The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Recording and Field Procedures
2.2. Statistical Analysis
3. Results
3.1. Monk Parakeet Nests and Their Communities of Tenants
3.2. Interactions between Monk Parakeets and Tenant Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wisz, M.S.; Pottier, J.; Kissling, W.D.; Pellissier, L.; Lenoir, J.; Damgaard, C.F.; Forchhammer, M.C.; Grytnes, J.A.; Guisan, A.; Heikkinen, R.K.; et al. The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 2013, 88, 15–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodge, D.M. Biological invasions: Lessons for ecology. Trends Ecol. Evol. 1993, 8, 133–137. [Google Scholar] [CrossRef]
- Wilcove, D.S.; Rothstein, D.; Dubow, J.; Phillips, A.; Losos, E. Quantifying threats to imperiled species in the United States. BioScience 1998, 48, 607–615. [Google Scholar] [CrossRef] [Green Version]
- Clavero, M.; García-Berthou, E. Invasive species are a leading cause of animal extinctions. Trends Ecol. Evol. 2005, 20, 110. [Google Scholar] [CrossRef] [Green Version]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef]
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 2017, 84, 468. [Google Scholar]
- Rodriguez, L.F. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol. Invasions 2006, 8, 927–939. [Google Scholar] [CrossRef]
- Braga, R.R.; Gómez, A.; Heger, T.; Vitule, J.R.S.; Jeschke, J.M. Invasional Meltdown Hypothesis. In Invasion Biology: Hypotheses and Evidence, 1st ed.; Jeschke, J.M., Heger, T., Eds.; CABI Publishing: Boston, MA, USA, 2018; Volume 10, pp. 79–91. [Google Scholar]
- Hernández-Brito, D.; Blanco, G.; Tella, J.L.; Carrete, M. A protective nesting association with native species counteracts biotic resistance for the spread of an invasive parakeet from urban into rural habitats. Front. Zool. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Abbreviated Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Cardador, L.; Lattuada, M.; Strubbe, D.; Tella, J.L.; Reino, L.; Figueira, R.; Carrete, M. Regional bans on wild-bird trade modify invasion risks at a global scale. Conserv. Lett. 2017, 10, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Calzada Preston, C.E.; Pruett-Jones, E.; Eberhard, J.R. Monk parakeets as a Globally Naturalized Species. In Naturalized Parrots of the World: Distribution, Ecology, and Impacts of the World’s Most Colorful Colonizers, 1st ed.; Pruett-Jones, E., Ed.; Princeton University Press: Princeton, NJ, USA, 2021; Volume 11, pp. 173–192. [Google Scholar]
- Calzada Preston, C.E.; Pruett-Jones, S. The number and distribution of naturalized parrots. Diversity 2021. pending acceptation. [Google Scholar]
- Navarro, J.L.; Martella, M.B.; Bucher, E.H. Breeding season and productivity of monk parakeets in Cordoba, Argentina. Wilson Bull. 1992, 104, 413–424. [Google Scholar]
- Eberhard, J.R. Breeding biology of the Monk Parakeet. Wilson Bull. 1998, 110, 463–473. [Google Scholar]
- Dawson Pell, F.S.; Senar, J.C.; Franks, D.W.; Hatchwell, B.J. Fine-scale genetic structure reflects limited and coordinated dispersal in the colonial monk parakeet, Myiopsitta Monachus. Mol. Ecol. 2021, 30, 1531–1544. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, H. Bird societies. In Handbook of Social Psychology, 1st ed.; Murchison, C., Ed.; Clark University Press: Worcester, MA, USA, 1935; Volume 5, pp. 142–185. [Google Scholar]
- Martella, M.B.; Bucher, E.H. Nesting of the Spot-winged Falconet in Monk Parakeet’s nests. Auk 1984, 101, 614–615. [Google Scholar] [CrossRef]
