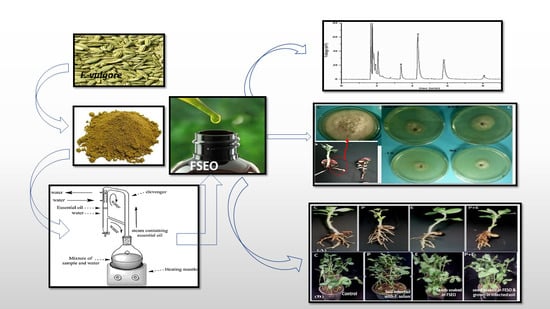
Essential Oil of Foeniculum vulgare Mill. as a Green Fungicide and Defense-Inducing Agent against Fusarium Root Rot Disease in Vicia faba L.
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction of Fennel Seed Essential Oil
2.2. Gas Chromatography/Mass Spectral Analysis
2.3. Isolation of Fusarium solani, Pathogenicity Test and Cultivation
2.4. In Vitro Evaluation of Antifungal Activity and Growth Inhibition
2.4.1. Agar Well Diffusion Method
2.4.2. Radial Growth Method
2.5. Pot Experiment
2.5.1. Preparation of Fungal Inoculum
2.5.2. Fennel Seeds, Growth Conditions and Treatments
2.6. Disease Assessments
2.7. Analysis of Plant Growth Parameters
2.8. Biochemical Analyses
2.8.1. Determination of 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.8.2. Total Phenolic Content
2.8.3. Total Flavonoid Content
2.8.4. Phenylalanine Lyase Assay
2.8.5. Polyphenol Oxidase (PPO)
2.8.6. Antioxidant Enzyme Quantification
2.8.7. Expression of Defense-Related Genes
2.9. Statistical Analysis
3. Results
3.1. Chemical Composition of Fennel Oil
3.2. In Vitro Control of F. Solani by Fennel Essential Oils
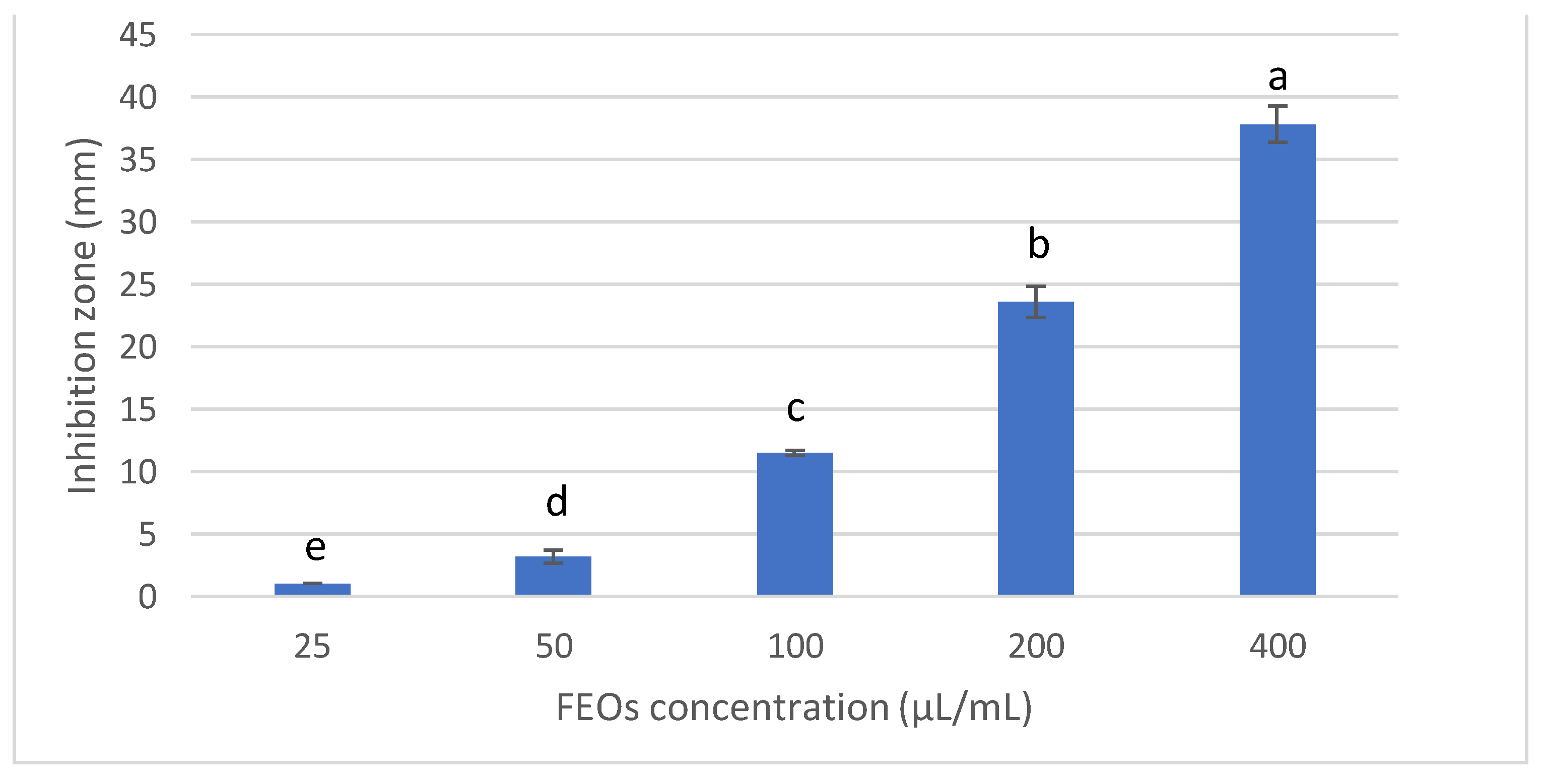
3.2.1. Antifungal Activity and Minimum Inhibitory Concentration of FSEO
3.2.2. Effect of FSEO on the Radial Growth of F. solani and Minimum Fungicidal Concentrations
3.3. In Vivo Control of F. solani KHA10 by Fennel Essential Oil
Efficacy of FSEO on Fusarium Root Rot Disease of Vicia faba L. under Pot Conditions
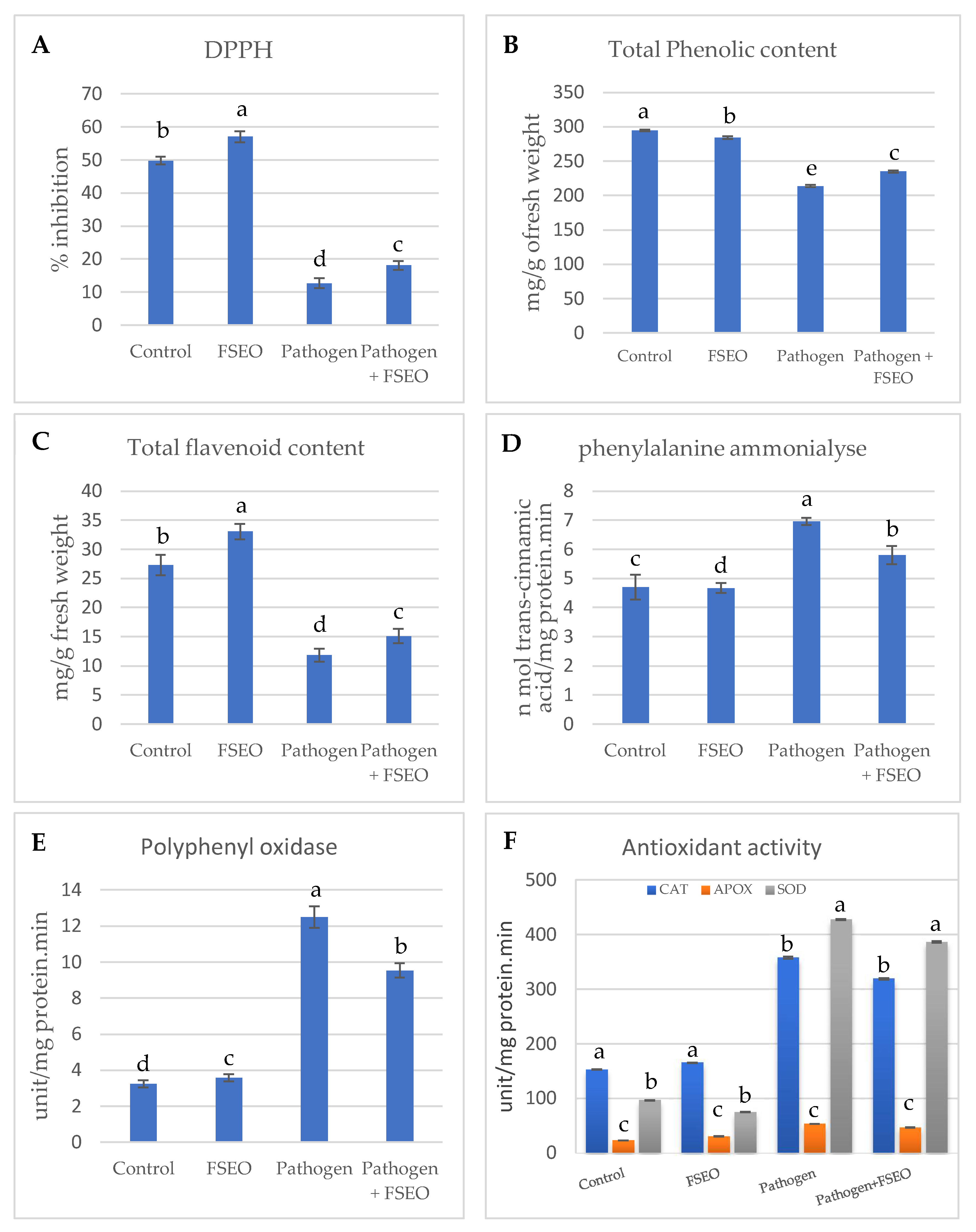
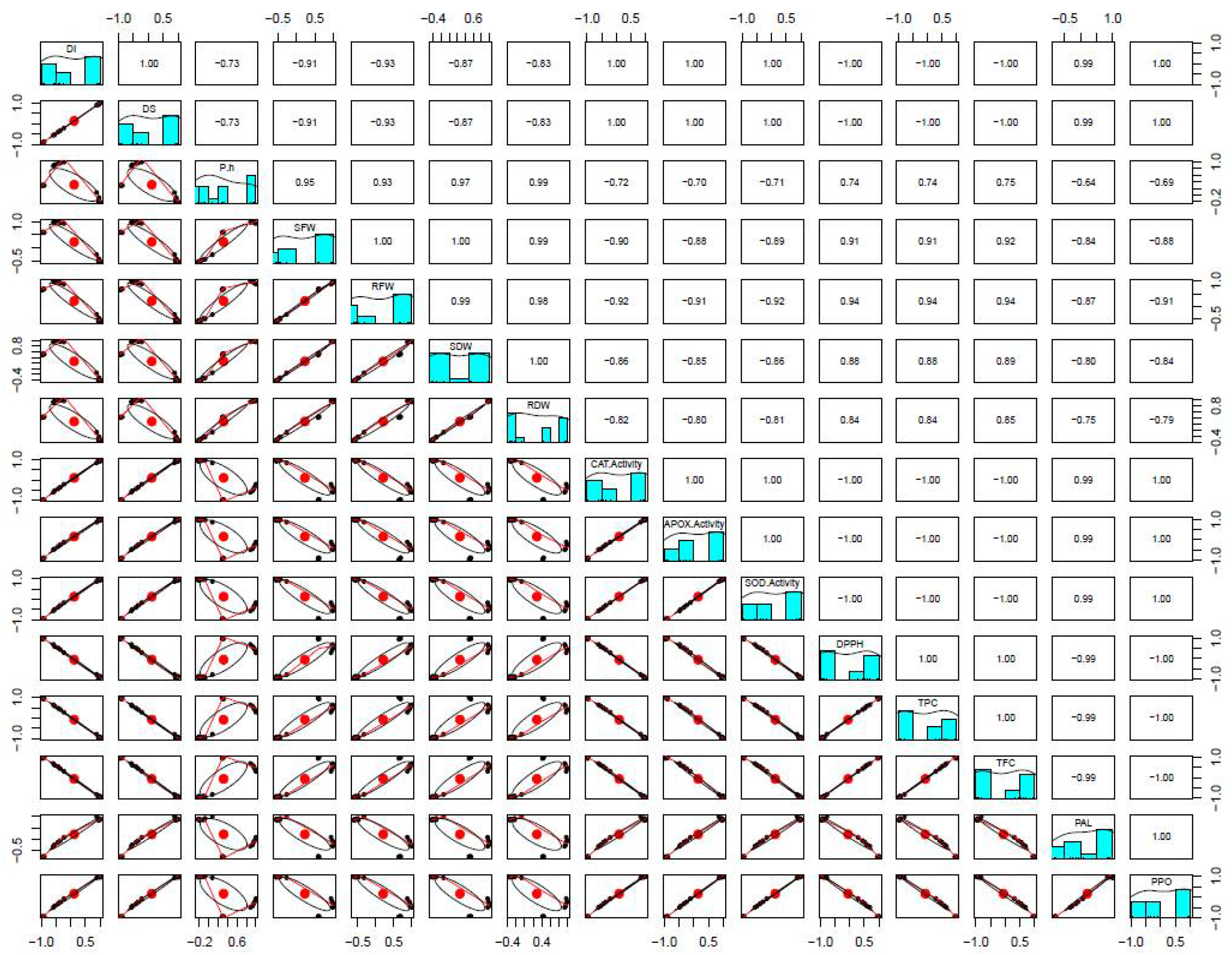
3.4. Physiological Characterization of FSEO Treated Faba Bean Plants
3.5. Influence of FSEO on Different Biochemical Parameters
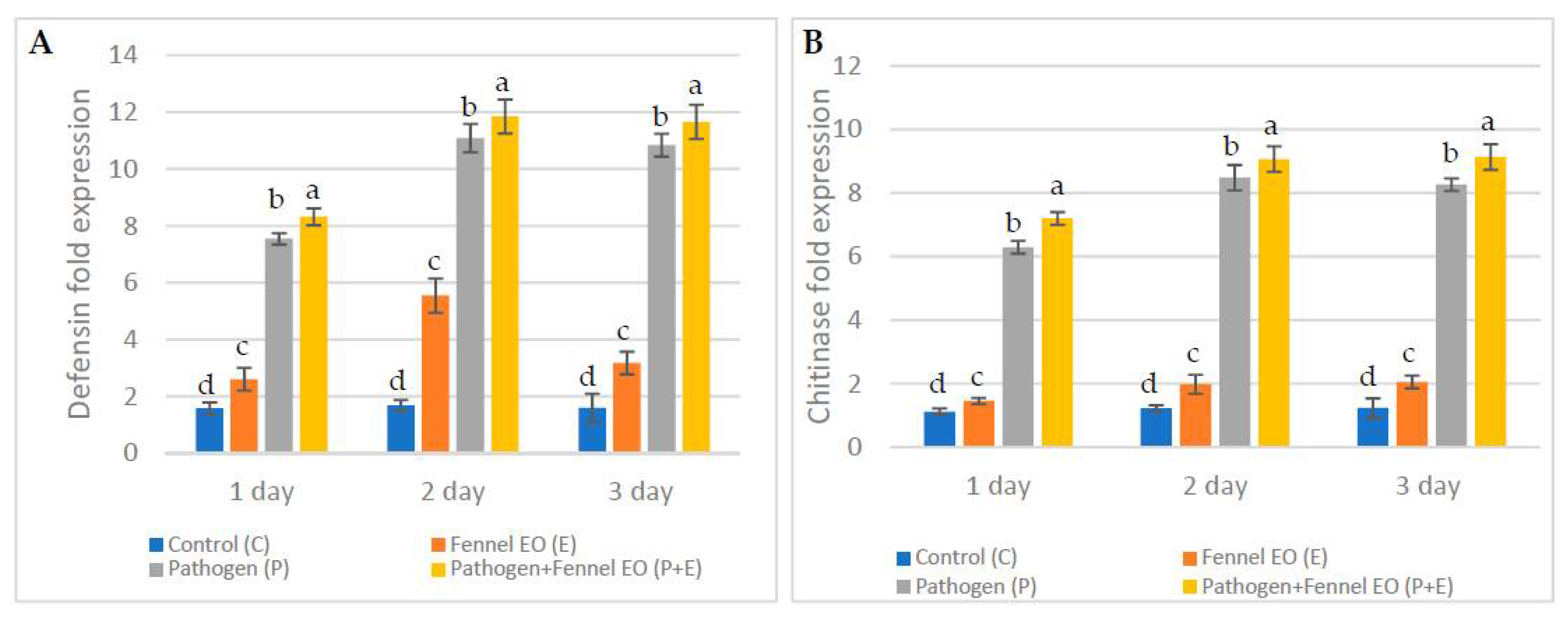
