Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Flies
2.2. Flight Performance
2.3. Climbing Performance
2.4. Statistical Analysis
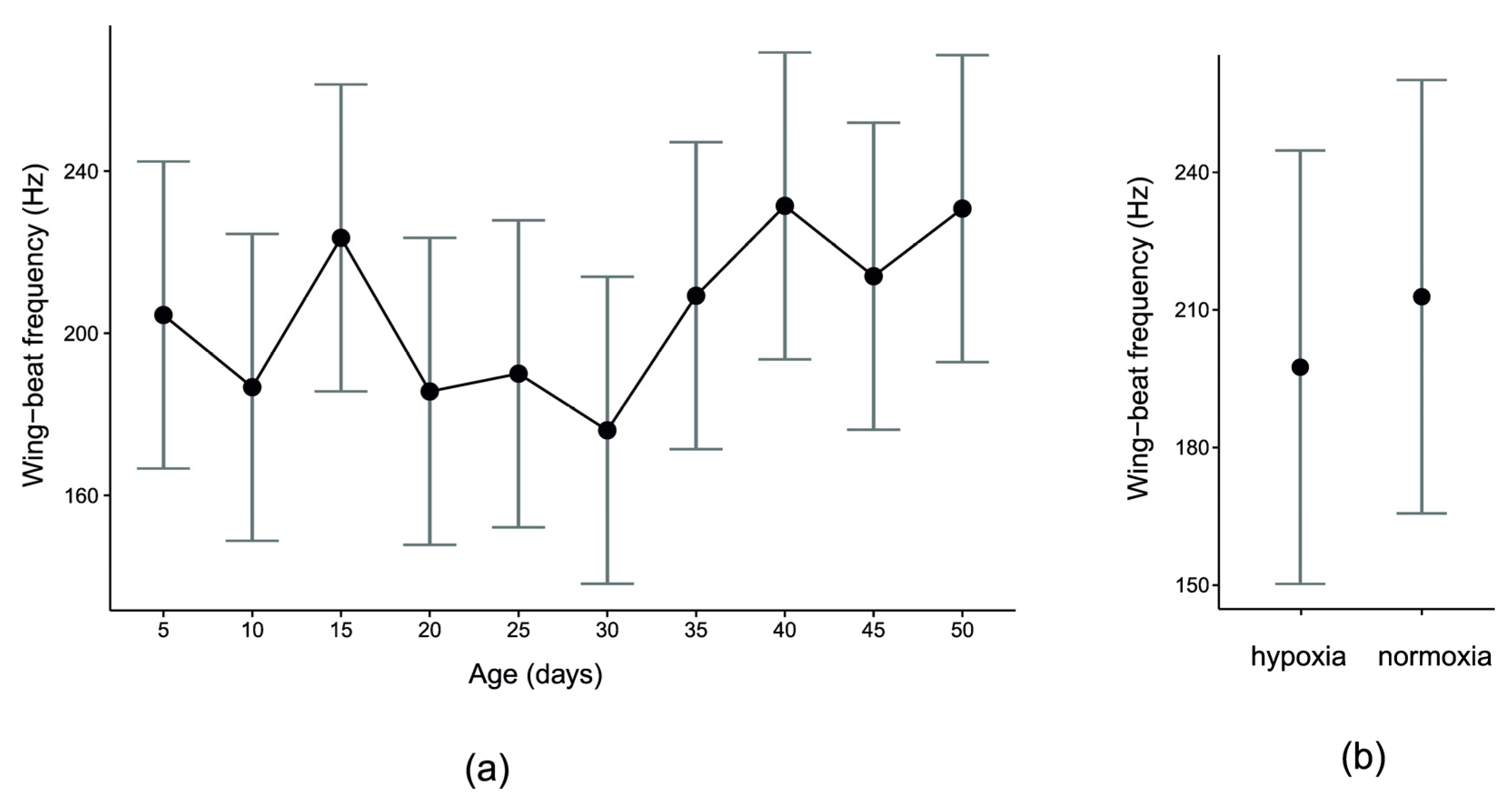
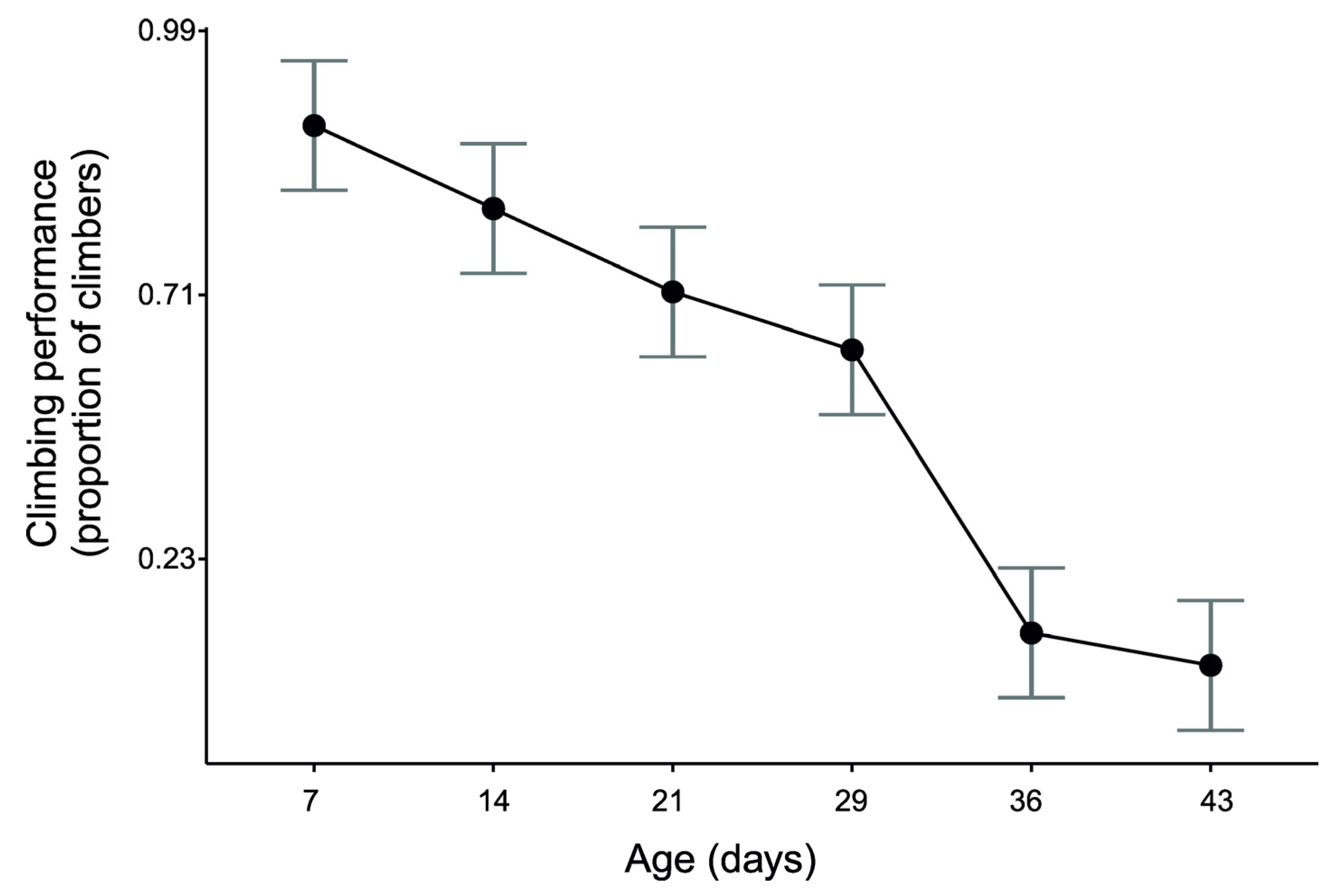
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, D.E. On the Wing; Oxford University Press: New York, NY, USA, 2015; ISBN 978-0199996773. [Google Scholar]
- Jenni-Eiermann, S.; Srygley, R.B. Physiological Aeroecology: Anatomical and Physiological Adaptations for Flight; Chilson, P., Frick, W., Kelly, J., Liechti, F., Eds.; Springer: Cham, Switzerland, 2017; ISBN 9783319685762. [Google Scholar]
- Harrison, J.F.; Roberts, S.P. Flight respiration and energetics. Annu. Rev. Physiol. 2000, 62, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Beenakkers, A.M.T.; Van der Horst, D.J.; Van Marrewijk, W.J.A. Insect flight muscle metabolism. Insect Biochem. 1984, 14, 243–260. [Google Scholar] [CrossRef]
- Bradley, T.J.; Briscoe, A.D.; Brady, S.G.; Contreras, H.L.; Danforth, B.N.; Dudley, R.; Grimaldi, D.; Harrison, J.F.; Kaiser, J.A.; Merlin, C.; et al. Episodes in insect evolution. Integr. Comp. Biol. 2009, 49, 590–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chown, S.L. Physiological variation in insects: Hierarchical levels and implications. J. Insect Physiol. 2001, 47, 649–660. [Google Scholar] [CrossRef]
- Ellington, B.Y.C.P. Limitations on Animal Flight Performance. J. Exp. Biol. 1991, 160, 71–91. [Google Scholar]
- Harrison, J.F.; Greenlee, K.J.; Verberk, W.C.E.P. Functional Hypoxia in Insects: Definition, Assessment, and Consequences for Physiology, Ecology, and Evolution. Annu. Rev. Entomol. 2018, 63, 303–325. [Google Scholar] [CrossRef] [PubMed]
- Klok, C.J.; Sinclair, B.J.; Chown, S.L. Upper thermal tolerance and oxygen limitation in terrestrial arthropods. J. Exp. Biol. 2004, 207, 2361–2370. [Google Scholar] [CrossRef] [Green Version]
- Chapelle, G.; Peck, L.S. Amphipod crustacean size spectra: New insights in the relationship between size and oxygen. Oikos 2004, 106, 167–175. [Google Scholar] [CrossRef]
- Hoefnagel, K.N.; Verberk, W.C.E.P. Is the temperature-size rule mediated by oxygen in aquatic ectotherms? J. Therm. Biol. 2015, 54, 56–65. [Google Scholar] [CrossRef]