- Martella, M.B.; Navarro, J.L.; Bucher, E.H. Vertebrados asociados a los nidos de la cotorra (Myiopsitta monachus) en Córdoba y La Rioja. Physis 1985, 43, 49–51. [Google Scholar]
- De Lucca, E.R. Nidificación del Halconcito Colorado (Falco sparverius) en nidos de Cotorra (Myiopsitta monachus). Hornero 1992, 13, 238–240. [Google Scholar]
- Port, J.L.; Brewer, G.L. Use of monk parakeet (Myiopsitta monachus) nests by speckled teal (Anas flavirostris) in eastern Argentina. Ornitol. Neotrop. 2004, 15, 209–218. [Google Scholar]
- Nores, M. Use of active Monk Parakeet nests by Common Pigeons and response by the host. Wilson J. Ornithol. 2009, 121, 812–815. [Google Scholar] [CrossRef]
- Wagner, N. Occupation of Monk Parakeet (Myiopsitta monachus) nest cavities by House Sparrows (Passer domesticus) in Rio Grande do Sul, Brazil. Bol. SAO 2012, 20, 1–6. [Google Scholar]
- Briceño, C.; Sandoval-Rodríguez, A.; Yévenes, K.; Larraechea, M.; Morgado, A.; Chappuzeau, C.; Muñoz, V.; Dufflocq, P.; Olivares, F. Interactions between Invasive Monk Parakeets (Myiopsitta monachus) and Other Bird Species during Nesting Seasons in Santiago, Chile. Animals 2019, 9, 923. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Riley, J.; Winch, J.M.; Stimson, A.F.; Pope, R.D. The association of Amphisbaena alba (Reptilia: Amphisbaenia) with the leaf-cutting ant Atta cephalotes in Trinidad. J. Nat. Hist. 1986, 20, 459–470. [Google Scholar] [CrossRef]
- Hugo, H.; Cristaldo, P.F.; DeSouza, O. Nonaggressive behavior: A strategy employed by an obligate nest invader to avoid conflict with its host species. Ecol. Evol. 2020, 10, 8741–8754. [Google Scholar] [CrossRef] [PubMed]
- Oschadleus, H.D. Birds adopting weaver nests for breeding in Africa. Ostrich 2018, 89, 131–138. [Google Scholar] [CrossRef]
- Delhey, K. Nest webs beyond woodpeckers. Ecology 2018, 99, 985–988. [Google Scholar] [CrossRef]
- van der Hoek, Y.; Gaona, G.V.; Ciach, M.; Martin, K. Global relationships between tree-cavity excavators and forest bird richness. R. Soc. Open Sci. 2020, 7, 192177. [Google Scholar] [CrossRef] [PubMed]
- Tella, J.L.; Romero-Vidal, P.; Dénes, F.V.; Hiraldo, F.; Toledo, B.; Rossetto, F.; Blanco, G.; Hernández-Brito, D.; Pacífico, E.; Díaz-Luque, J.A.; et al. Roadside Car Surveys: Methodological Constraints and Solutions for Estimating Parrot Abundances across the World. Diversity 2021, 13, 300. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Carrete, M.; Popa-Lisseanu, A.G.; Ibáñez, C.; Tella, J.L. Crowding in the city: Losing and winning competitors of an invasive bird. PLoS ONE 2014, 9, e100593. [Google Scholar] [CrossRef]
- Mori, E.; Ancillotto, L.; Groombridge, J.; Howard, T.; Smith, V.S.; Menchetti, M. Macroparasites of introduced parakeets in Italy: A possible role for parasite-mediated competition. Parasitol. Res. 2015, 114, 3277–3281. [Google Scholar] [CrossRef]
- Senar, J.C.; Carrillo-Ortiz, J.G.; Ortega-Segalerva, A.; Dawson Pell, F.S.E.; Pascual, J.; Arroyo, L.; Mazzoni, D.; Montalvo, T.; Hatchwell, B.J. The reproductive capacity of Monk Parakeets Myiopsitta monachus is higher in their invasive range. Bird Study 2019, 66, 136–140. [Google Scholar] [CrossRef]
- Hardin, J.W.; Hilbe, J.M. Generalized Linear Models and Extensions, 2nd ed.; Stata Press: College Station, TX, USA, 2007. [Google Scholar]
- Dunning, J.J.B. CRC Handbook of Avian Body Masses, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: Berlin, Germany, 2002; pp. 323–324. [Google Scholar]
- Bartón, K. MuMIn: Multi-Model Inference. R Package Version 1.40.0. 2017. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 April 2021).