3.6. Expression Levels of Defense-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashem, A.H.; Abdelaziz, A.M.; Askar, A.A.; Fouda, H.M.; Khalil, A.M.A.; Abd-elsalam, K.A.; Khaleil, M.M. Bacillus megaterium -Mediated Synthesis of Selenium Nanoparticles and Their Antifungal Activity against Rhizoctonia solani in Faba Bean Plants. J. Fungi 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Alsiņa, I.; Kronberga, A.; Balliu, A.; Olle, M. Faba bean cultivation–revealing novel managing practices for more sustainable and competitive European cropping systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.A.; Abou-Taleb, E.M.; Okasha, A.M. Acetone inhibition of Rhizoctonia solani growth. J. Phytopathol. 1983, 107, 111–116. [Google Scholar] [CrossRef]
- Belay, H.; Anteneh, B. Integrated management of faba bean black root rot (Fusarium solani) through varietal resistance, drainage and adjustment of planting time. J. Plant Pathol. Microbiol. 2016, 7. [Google Scholar]
- Rose, T.J.; Julia, C.C.; Shepherd, M.; Rose, M.T.; Van Zwieten, L. Faba bean is less susceptible to fertiliser N impacts on biological N2 fixation than chickpea in monoculture and intercropping systems. Biol. Fertil. Soils 2016, 52, 271–276. [Google Scholar] [CrossRef]
- Hafizi, R.; Salleh, B.; Latiffah, Z. Morphological and molecular characterization of Fusarium. solani and F. oxysporum associated with crown disease of oil palm. Braz. J. Microbiol. 2013, 44, 959–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsyula, R.M.; Ajanga, S.I.; Buruchara, R.A.; Wortmann, C.S. Development of an integrated bean root rot control strategy for western Kenya. Afr. Crop Sci. J. 1998, 6, 61–67. [Google Scholar] [CrossRef]
- El-Mougy, N.S.; Abdel-Kader, M.M. Seed and soil treatments as integrated control measure against faba bean root rot pathogens. Plant Pathol. Bull. 2009, 18, 75–87. [Google Scholar]