- Antoł, A.; Rojek, W.; Singh, S.; Piekarski, D.; Czarnoleski, M. Hypoxia causes woodlice (Porcellio scaber) to select lower temperatures and impairs their thermal performance and heat tolerance. PLoS ONE 2019, 14, e0220647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walczyńska, A.; Labecka, A.M.; Sobczyk, M.; Czarnoleski, M.; Kozłowski, J. The Temperature-Size Rule in Lecane inermis (Rotifera) is adaptive and driven by nuclei size adjustment to temperature and oxygen combinations. J. Therm. Biol. 2015, 54, 78–85. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Ejsmont-Karabin, J.; Angilletta, M.J.; Kozlowski, J. Colder rotifers grow larger but only in oxygenated waters. Ecosphere 2015, 6, 164. [Google Scholar] [CrossRef]
- Verberk, W.C.E.P.; Overgaard, J.; Ern, R.; Bayley, M.; Wang, T.; Boardman, L.; Terblanche, J.S. Does oxygen limit thermal tolerance in arthropods? A critical review of current evidence. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 192, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Pörtner, H.O. Oxygen- And capacity-limitation of thermal tolerance: A matrix for integrating climate-related stressor effects in marine ecosystems. J. Exp. Biol. 2010, 213, 881–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiehzadegan, S.; Le Vinh Thuy, J.; Szabla, N.; Angilletta, M.J.; VandenBrooks, J.M. More oxygen during development enhanced flight performance but not thermal tolerance of Drosophila melanogaster. PLoS ONE 2017, 12, e0177827. [Google Scholar] [CrossRef]
- Frazier, M.R.; Woods, H.A.; Harrison, J.F. Interactive effects of rearing temperature and oxygen on the development of Drosophila melanogaster. Physiol. Biochem. Zool. 2001, 74, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.M.; Jackson, S.; Bester, S.A.; Terblanche, J.S.; Chown, S.L. Oxygen limitation and thermal tolerance in two terrestrial arthropod species. J. Exp. Biol. 2010, 213, 2209–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillon, M.E.; Frazier, M.R. Drosophila melanogaster locomotion in cold thin air. J. Exp. Biol. 2006, 209, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Gatehouse, A.G. Behavior and ecological genetics of wind-borne migration by insects. Annu. Rev. Entomol. 1997, 42, 475–502. [Google Scholar] [CrossRef]
