B. Biomaterials and Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The Extracellular Matrix in Development and Morphogenesis: A Dynamic View. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Algan, C.; Jacobs, V.; John, M.; Oksman, K.; Mathew, A.P. Electrospun chitosan-based nanocomposite mats reinforced with chitin nanocrystals for wound dressing. Carbohydr. Polym. 2014, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, R.; Venugopal, J.R.; Sundarrajan, S.; Mukherjee, S.; Sridhar, R.; Ramakrishna, S. Composite poly-l-lactic acid/poly-(α,β)-dl-aspartic acid/collagen nanofibrous scaffolds for dermal tissue regeneration. Mater. Sci. Eng. C 2012, 32, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Shi, J.; Zhu, R.; Zhang, J.; Zhang, Z.; Ma, D.; Hou, Y.; Lin, F.; Yang, J.; et al. A Biomimetic Silk Fibroin/Sodium Alginate Composite Scaffold for Soft Tissue Engineering. Sci. Rep. 2016, 6, 39477. [Google Scholar] [CrossRef] [PubMed]
- Fiocco, L.; Li, S.; Stevens, M.; Bernardo, E.; Jones, J. Biocompatibility and bioactivity of porous polymer-derived Ca-Mg silicate ceramics. Acta Biomater. 2017, 50, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.D.; Rüppell, C.; Tomakidi, P.; Steinberg, T.; Reichl, F.-X.; Hellwig, E.; Polydorou, O. Gene expression analysis of conventional and interactive human gingival cell systems exposed to dental composites. Dent. Mater. 2015, 31, 1321–1334. [Google Scholar] [CrossRef]
- Zhu, Y.; Ma, Z.; Kong, L.; He, Y.; Chan, H.F.; Li, H. Modulation of macrophages by bioactive glass/sodium alginate hydrogel is crucial in skin regeneration enhancement. Biomaterials 2020, 256, 120216. [Google Scholar] [CrossRef] [PubMed]
- Naeimi, M.; Fathi, M.; Rafienia, M.; Bonakdar, S. Silk fibroin-chondroitin sulfate-alginate porous scaffolds: Structural properties andin vitrostudies. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Hu, W.; Wang, Z.; Zha, Y.; Gu, X.; You, W.; Xiao, Y.; Wang, X.; Zhang, S.; Wang, J. High Flexible and Broad Antibacterial Nanodressing Induces Complete Skin Repair with Angiogenic and Follicle Regeneration. Adv. Heal. Mater. 2020, 9, e2000035. [Google Scholar] [CrossRef]
- Wray, L.S.; Rnjak-Kovacina, J.; Mandal, B.B.; Schmidt, D.F.; Gil, E.S.; Kaplan, D.L. A silk-based scaffold platform with tunable architecture for engineering critically-sized tissue constructs. Biomaterials 2012, 33, 9214–9224. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kapoor, S.; Kundu, S.C. Silk fibroin/polyacrylamide semi-interpenetrating network hydrogels for controlled drug release. Biomaterials 2009, 30, 2826–2836. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y.; Xing, F.; Liang, J.; Wang, Q.; Fan, Y.; Zhang, X. Cell-free scaffolds functionalized with bionic cartilage acellular matrix microspheres to enhance the microfracture treatment of articular cartilage defects. J. Mater. Chem. B 2021, 9, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef]
- Commo, S.; Gaillard, O.; Bernard, B.A. The Human Hair Follicle Contains Two Distinct K19 Positive Compartments in the Outer Root Sheath: A Unifying Hypothesis for Stem Cell Reservoir? Differentiation 2000, 66, 157–164. [Google Scholar] [CrossRef]
- Park, S.; Bivona, B.J.; Harrison-Bernard, L.M. Compromised renal microvascular reactivity of angiotensin type 1 double null mice. Am. J. Physiol. Physiol. 2007, 293, F60–F67. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.J.; Chaponnier, C.; Jahoda, C.A.; Gabbiani, G. A Quantitative Study of the Differential Expression of Alpha-Smooth Muscle Actin in Cell Populations of Follicular and Non-Follicular Origin. J. Investig. Dermatol. 1993, 101, 577–583. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Banerjee, R. Biopolymer-Based Hydrogels for Cartilage Tissue Engineering. Chem. Rev. 2011, 111, 4453–4474. [Google Scholar] [CrossRef] [PubMed]
- Gharzi, A.; Reynolds, A.J.; Jahoda, C.A.B. Plasticity of hair follicle dermal cells in wound healing and induction. Exp. Dermatol. 2003, 12, 126–136. [Google Scholar] [CrossRef] [PubMed]




| Sample | Porosity (%) | Pore Diameter (μm) |
|---|---|---|
| FCC | 81.23 ± 1.189 | 247.028 ± 64.277 |
| AC | 43.925 ± 2.288 | 219.779 ± 35.912 |
| FA | 72.845 ± 1.537 | 173.35 ± 20.259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, K.; Wang, X.; Shen, Y.; Wang, Y.; Li, B.; Cai, C.; Shen, L.; Guo, Y. Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration. Biology 2021, 10, 269. https://doi.org/10.3390/biology10040269
Dong K, Wang X, Shen Y, Wang Y, Li B, Cai C, Shen L, Guo Y. Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration. Biology. 2021; 10(4):269. https://doi.org/10.3390/biology10040269
Chicago/Turabian StyleDong, Kuo, Xinyu Wang, Ying Shen, Yiyu Wang, Binbin Li, Cuiling Cai, Linyi Shen, and Yajin Guo. 2021. "Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration" Biology 10, no. 4: 269. https://doi.org/10.3390/biology10040269
APA StyleDong, K., Wang, X., Shen, Y., Wang, Y., Li, B., Cai, C., Shen, L., & Guo, Y. (2021). Maintaining Inducibility of Dermal Follicle Cells on Silk Fibroin/Sodium Alginate Scaffold for Enhanced Hair Follicle Regeneration. Biology, 10(4), 269. https://doi.org/10.3390/biology10040269