- Juroszek, P.; Von Tiedemann, A. Potential strategies and future requirements for plant disease management under a changing climate. Plant Pathol. 2011, 60, 100–112. [Google Scholar] [CrossRef]
- Mehta, S.; Sharma, K. Natural resources: An ecofriendly and safer alternate to control plant diseases. Int. J. Pharm. Sci. Res. 2016, 7, 4327. [Google Scholar]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Hmaied, M.; Bouafif, H.; Magdouli, S.; Braghiroli, F.L.; Koubaa, A. Effect of Forest Biomass Pretreatment on Essential Oil Yield and Properties. Forests 2019, 10, 1042. [Google Scholar] [CrossRef] [Green Version]
- Terzi, V.; Morcia, C.; Faccioli, P.; Vale, G.; Tacconi, G.; Malnati, M. In vitro antifungal activity of the tea tree (Melaleuca alternifolia) essential oil and its major components against plant pathogens. Lett. Appl. Microbiol. 2007, 44, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential oils as potential alternative biocontrol products against plant pathogens and weeds: A review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ultee, A.; Smid, E.J. Influence of carvacrol on growth and toxin production by Bacillus cereus. Int. J. Food Microbiol. 2001, 64, 373–378. [Google Scholar] [CrossRef]
- Akthar, M.S.; Degaga, B.; Azam, T. Antimicrobial activity of essential oils extracted from medicinal plants against the pathogenic microorganisms: A review. Biol. Sci. Pharm. Res. 2014, 2350, 1588. [Google Scholar]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. Biomed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [Green Version]
- Kooti, W.; Moradi, M.; Ali-Akbari, S.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Therapeutic and pharmacological potential of Foeniculum vulgare Mill: A review. J. HerbMed Pharmacol. 2015, 4, 1–9. [Google Scholar]