- Hoback, W.W.; Stanley, D.W. Insects in hypoxia. J. Insect Physiol. 2001, 47, 1879–1884. [Google Scholar] [CrossRef]
- Callier, V.; Hand, S.C.; Campbell, J.B.; Biddulph, T.; Harrison, J.F. Developmental changes in hypoxic exposure and responses to anoxia in Drosophila melanogaster. J. Exp. Biol. 2015, 218, 2927–2934. [Google Scholar] [CrossRef] [Green Version]
- Chapman, J.W.; Reynolds, D.R.; Wilson, K. Long-range seasonal migration in insects: Mechanisms, evolutionary drivers and ecological consequences. Ecol. Lett. 2015, 18, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.F.; Kaiser, A.; VandenBrooks, J.M. Atmospheric oxygen level and the evolution of insect body size. Proc. R. Soc. B Biol. Sci. 2010, 277, 1937–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bong, C.F.J.; Er, C.C.; Yiu, P.H.; Rajan, A. Growth performance of the red-stripe weevil Rhynchophorus schach Oliv. (Insecta: Coleoptera: Curculionidae) on meridic diets. Am. J. Agric. Biol. Sci. 2008, 3, 403–409. [Google Scholar] [CrossRef]
- Burrows, M. Jumping performance of froghopper insects. J. Exp. Biol. 2006, 209, 4607–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, K.; Terblanche, J.S. Insect thermal tolerance: What is the role of ontogeny, ageing and senescence? Biol. Rev. 2008, 83, 339–355. [Google Scholar] [CrossRef]
- Lalouette, L.; Vernon, P.; Amat, H.; Renault, D. Ageing and thermal performance in the sub-Antarctic wingless fly Anatalanta aptera (diptera: Sphaeroceridae): Older is better. Biol. Lett. 2010, 6, 346–349. [Google Scholar] [CrossRef] [Green Version]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Tamura, T.; Chiang, A.S.; Ito, N.; Liu, H.P.; Horiuchi, J.; Tully, T.; Saitoe, M. Aging Specifically Impairs amnesiac-Dependent Memory in Drosophila. Neuron 2003, 40, 1003–1011. [Google Scholar] [CrossRef]
- Cook-Wiens, E.; Grotewiel, M.S. Dissociation between functional senescence and oxidative stress resistance in Drosophila. Exp. Gerontol. 2002, 37, 1347–1357. [Google Scholar] [CrossRef]
- Economos, A.C.; Miquel, J.; Binnard, R.; Kessler, S. Quantitative analysis of mating behavior in aging male Drosophila melanogaster. Mech. Ageing Dev. 1979, 10, 233–240. [Google Scholar] [CrossRef]