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.1.3. 2018. Available online: https://cran.r-project.org/package=emmeans (accessed on 1 April 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Traveset, A.; Richardson, D.M. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Bronstein, J.L. The Gift That Keeps on Giving: Why Does Biological Diversity Accumulate Around Mutualisms? In Plant-Animal Interactions, 1st ed.; Del-Claro, K., Torezan-Silingardi, H.M., Eds.; Springer: Cham, Switzerland, 2021; Volume 11, pp. 283–306. [Google Scholar]
- Martin, K.; Eadie, J.M. Nest webs: A community-wide approach to the management and conservation of cavity-nesting forest birds. For. Ecol. Manag. 1999, 115, 243–257. [Google Scholar] [CrossRef]
- Caccamise, D.F.; Weathers, W.W. Winter nest microclimate of monk parakeets. Wilson Bull. 1977, 89, 346–349. [Google Scholar]
- Wiebe, K.L. Microclimate of tree cavity nests: Is it important for reproductive success in Northern Flickers? Auk 2001, 118, 412–421. [Google Scholar] [CrossRef]
- Lloyd, J.D.; Martin, T.E. Nest-site preference and maternal effects on offspring growth. Behav. Ecol. 2004, 15, 816–823. [Google Scholar] [CrossRef]
- Aramburú, R.; Calvo, S.; Alzugaray, M.E.; Cicchino, A. Ectoparasitic load of monk parakeet (Myiopsitta monachus, Psittacidae) nestlings. Ornitol. Neotrop. 2003, 14, 415–418. [Google Scholar]
- Briceño, C.; Surot, D.; González-Acuña, D.; Martínez, F.J.; Fredes, F. Parasitic survey on introduced monk parakeets (Myiopsitta monachus) in Santiago, Chile. Rev. Bras. Parasitol. Vet. 2017, 26, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Ancillotto, L.; Studer, V.; Howard, T.; Smith, V.S.; McAlister, E.; Beccaloni, J.; Manzia, F.; Renzopaoli, F.; Bosso, L.; Russo, D.; et al. Environmental drivers of parasite load and species richness in introduced parakeets in an urban landscape. Parasitol. Res. 2018, 117, 3591–3599. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Pascual, J.; Fattorini, N.; Menchetti, M.; Montalvo, T.; Senar, J.C. Ectoparasite sharing among native and invasive birds in a metropolitan area. Parasitol. Res. 2019, 118, 399–409. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Díez-Fernández, A.; Montalvo, T.; Bueno-Marí, R.; Pangrani, Q.; Soriguer, R.C.; Senar, J.C.; Figuerola, J. Do invasive mosquito and bird species alter avian malaria parasite transmission? Diversity 2020, 12, 111. [Google Scholar] [CrossRef] [Green Version]
- Morinha, F.; Carrete, M.; Tella, J.L.; Blanco, G. High Prevalence of Novel Beak and Feather Disease Virus in Sympatric Invasive Parakeets Introduced to Spain from Asia and South America. Diversity 2020, 12, 192. [Google Scholar] [CrossRef]
- Sandoval-Rodríguez, A.; Marcone, D.; Alegría-Morán, R.; Larraechea, M.; Yévenes, K.; Fredes, F.; Briceño, C. Cryptosporidium spp. and Giardia spp. in Free-Ranging Introduced Monk Parakeets from Santiago, Chile. Animals 2021, 11, 801. [Google Scholar] [CrossRef]
- Viana, I.R.; Strubbe, D.; Zocche, J.J. Monk parakeet invasion success: A role for nest thermoregulation and bactericidal potential of plant nest material? Biol. Invasions 2016, 18, 1305–1315. [Google Scholar] [CrossRef]
- Rowley, I. The Galah: Behavioral Ecology of Galahs, 1st ed.; Surrey Beatty and Sons Pty Ltd.: Chipping Norton, Australia, 1990; pp. 77–79. [Google Scholar]