- Saharkhiz, M.J.; Tarakeme, A. Essential oil content and composition of fennel (Foeniculum vulgare L.) fruits at different stages of development. J. Essent. Oil Bear. Plants 2011, 14, 605–609. [Google Scholar] [CrossRef]
- Anwar, F.; Ali, M.; Hussain, A.I.; Shahid, M. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare Mill.) seeds from Pakistan. Flavour Fragr. J. 2009, 24, 170–176. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Khan, M.A.; Ahmad, M.; Ahmad, M.M.; Abdin, M.Z.; Musarrat, J.; Javed, S. Chitinases: An update. J. Pharm. Bioallied Sci. 2013, 5, 21. [Google Scholar] [PubMed]
- Sara, M.; Rouissi, T.; Brar, S.K.; Blais, J.F. Life cycle analysis of potential substrates of sustainable biorefinery. In Platform Chemical Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 55–76. [Google Scholar]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- Tomazoni, E.Z.; Griggio, G.S.; Broilo, E.P.; da Silva Ribeiro, R.T.; Soares, G.L.G.; Schwambach, J. Screening for inhibitory activity of essential oils on fungal tomato pathogen Stemphylium solani Weber. Biocatal. Agric. Biotechnol. 2018, 16, 364–372. [Google Scholar] [CrossRef]
- Nash, S.M.; Snyder, W.C. Quantitative estimations by plate counts of propagules of the bean root rot Fusarium in field soils. Phytopathology 1962, 52. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0470276460. [Google Scholar]
- Djalali Farahani-Kofoet, R.; Witzel, K.; Graefe, J.; Grosch, R.; Zrenner, R. Species-specific impact of Fusarium infection on the root and shoot characteristics of asparagus. Pathogens 2020, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Belabid, L.; Simoussa, L.; Bayaa, B. Effect of some plant extracts on the population of Fusarium oxysporum f. sp. lentis, the causal organism of lentil wilt. Adv. Environ. Biol. 2010, 4, 95–101. [Google Scholar]