- Leffelaar, D.; Grigliatti, T. Age-dependent behavior loss in adult Drosophila melanogaster. Dev. Genet. 1983, 4, 211–227. [Google Scholar] [CrossRef]
- Haddadi, M.; Jahromi, S.R.; Sagar, B.K.C.; Patil, R.K.; Shivanandappa, T.; Ramesh, S.R. Brain aging, memory impairment and oxidative stress: A study in Drosophila melanogaster. Behav. Brain Res. 2014, 259, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M. Drosophila melanogaster as a model system for the evaluation of anti-aging compounds. Fly 2010, 4, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, A.; Vilcinskas, A. Synthetic The Fruit Fly Drosophila melanogaster as a Model for Aging Research in Molecular Imprinting. Adv. Biochem. Eng. Biotechnol. 2013, 135, 63–77. [Google Scholar] [CrossRef]
- Grotewiel, M.S.; Martin, I.; Bhandari, P.; Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005, 4, 372–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kaufmann, C.; Scherm, H. Laboratory evaluation of flight performance of the plum curculio (Coleoptera: Curculionidae). J. Econ. Entomol. 2006, 99, 2065–2071. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.T.; Williams, J.B.; Elekonich, M.M.; Roberts, S.R. The effects of age and behavioral development on honey bee (Apis mellifera) flight performance. J. Exp. Biol. 2009, 212, 2604–2611. [Google Scholar] [CrossRef] [Green Version]
- Carey, J.R.; Papadopoulos, N.; Kouloussis, N.; Katsoyannos, B.; Müller, H.G.; Wang, J.L.; Tseng, Y.K. Age-specific and lifetime behavior patterns in Drosophila melanogaster and the Mediterranean fruit fly, Ceratitis capitata. Exp. Gerontol. 2006, 41, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Wigglesworth, V.B. The utilization of reserve substances in Drosophila during flight. J. Exp. Biol. 1949, 26, 150–163. [Google Scholar]
- Miller, M.S.; Lekkas, P.; Braddock, J.M.; Farman, G.P.; Ballif, B.A.; Irving, T.C.; Maughan, D.W.; Vigoreaux, J.O. Aging enhances indirect flight muscle fiber performance yet decreases flight ability in Drosophila. Biophys. J. 2008, 95, 2391–2401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lane, S.J.; Frankino, W.A.; Elekonich, M.M.; Roberts, S.P. The effects of age and lifetime flight behavior on flight capacity in Drosophila melanogaster. J. Exp. Biol. 2014, 217, 1437–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coquin, L.; Feala, J.D.; McCulloch, A.D.; Paternostro, G. Metabolomic and flux-balance analysis of age-related decline of hypoxia tolerance in Drosophila muscle tissue. Mol. Syst. Biol. 2008, 4, 233. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Marce, M.; Okuyama, H.; Wesley, J.B.; Sarkar, K.; Kimura, H.; Liu, Y.V.; Zhang, H.; Strazza, M.; Rey, S.; Savino, L.; et al. Effects of aging and hypoxia-inducible factor-1 activity on angiogenic cell mobilization and recovery of perfusion after limb ischemia. Circ. Res. 2007, 101, 1310–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Xu, B.; Cavalieri, T.A.; Hock, C.E. Age-related difference in myocardial function and inflammation in a rat model of myocardial ischemia-reperfusion. Cardiovasc. Res. 2002, 56, 443–453. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.F.; Haddad, G.G. Effects of oxygen on growth and size: Synthesis of molecular, organismal, and evolutionary studies with Drosophila melanogaster. Annu. Rev. Physiol. 2011, 73, 95–113. [Google Scholar] [CrossRef]
- Rhodenizer, D.; Martin, I.; Bhandari, P.; Pletcher, S.D.; Grotewiel, M. Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp. Gerontol. 2008, 43, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.A.; Grotewiel, M. Drosophila as a model for age-related impairment in locomotor and other behaviors. Exp. Gerontol. 2011, 46, 320–325. [Google Scholar] [CrossRef] [Green Version]
- Unwin, D.M.; Ellington, C.P. An optical tachometer for measurement of the wing-beat frequency of free-flying insects. J. Exp. Biol. 1979, 82, 377–378. [Google Scholar]
- Starmer, W.T.; Wolf, L.L. Causes of variation in wing loading among Drosophila species. Biol. J. Linn. Soc. 1989, 37, 247–261. [Google Scholar] [CrossRef]
- Azevedo, R.B.R.; James, A.C.; McCabe, J.; Partridge, L. Latitudinal variation of wing:thorax size ratio and wing-aspect ratio in Drosophila melanogaster. Evolution 1998, 52, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Benzer, S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA 1967, 58, 1112–1119. [Google Scholar] [CrossRef] [Green Version]
- R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 5 February 2021).
