Is the Life History Flexibility of Cold Desert Annuals Broad Enough to Cope with Predicted Climate Change? The Case of Erodium oxyrhinchum in Central Asia
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
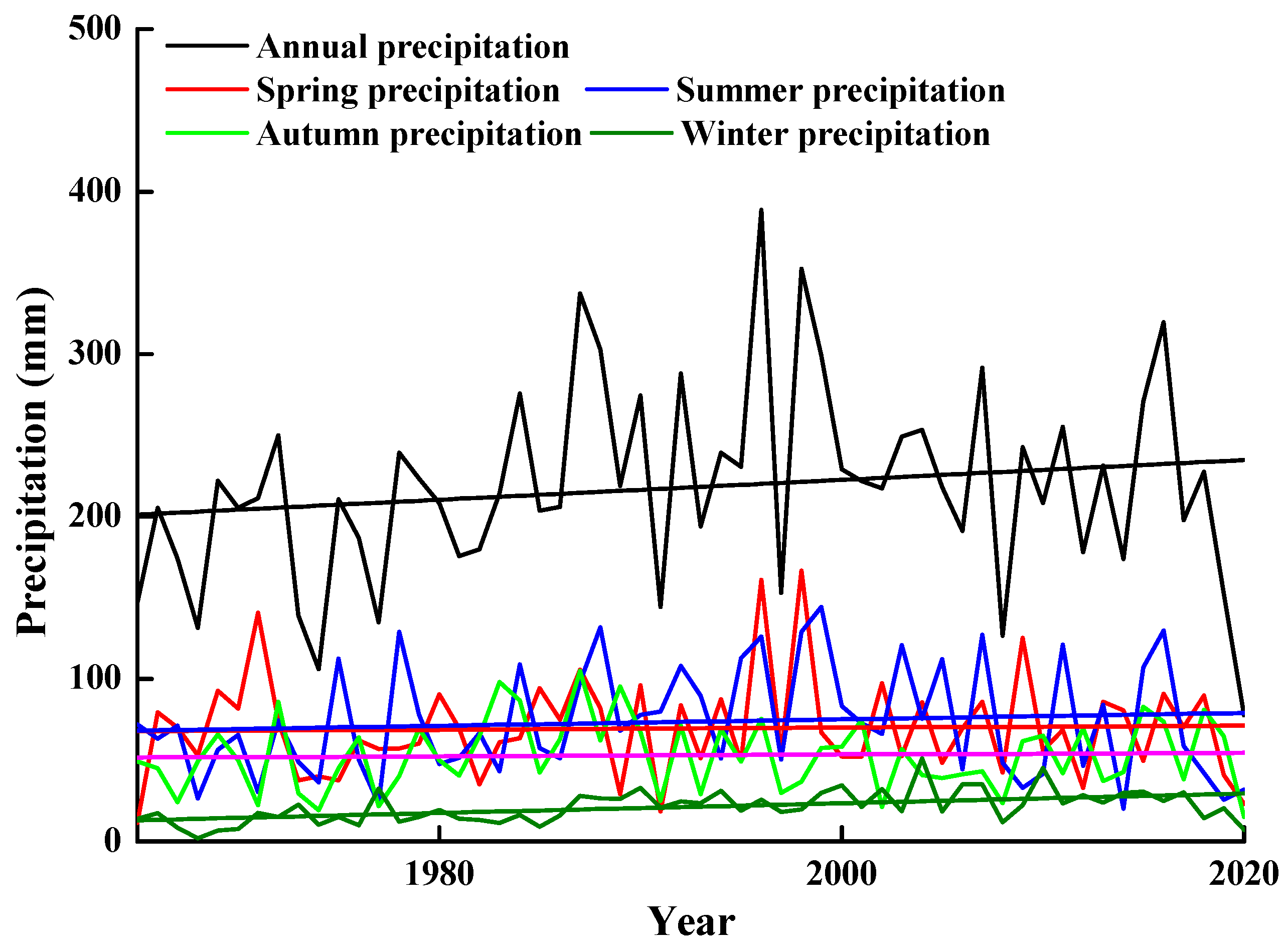
2.2. Climate Data for the Last Half Century in the Gurbantunggut Desert
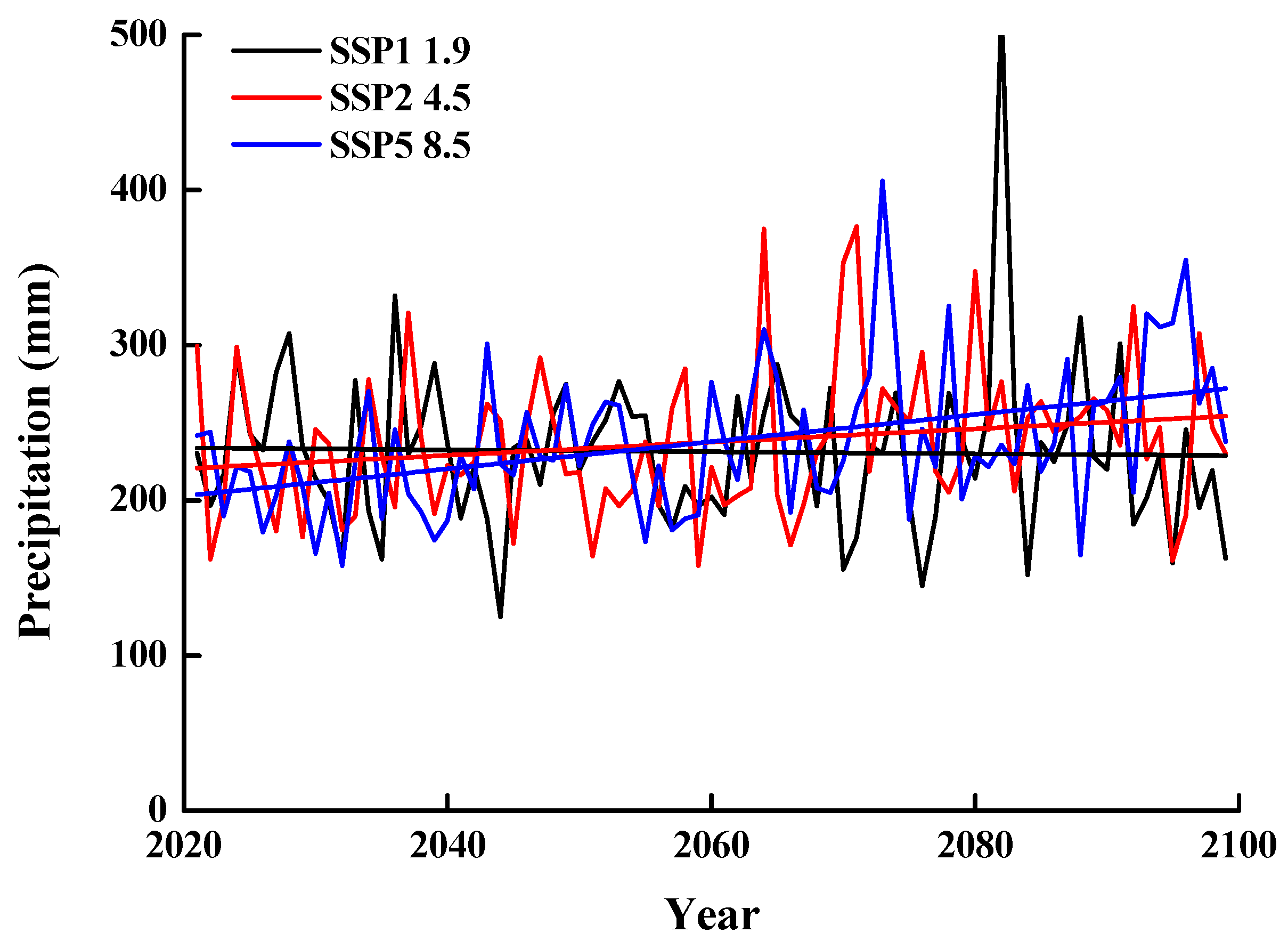
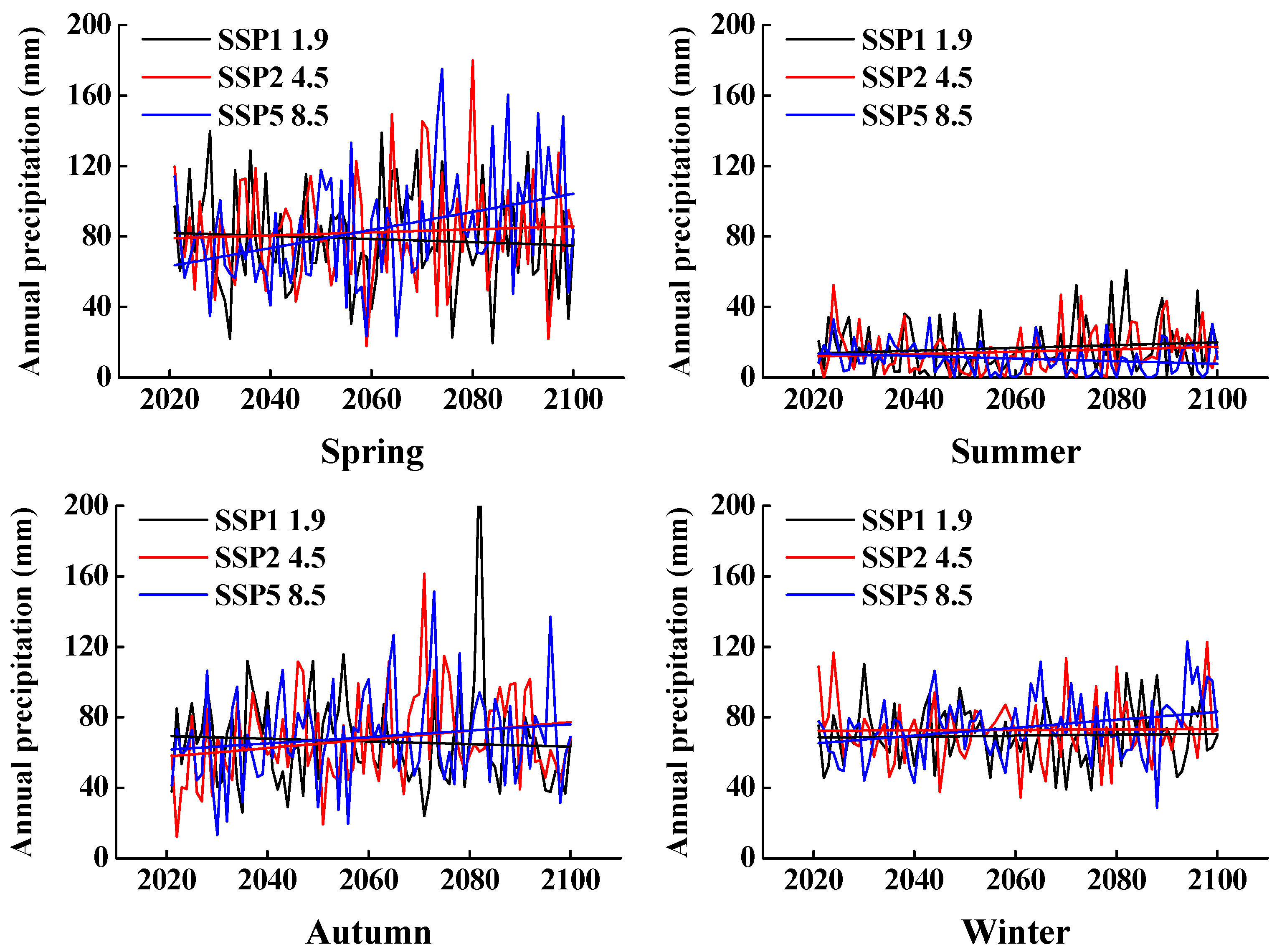
2.3. Predictions of Climate Change Models