- Büttner, G.; Pfähler, B.; Märländer, B. Greenhouse and field techniques for testing sugar beet for resistance to Rhizoctonia root and crown rot. Plant Breed. 2004, 123, 158–166. [Google Scholar] [CrossRef]
- Kalleli, F.; Ghassen, A.; Salem, I.B.; BOUGHALLEB-M’HAMDI, N.; M’HAMDI, M. Essential oil from fennel seeds (Foeniculum vulgare) reduces Fusarium wilt of tomato (Solanum lycopersicon). Phytopathol. Mediterr. 2020, 59, 63–76. [Google Scholar]
- Filion, M.; St-Arnaud, M.; Jabaji-Hare, S.H. Quantification of Fusarium solani f. sp. phaseoli in mycorrhizal bean plants and surrounding mycorrhizosphere soil using real-time polymerase chain reaction and direct isolations on selective media. Phytopathology 2003, 93, 229–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, A.M.A.; Abdelaziz, A.M.; Khaleil, M.M.; Hashem, A.H. Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Lett. Appl. Microbiol. 2020, 2, 1–12. [Google Scholar] [CrossRef]
- Whetten, R.W.; Sederoff, R.R. Phenylalanine ammonia-lyase from loblolly pine: Purification of the enzyme and isolation of complementary DNA clones. Plant Physiol. 1992, 98, 380–386. [Google Scholar] [CrossRef]
- Yingsanga, P.; Srilaong, V.; Kanlayanarat, S.; Noichinda, S.; McGlasson, W.B. Relationship between browning and related enzymes (PAL, PPO and POD) in rambutan fruit (Nephelium lappaceum Linn.) cvs. Rongrien and See-Chompoo. Postharvest Biol. Technol. 2008, 50, 164–168. [Google Scholar] [CrossRef]
- Kono, Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch. Biochem. Biophys. 1978, 186, 189–195. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods of Enzymatic Analysis; Elsevier: Amsterdam, The Netherlands, 1974; pp. 673–684. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Rawat, S.; Ali, S.; Mittra, B.; Grover, A. Expression analysis of chitinase upon challenge inoculation to Alternaria wounding and defense inducers in Brassica juncea. Biotechnol. Rep. 2017, 13, 72–79. [Google Scholar] [CrossRef]