- van Dijk, R.E.; Kaden, J.C.; Argüelles-Ticó, A.; Beltran, L.M.; Paquet, M.; Covas, R.; Doutrelant, C.; Hatchwell, B.J. The thermoregulatory benefits of the communal nest of sociable weavers Philetairus socius are spatially structured within nests. J. Avian Biol. 2013, 44, 102–110. [Google Scholar] [CrossRef]
- Covas, R.; Huyser, O.; Doutrelant, C. Pygmy Falcon predation of nestlings of their obligate host, the Sociable Weaver. Ostrich 2004, 75, 325–326. [Google Scholar] [CrossRef]
- Quinn, J.L.; Ueta, M. Protective nesting associations in birds. Ibis 2008, 150, 146–167. [Google Scholar] [CrossRef]
- Lima, S.L. Predators and the breeding bird: Behavioral and reproductive flexibility under the risk of predation. Biol. Rev. 2009, 84, 485–513. [Google Scholar] [CrossRef]
- Senar, J.C.; Domènech, J.; Arroyo, L.; Torre, I.; Gordo, O. An evaluation of monk parakeet damage to crops in the metropolitan area of Barcelona. Anim. Biodivers. Conserv. 2016, 39, 141–145. [Google Scholar] [CrossRef]
- Simberloff, D.; Von Holle, B. Positive interactions of nonindigenous species: Invasional meltdown? Biol. Invasions 1999, 1, 21–32. [Google Scholar] [CrossRef]
- Tracey, K.F.; Miller, K.E. Monk Parakeets Provide Nesting Opportunities for the Threatened Southeastern American Kestrel. J. Raptor Res. 2018, 52, 389–392. [Google Scholar] [CrossRef]
- Blanco, J.C.; González, J.L. Libro Rojo de los Vertebrados de España. In Ministerio Agricultura, Pesca y Alimentación, 1st ed.; ICONA: Madrid, Spain, 1992. [Google Scholar]
- Blanco, G.; Frías, Ó.; Cuevas, J.A.; González, J.L.; Martínez, F. Commonness of not-so-common birds: The need for baseline knowledge of actual population size for the validation of population size predictions. Bird Study 2014, 61, 351–360. [Google Scholar] [CrossRef]
- Richardson, J.E.; Lees, A.; Marsden, S. Landscape -scale habitat associations in an urban Stock Dove Columba oenas population. Research Sqare: Rs-320608/v1, preprint. Available online: https://www.researchsquare.com/article/rs-320608/v1 (accessed on 10 July 2021).
- White, E.M.; Wilson, J.C.; Clarke, A.R. Biotic indirect effects: A neglected concept in invasion biology. Divers. Distrib. 2006, 12, 443–455. [Google Scholar] [CrossRef]
- Feit, B.; Gordon, C.E.; Webb, J.K.; Jessop, T.S.; Laffan, S.W.; Dempster, T.; Letnic, M. Invasive cane toads might initiate cascades of direct and indirect effects in a terrestrial ecosystem. Biol. Invasions 2018, 20, 1833–1847. [Google Scholar] [CrossRef] [Green Version]
- Postigo, J.L.; Strubbe, D.; Mori, E.; Ancillotto, L.; Carneiro, I.; Latsoudis, P.; Menchetti, M.; Pârâu, L.G.; Parrott, D.; Reino, L.; et al. Mediterranean versus Atlantic monk parakeets Myiopsitta monachus: Towards differentiated management at the European scale. Pest. Manag. Sci. 2019, 75, 915–922. [Google Scholar] [CrossRef]
- Ballari, S.A.; Kuebbing, S.E.; Nuñez, M.A. Potential problems of removing one invasive species at a time: A meta-analysis of the interactions between invasive vertebrates and unexpected effects of removal programs. PeerJ 2016, 4, e2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catford, J.A.; Jaimie, T.A.D.; Hulme, P.E.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environ. Rev. 2020, 29, 119–141. [Google Scholar] [CrossRef]
- Hartig, F. Emmeans: DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.2.0. 2018. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 1 April 2021).