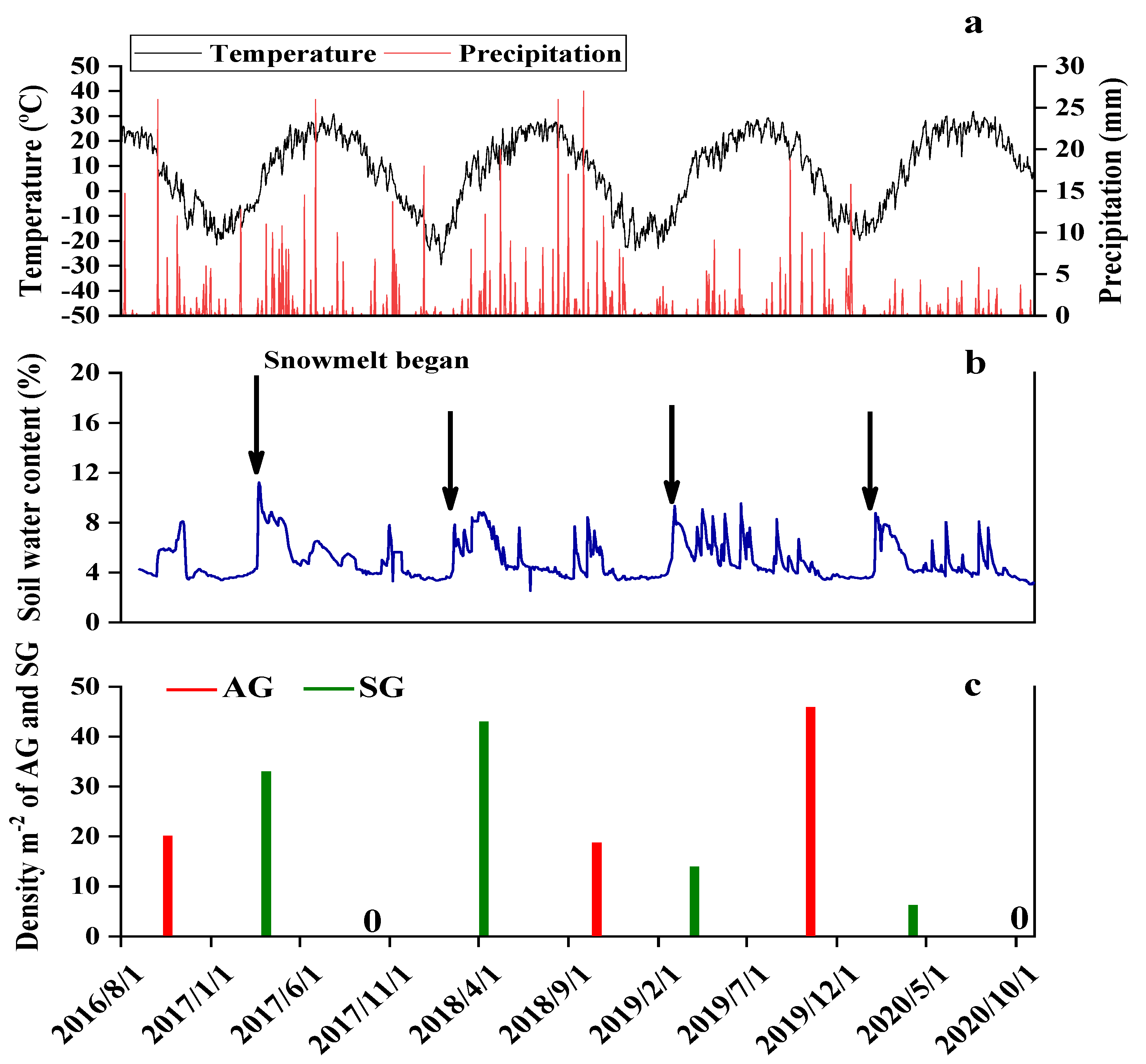
2.4. Precipitation, Temperature, and Soil Water Content
2.5. Emergence and Density of AG and SG Plants
2.6. Morphological Characters of SG and AG Plants