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE Publications: Sauzend Oaks, CA, USA, 2018; ISBN 9781544336473. [Google Scholar]
- Wickham, H. ggplot2 Book; Springer: New York, NY, USA, 2015; ISBN 9780387981406. [Google Scholar]
- Lenth, R.; Buerkner, P.; Herve, M.; Love, J.; Lenth, M.R. Package ‘Emmeans’: Estimated Marginal Means, Aka Least-Squares Means. Available online: https://cran.r-project.org/package=emmeans (accessed on 10 December 2020).
- Sokal, R.R.; Rohlf, F.J. Biometry, 4th ed.; W. H. Freeman and Company: New York, NY, USA, 2011; ISBN 0716786044. [Google Scholar]
- Mołoń, M.; Dampc, J.; Kula-Maximenko, M.; Zebrowski, J.; Mołoń, A.; Dobler, R.; Durak, R.; Skoczowski, A. Effects of Temperature on Lifespan of Drosophila melanogaster from Different Genetic Backgrounds: Links between Metabolic Rate and Longevity. Insects 2020, 11, 470. [Google Scholar] [CrossRef]
- Gibert, P.; Huey, R.B.; Gilchrist, G.W. Locomotor performance of Drosophila melanogaster: Interactions among developmental and adult temperatures, age, and geography. Evolution 2001, 55, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.J.; Bijlsma, R. Changes in mortality patterns and temperature dependence of lifespan in Drosophila melanogaster caused by inbreeding. Heredity 2004, 92, 275–281. [Google Scholar] [CrossRef]
- Paternostro, G.; Vignola, C.; Bartsch, D.; Omens, J.H.; Mcculloch, A.D.; Reed, J.C. Integrative Physiology Age-Associated Cardiac Dysfunction in Drosophila melanogaster. Circ. Res. 2001, 88, 1053–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Muñoz, R.; Boonekamp, J.J.; Liu, X.P.; Skicko, I.; Fisher, D.N.; Hopwood, P.; Tregenza, T. Testing the effect of early-life reproductive effort on age-related decline in a wild insect. Evolution 2019, 73, 317–328. [Google Scholar] [CrossRef] [Green Version]
- Ridgel, A.L.; Ritzmann, R.E.; Schaefer, P.L. Effects of aging on behavior and leg kinematics during locomotion in two species of cockroach. J. Exp. Biol. 2003, 206, 4453–4465. [Google Scholar] [CrossRef] [Green Version]
- Dukas, R. Life history of learning: Performance curves of honeybees in the wild. Ethology 2008, 114, 1195–1200. [Google Scholar] [CrossRef]
- Petrosyan, A.; Hsieh, I.H.; Saberi, K. Age-dependent stability of sensorimotor functions in the life-extended Drosophila mutant Methuselah. Behav. Genet. 2007, 37, 585–594. [Google Scholar] [CrossRef] [PubMed]
- McInnis, D.O.; Schaffer, H.E.; Mettler, L.E. Field Dispersal and Population Sizes of Native Drosophila from North Carolina. Am. Nat. 1982, 119, 319–330. [Google Scholar] [CrossRef]
- Coyne, J.A.; Milstead, B. Long-Distance Migration of Drosophila. 3. Dispersal of D. melanogaster Alleles from a Maryland Orchard. Am. Nat. 1987, 130, 70–82. [Google Scholar] [CrossRef]
- Yerington, A.P.; Warner, R.M. Flight Distances of Drosophila Determined with Radioactive Phosphorus. J. Econ. Entomol. 1961, 54, 425–428. [Google Scholar] [CrossRef]
- Soto-Yéber, L.; Soto-Ortiz, J.; Godoy, P.; Godoy-Herrera, R. The behavior of adult Drosophila in the wild. PLoS ONE 2018, 13, e0209917. [Google Scholar] [CrossRef]