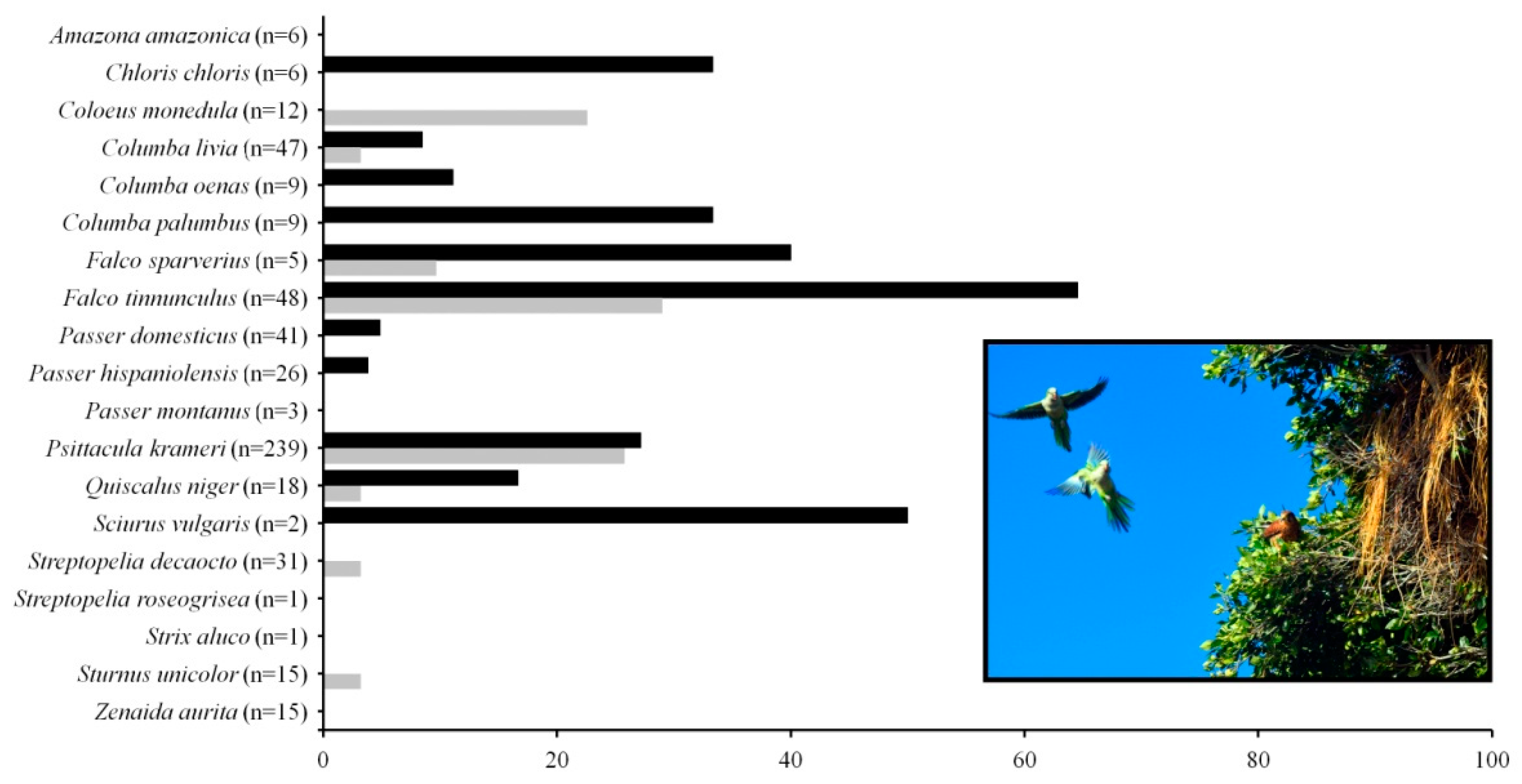
| Tenant Species | Invaded Areas | Native Areas | Total Records | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Italy | P. Rico | C. Spain | Tenerife | Argentina | Brazil | Paraguay | Uruguay | ||
| BIRDS | |||||||||
| Agelaioides badiusV,O/C | 4 (4) | 1 (1) | 5 | ||||||
| Amazona amazonicaV,C | 1 * | 1 | |||||||
| Anumbius annumbiS | 2 (2) | 2 | |||||||
| Caracara plancusP,S | 6 (2) | 6 | |||||||
| Chloris chlorisS | 11 (6) | 11 | |||||||
| Ciconia ciconiaP,S | 2 | 2 | |||||||
| Columba livia var. domestica V,C | 4 (4) * | 185 (86) | 4 | 7 (7) * | 200 | ||||
| Columba oenasV,C | 268 (142) | 268 | |||||||
| Columba palumbusS | 6 (2) | 6 | |||||||
| Coloeus monedulaV,P,C | 196 (123) | 196 | |||||||
| Coryphistera alaudinaC | 1 (1) | 1 | |||||||
| Dendrocygna autumnalisV,C | 1 (1) | 1 | |||||||
| Falco sparveriusV,P,C | 2 (2) | 3 (2) | 1 (1) | 1 (1) | 7 | ||||
| Falco tinnunculusV,P,C | 20 (12) | 9 (1) | 29 | ||||||
| Geranoaetus polyosomaP,S | 2 | 2 | |||||||
| Machetornis rixosaO/C | 10 (10) | 1 (1) | 2 (2) | 1 (1) | 14 | ||||
| Mimus saturninusC | 1 (1) | 1 | |||||||
| Molothrus bonariensisC | 1 (1) * † | 1 | |||||||
| Otus scopsV,P,C | 1 | 1 | |||||||
| Passer domesticusV,O/C | 27 (27) * | 852 (492) | 94 (87) * | 10 (10) * | 33 (33) * | 1016 | |||
| Passer hispaniolensisS | 285 (133) | 39 (4) | 324 | ||||||
| Passer italiaeV,O/C | 4 | 4 | |||||||
| Passer montanusV,O/C | 278 (122) | 278 | |||||||
| Pitangus sulphuratusS/C | 3 (2) | 2(1) | 4 (4) | 9 | |||||
| Psittacara leucophthalmusV/C | 1 (1) | 1 | |||||||
| Patagioenas picazuroS | 1 | 1 | |||||||
| Psittacula krameriV/C | 35 (6) * | 35 | |||||||
| Quiscalus nigerS | 42 (42) | 42 | |||||||
| Schoeniophylax phryganophilusS/C | 2 (2) | 2 | |||||||
| Sicalis flaveolaV,O/C | 4 (4) | 1 (1) | 7 (7) | 12 | |||||
| Sicalis luteolaO/C | 2 (2) | 2 | |||||||
| Streptopelia decaoctoS/C | 28 (10) | 4 | 32 | ||||||
| Streptopelia roseogriseaS | 4 * | 4 | |||||||
| Strix alucoV,P,C | 1 (1) | 1 | |||||||
| Sturnus unicolorV,O/C | 158 (100) | 158 | |||||||
| Thraupis palmarumC | 2 (2) | 2 | |||||||