- Damjanović, B.; Lepojević, Ž.; Živković, V.; Tolić, A. Extraction of fennel (Foeniculum vulgare Mill.) seeds with supercritical CO2: Comparison with hydrodistillation. Food Chem. 2005, 92, 143–149. [Google Scholar] [CrossRef]
- Hegazi, M.A.; El-Kot, G.A. Biological control of powdery mildew on zinnia (Zinnia elegans, L) using some biocontrol agents and plant extracts. J. Agric. Sci. 2010, 2, 221. [Google Scholar]
- Regmi, S.; Jha, S.K. Antifungal activity of plant essential oils against Fusarium oxysporum schlecht. and Aspergillus niger van tiegh. from papaya. Int. J. Curr. Trends Sci. Technol. 2017, 8, 2019620204. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid.-Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crops Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef] [Green Version]


| Primer | Primer Sequence | Annealing Temp. |
|---|---|---|
| Chitinase-F | 5′-GCGGATCCCAACGCACTGCAACCGATTAT-3′ | 60 °C |
| Chitinase-R | 5′-GCCCATGGAAGGAATCAGTTATGCGCAAAT-3′ | |
| Defensin-F | 5′-CCAAATGCCTCGTCATCT-3′ | |
| Defensin-R | 5′-ATTAGAGTCAAGCTCAAAAGG-3′ | |
| β-Actin | 5′-GTGGGCCGCTCTAGGCACCAA-3′ | |
| β-Actin | 5′-CTCTTTGATGTCACGCACGATTTC-3′ |
| Quantitative ID | Component Identified | Retention Time (min) | Area (%) |
|---|---|---|---|
| 1 | D-limonene | 11.81 | 2.93 |
| 2 | Menthol | 18.44 | 1.89 |
| 3 | Estragole | 19.66 | 4.39 |
| 4 | 2-Decenal | 22.51 | 1.58 |
| 5 | 2,4-Decadienal | 25.1 | 1.68 |
| 6 | Pentadecanoic acid | 50.64 | 7.51 |
| 7 | 9,12-Octadecadienoic acid | 56.45 | 29.49 |
| 8 | Cis-vaccenic acid | 56.63 | 31.23 |
| 9 | Octadecadienoic acid | 57.15 | 3.92 |
| 10 | 9-Octadecadienoic acid | 72 | 3.75 |
| Treatments | Pre-Emergence Damping off % | Post-Emergence Damping off % | Survival Plant % | Disease Severity (DS) (%) | Disease Incidence (DI) % | Protection % |