- Markow, T.A. The secret lives of Drosophila flies. Elife 2015, 4, e06793. [Google Scholar] [CrossRef]
- Batista, M.R.D.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, e3063. [Google Scholar] [CrossRef] [Green Version]
- Harrison, R.G. Dispersal Polymorphisms in Insects. Annu. Rev. Ecol. Syst. 1980, 11, 95–118. [Google Scholar] [CrossRef]
- Matthysen, E. Density-dependent dispersal in birds and mammals. Ecography 2005, 28, 403–416. [Google Scholar] [CrossRef]
- Plazio, E.; Margol, T.; Nowicki, P. Intersexual differences in density-dependent dispersal and their evolutionary drivers. J. Evol. Biol. 2020, 33, 1495–1506. [Google Scholar] [CrossRef]
- Simon, J.C.; Dickson, W.B.; Dickinson, M.H. Prior mating experience modulates the dispersal of drosophila in males more than in females. Behav. Genet. 2011, 41, 754–767. [Google Scholar] [CrossRef] [Green Version]
- Flatt, T. Life-history evolution and the genetics of fitness components in Drosophila melanogaster. Genetics 2020, 214, 3–48. [Google Scholar] [CrossRef] [Green Version]
- Cao, T.; Jin, J.-P. Evolution of Flight Muscle Contractility and Energetic Efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H. Structure, function and evolution of insect flight muscle. Biophysics 2011, 7, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.F.; Woods, H.A.; Roberts, S.P. Ecological and Environmental Physiology of Insects; Oxford University Press: New York, NY, USA, 2012; ISBN 9780199225958. [Google Scholar]
- Gordon, S.; Dickinson, M.H. Role of calcium in the regulation of mechanical power in insect flight. Proc. Natl. Acad. Sci. USA 2006, 103, 4311–4315. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.-J.; Kim, Y.-S.; Park, J.-S.; Pyo, J.-H.; Na, H.-J.; Kim, I.-J.; Kim, C.-M.; Chung, H.Y.; Kim, N.D.; Arking, R.; et al. Age-related change in γH2AX of Drosophila muscle: Its significance as a marker for muscle damage and longevity. Biogerontology 2015, 16, 503–516. [Google Scholar] [CrossRef]
- Ambrioso, G.; Weisfeldt, M.L.; Jacobus, W.E.; Flaherty, J.T. Evidence for a reversible oxygen radical-mediated component of reperfusion injury: Reduction by recombinant human superoxide dismutase administered at the time of reflow. Circulation 1987, 75, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Steenbergen, C.; Murphy, E.; Levy, L.; London, R.E. Elevation in cytosolic free calcium concentration early in myocardial ischemia in perfused rat heart. Circ. Res. 1987, 60, 700–707. [Google Scholar] [CrossRef] [Green Version]
- Hochachka, P.W.; Somero, G.N. Biochemical Adaptation: Mechanism and Process in Physiological Evolution; Oxford University Press: New York, NY, USA, 2002; ISBN 0-195-11703-4. [Google Scholar]
- Joos, B.; Lighton, J.R.B.; Harrison, J.F.; Suarez, R.K.; Roberts, S.P. Effects of ambient oxygen tension on flight performance, metabolism, and water loss of the honeybee. Physiol. Zool. 1997, 70, 167–174. [Google Scholar] [CrossRef]
- Chadwick, L.E.; Williams, C.M. The effects of atmospheric pressure and composition on the flight of Drosophila. Biol. Bull. 1949, 97, 115–137. [Google Scholar] [CrossRef]
- Lighton, J.R.B.; Schilman, P.E. Oxygen reperfusion damage in an insect. PLoS ONE 2007, 2, e1267. [Google Scholar] [CrossRef] [Green Version]