| Tyrannus melancholicusC | 1 (1) | 1 | |||||||
| Tyrannus savanaS | 1 | 1 | |||||||
| Upupa epopsV,C | 1 | 1 | |||||||
| Zenaida auritaS | 3 (3) | 3 | |||||||
| MAMMALS | |||||||||
| Sciurus vulgarisV,P,C | 4 (2) | 4 | |||||||
| INSECTS | |||||||||
| Unknown bee (Apoidea) V,C | 1 (1) | 1 | |||||||
| UNKNOWN TENANT | 1 (1) | 1 (1) | 2 | ||||||
| Models of Presence | k | ΔAICc | w | Variables | Estimate | 2.50% | 97.50% |
| A + B + C + D + E | 9 | 0.00 | 0.73 | A | 22.77 | −6219.85 | 6265.39 |
| A + B + C + F + D + E | 10 | 1.95 | 0.27 | B | 1.59 | 1.40 | 1.78 |
| A + B + D + E | 8 | 16.91 | 0.00 | C(urban) | −1.39 | −2.05 | −0.74 |
| A + B + F + D + E | 9 | 18.91 | 0.00 | D(Puerto Rico) | 3.14 | 2.50 | 3.77 |
| A + B + C + D | 7 | 68.19 | 0.00 | D(C. Spain) | 2.94 | 2.23 | 3.65 |
| A + B + C + F + D | 8 | 69.62 | 0.00 | D(Tenerife) | 1.27 | 0.03 | 2.51 |
| A + B + D | 6 | 69.94 | 0.00 | E(roof) | 3.89 | 2.58 | 5.20 |
| A + B + F + D | 7 | 71.51 | 0.00 | E(tree) | −0.66 | −1.34 | 0.03 |
| A + B + C + E | 6 | 123.91 | 0.00 | F | 0.02 | −0.11 | 0.15 |
| A + B + C + F + E | 7 | 125.30 | 0.00 | ||||
| Models of Abundance | k | ΔAICc | w | Variables | Estimate | 2.50% | 97.50% |
| A + B + C + D + E | 11 | 0.00 | 0.72 | A | 1.54 | 1.28 | 1.80 |
| A + B + C + F + D + E | 12 | 1.85 | 0.28 | B | 0.87 | 0.79 | 0.95 |
| A + B + D + E | 10 | 44.58 | 0.00 | C(urban) | −0.97 | −1.25 | −0.69 |
| A + B + F + D + E | 11 | 45.17 | 0.00 | D(Puerto Rico) | 1.80 | 1.43 | 2.17 |
| A + B + C + D | 9 | 57.59 | 0.00 | D(C. Spain) | 1.67 | 1.37 | 1.98 |
| A + B + C + F + D | 10 | 59.61 | 0.00 | D(Tenerife) | 0.61 | −0.14 | 1.36 |
| A + B + F + D | 9 | 121.88 | 0.00 | E(roof) | 1.40 | 0.70 | 2.10 |
| A + B + D | 8 | 122.45 | 0.00 | E(tree) | −0.68 | −0.99 | −0.37 |
| B + C + F + D + E | 11 | 136.62 | 0.00 | F | 0.02 | −0.06 | 0.10 |
| B + C + D + E | 10 | 137.88 | 0.00 | ||||
| Models of richness | k | ΔAICc | w | Variables | Estimate | 2.50% | 97.50% |
| A + B + C + D + E | 10 | 0.00 | 0.73 | A | 1.07 | 0.85 | 1.29 |
| A + B + C + F + D + E | 11 | 2.01 | 0.27 | B | 0.32 | 0.29 | 0.35 |