|---|---|---|---|---|---|---|
| Healthy control (C) | 0 | 0 | 100 | 0 | 0 | - |
| Treated with FSEO (T) | 0 | 0 | 100 | 0 | 0 | - |
| Infected control (P) | 46.5 | 33.8 | 19.71 | 53.1 | 67.4 | 0 |
| Treated +infected (T+P) | 21.8 | 14.5 | 63.7 | 20.6 | 33.5 | 32.5 |
| Treatments | Plant Height (cm) | Shoot F. wt. (g) | Root F. wt. (g) | Shoot D. wt. (g) | Root D. wt. (g) |
|---|---|---|---|---|---|
| Healthy control (C) | 43.2 ± 0.18 d | 4.37 ± 0.02 b | 2.16 ± 0.03 a | 0.56 ± 0.03 a | 0.34 ± 0.07 a |
| Treated with FSEO (T) | 49.92 ± 1.2 a | 4.75 ± 0.03 a | 2. 43 ± 0.03 a | 0.66 ± 0.02 b | 0.38 ± 0.06 b |
| Infected control (P) | 36.7 ± 1.0 b | 2.69 ± 0.02 b | 1.59 ± 0.02 b | 0.38 ± 0.01 c | 0.24 ± 0.02 c |
| Treated + infected (T+P) | 39.4 ± 0.52 c | 3.27 ± 0.02 b | 1.74 ± 0.03 a | 0.41 ± 0.01 d | 0.28 ± 0.01 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khaleil, M.M.; Alnoman, M.M.; Elrazik, E.S.A.; Zagloul, H.; Khalil, A.M.A. Essential Oil of Foeniculum vulgare Mill. as a Green Fungicide and Defense-Inducing Agent against Fusarium Root Rot Disease in Vicia faba L. Biology 2021, 10, 696. https://doi.org/10.3390/biology10080696
Khaleil MM, Alnoman MM, Elrazik ESA, Zagloul H, Khalil AMA. Essential Oil of Foeniculum vulgare Mill. as a Green Fungicide and Defense-Inducing Agent against Fusarium Root Rot Disease in Vicia faba L. Biology. 2021; 10(8):696. https://doi.org/10.3390/biology10080696
Chicago/Turabian StyleKhaleil, Mona M., Maryam M. Alnoman, Elsayed S. Abd Elrazik, Hayat Zagloul, and Ahmed Mohamed Aly Khalil. 2021. "Essential Oil of Foeniculum vulgare Mill. as a Green Fungicide and Defense-Inducing Agent against Fusarium Root Rot Disease in Vicia faba L." Biology 10, no. 8: 696. https://doi.org/10.3390/biology10080696