- Dillon, M.E. Into thin air: Physiology and evolution of alpine insects. Integr. Comp. Biol. 2006, 46, 49–61. [Google Scholar] [CrossRef] [Green Version]
- Dillon, M.E.; Dudley, R. Surpassing Mt. Everest: Extreme flight performance of alpine bumble-bees. Biol. Lett. 2014, 10, 20130922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodkinson, I.D. Terrestrial insects along elevation gradients: Species and community responses to altitude. Biol. Rev. Camb. Philos. Soc. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzmenko, N.V. Seasonal Variations in Atmospheric Pressure, Partial Oxygen Density, and Geomagnetic Activity as Additional Synchronizers of Circannual Rhythms. Biophysics 2019, 64, 599–609. [Google Scholar] [CrossRef]
- Harrison, J.F.; Frazier, M.R.; Henry, J.R.; Kaiser, A.; Klok, C.J.; Rascón, B. Responses of terrestrial insects to hypoxia or hyperoxia. Respir. Physiol. Neurobiol. 2006, 154, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D.; Morley, S.A.; Hughes, R.N. From cells to colonies: At what levels of body organization does the ‘temperature-size rule’ apply? Evol. Dev. 2006, 8, 202–214. [Google Scholar] [CrossRef]
- Kozłowski, J.; Czarnoleski, M.; Dańko, M. Can optimal resource allocation models explain why ectotherms grow larger in cold? Integr. Comp. Biol. 2004, 44, 480–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, H.A. Egg-mass size and cell size: Effects of temperature on oxygen distribution. Am. Zool. 1999, 39, 244–252. [Google Scholar] [CrossRef]
- Angilletta, M.J.; Steury, T.D.; Sears, M.W. Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integr. Comp. Biol. 2004, 44, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, D. Temperature and Organism Size—A Biological Law for Ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar] [CrossRef]
- Czarnoleski, M.; Cooper, B.S.; Kierat, J.; Angilletta, M.J. Flies developed small bodies and small cells in warm and in thermally fluctuating environments. J. Exp. Biol. 2013, 216, 2896–2901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, R.B.R.; French, V.; Partridge, L. Temperature modulates epidermal cell size in Drosophila melanogaster. J. Insect Physiol. 2002, 48, 231–237. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Privalova, V.; Szlachcic, E.; Sobczyk, Ł.; Szabla, N.; Czarnoleski, M. Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster. Biology 2021, 10, 327. https://doi.org/10.3390/biology10040327
Privalova V, Szlachcic E, Sobczyk Ł, Szabla N, Czarnoleski M. Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster. Biology. 2021; 10(4):327. https://doi.org/10.3390/biology10040327
Chicago/Turabian StylePrivalova, Valeriya, Ewa Szlachcic, Łukasz Sobczyk, Natalia Szabla, and Marcin Czarnoleski. 2021. "Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster" Biology 10, no. 4: 327. https://doi.org/10.3390/biology10040327
APA StylePrivalova, V., Szlachcic, E., Sobczyk, Ł., Szabla, N., & Czarnoleski, M. (2021). Oxygen Dependence of Flight Performance in Ageing Drosophila melanogaster. Biology, 10(4), 327. https://doi.org/10.3390/biology10040327