| A + B + C + D | 8 | 30.82 | 0.00 | C(urban) | −0.85 | −1.10 | −0.61 |
| A + B + C + F + D | 9 | 32.46 | 0.00 | D(Puerto Rico) | 1.20 | 0.86 | 1.55 |
| A + B + D + E | 9 | 44.19 | 0.00 | D(C. Spain) | 1.43 | 1.14 | 1.72 |
| A + B + F + D + E | 10 | 45.66 | 0.00 | D(Tenerife) | 1.79 | 1.32 | 2.27 |
| A + B + D | 7 | 55.59 | 0.00 | E(roof) | 1.94 | 1.35 | 2.52 |
| A + B + F + D | 8 | 57.57 | 0.00 | E(tree) | 0.19 | −0.08 | 0.45 |
| B + C + D + E | 9 | 78.17 | 0.00 | ||||
| B + C + F + D + E | 10 | 79.45 | 0.00 |
| Model | k | ΔAICc | w | Variables | Estimate | 2.50% | 97.50% |
|---|---|---|---|---|---|---|---|
| G + A + B + C + F | 6 | 0.00 | 0.51 | G | −7.98 | −8.92 | −7.03 |
| G + A + B + C + F + E | 7 | 1.54 | 0.24 | A | 2.03 | 0.93 | 3.13 |
| G + A + B + C + F + D | 8 | 2.18 | 0.17 | B | 9.88 | 8.84 | 10.92 |
| G + A + B + C + F + D + E | 9 | 3.77 | 0.08 | C(urban) | −5.30 | −7.95 | −2.66 |
| G + B + C + F | 5 | 16.70 | 0.00 | F | −0.48 | −0.62 | −0.33 |
| G + B + C + F + E | 6 | 18.65 | 0.00 | E(tree) | 0.97 | −1.84 | 3.78 |
| G + B + C + F + D | 7 | 18.99 | 0.00 | ||||
| G + B + C + F + D + E | 8 | 20.97 | 0.00 | ||||
| G + A + B + C + D | 7 | 30.39 | 0.00 | ||||
| G + A + B + F + E | 6 | 31.20 | 0.00 |
| Aggressive Interactions | k | ΔAICc | w | Variables | Estimate | 2.50% | 97.50% |
| T | 4 | 0.00 | 0.25 | T(Puerto Rico) | −0.89 | −2.03 | 0.25 |
| U + T | 5 | 0.09 | 0.24 | T(Seville) | −1.14 | −2.37 | 0.10 |
| V + T | 5 | 1.57 | 0.11 | T(Tenerife) | 0.63 | −0.23 | 1.49 |
| U + V + T | 6 | 1.65 | 0.11 | U | 0.05 | −0.02 | 0.13 |
| W + T | 5 | 1.72 | 0.11 | V(urban) | 0.41 | −0.76 | 1.58 |
| W + U + T | 6 | 1.87 | 0.10 | W | 0.00 | 0.00 | 0.00 |
| W + V + T | 6 | 3.43 | 0.04 | ||||
| W + U + V + T | 7 | 3.56 | 0.04 | ||||
| V | 2 | 22.90 | 0.00 | ||||
| U + V | 3 | 24.33 | 0.00 | ||||
| Winning an Aggression | k | delta | w | Variables | Estimate | 2.50% | 97.50% |
| W + T | 5 | 0.00 | 0.16 | W | 0.01 | 0.00 | 0.01 |
| U + T | 5 | 0.53 | 0.12 | T(Puerto Rico) | −0.02 | −3.19 | 3.14 |
| W + V | 3 | 0.72 | 0.11 | T(Seville) | −1.33 | −4.32 | 1.66 |
| W + U + T | 6 | 0.83 | 0.11 | T(Tenerife) | −2.04 | −4.33 | 0.25 |
| W | 2 | 1.25 | 0.09 | U | 0.09 | −0.04 | 0.22 |
| T | 4 | 1.25 | 0.08 | V(urban) | −1.68 | −3.97 | 0.60 |
| W + U + V | 4 | 1.76 | 0.07 | ||||
| W + V + T | 6 | 2.10 | 0.06 | ||||
| W + U | 3 | 2.35 | 0.05 | ||||
| U + V + T | 6 | 2.59 | 0.04 | ||||
| Expelling a Predator | k | delta | w | Variables | Estimate | 2.50% | 97.50% |
| T + X | 6 | 0.00 | 0.15 | T(Madrid) | −19.87 | −25,255.80 | 25,216.06 |
| T + Y | 6 | 1.53 | 0.07 | T(Puerto Rico) | −18.21 | −25,254.14 | 25,217.72 |
| T + Y + X | 7 | 1.78 | 0.06 | T(Seville) | −18.78 | −25,254.71 | 25,217.15 |
| V + X | 3 | 1.98 | 0.05 | T(Tenerife) | −18.53 | −25,254.46 | 25,217.40 |
| T + V + X | 7 | 1.99 | 0.05 | X | 2.23 | 0.66 | 3.79 |
| X | 2 | 2.09 | 0.05 | Y | 1.30 | −0.77 | 3.37 |
| T + Z + X | 7 | 2.23 | 0.05 | V(urban) | 0.56 | −0.66 | 1.78 |
| T + U + X | 7 | 2.31 | 0.05 | ||||
| Y | 2 | 3.19 | 0.03 | ||||
| U + V + X | 4 | 3.27 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Brito, D.; Carrete, M.; Blanco, G.; Romero-Vidal, P.; Senar, J.C.; Mori, E.; White, T.H., Jr.; Luna, Á.; Tella, J.L. The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas. Biology 2021, 10, 683. https://doi.org/10.3390/biology10070683
Hernández-Brito D, Carrete M, Blanco G, Romero-Vidal P, Senar JC, Mori E, White TH Jr., Luna Á, Tella JL. The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas. Biology. 2021; 10(7):683. https://doi.org/10.3390/biology10070683
Chicago/Turabian StyleHernández-Brito, Dailos, Martina Carrete, Guillermo Blanco, Pedro Romero-Vidal, Juan Carlos Senar, Emiliano Mori, Thomas H. White, Jr., Álvaro Luna, and José L. Tella. 2021. "The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas" Biology 10, no. 7: 683. https://doi.org/10.3390/biology10070683
APA StyleHernández-Brito, D., Carrete, M., Blanco, G., Romero-Vidal, P., Senar, J. C., Mori, E., White, T. H., Jr., Luna, Á., & Tella, J. L. (2021). The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas. Biology, 10(7), 683. https://doi.org/10.3390/biology10070683