2.7. Dry Mass Accumulation and Allocation in SG and AG Plants
2.8. Offspring (F2) Seed Dormancy and Germination of AG and SG Plants
2.9. Statistical Analyses
3. Results
3.1. Climate Data for the Last Half Century in the Gurbantunggut Desert
3.2. Predictions from Climate Change Models
3.3. Precipitation and Temperature in Autumn and Spring
3.4. Emergence and Density of AG and SG plants
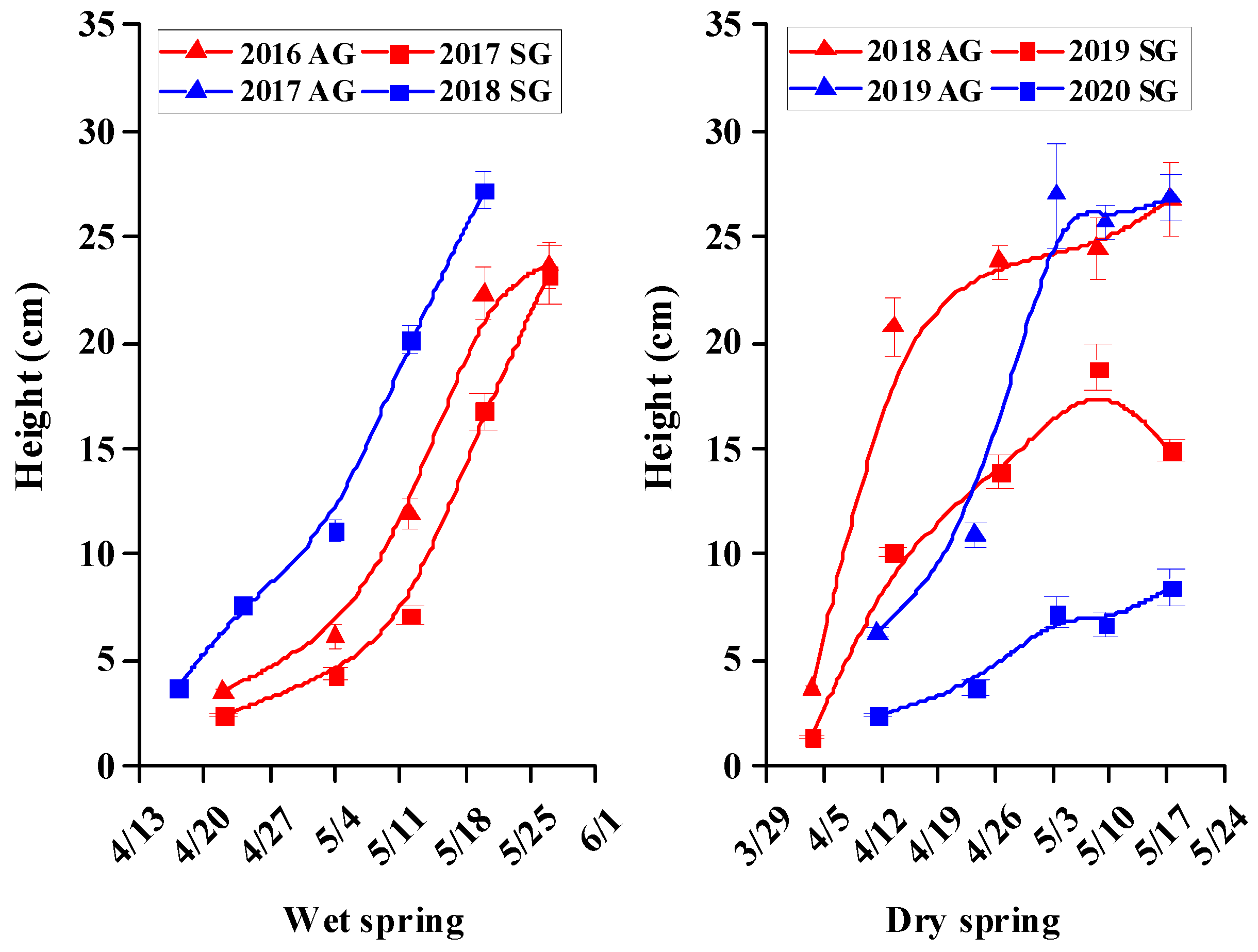
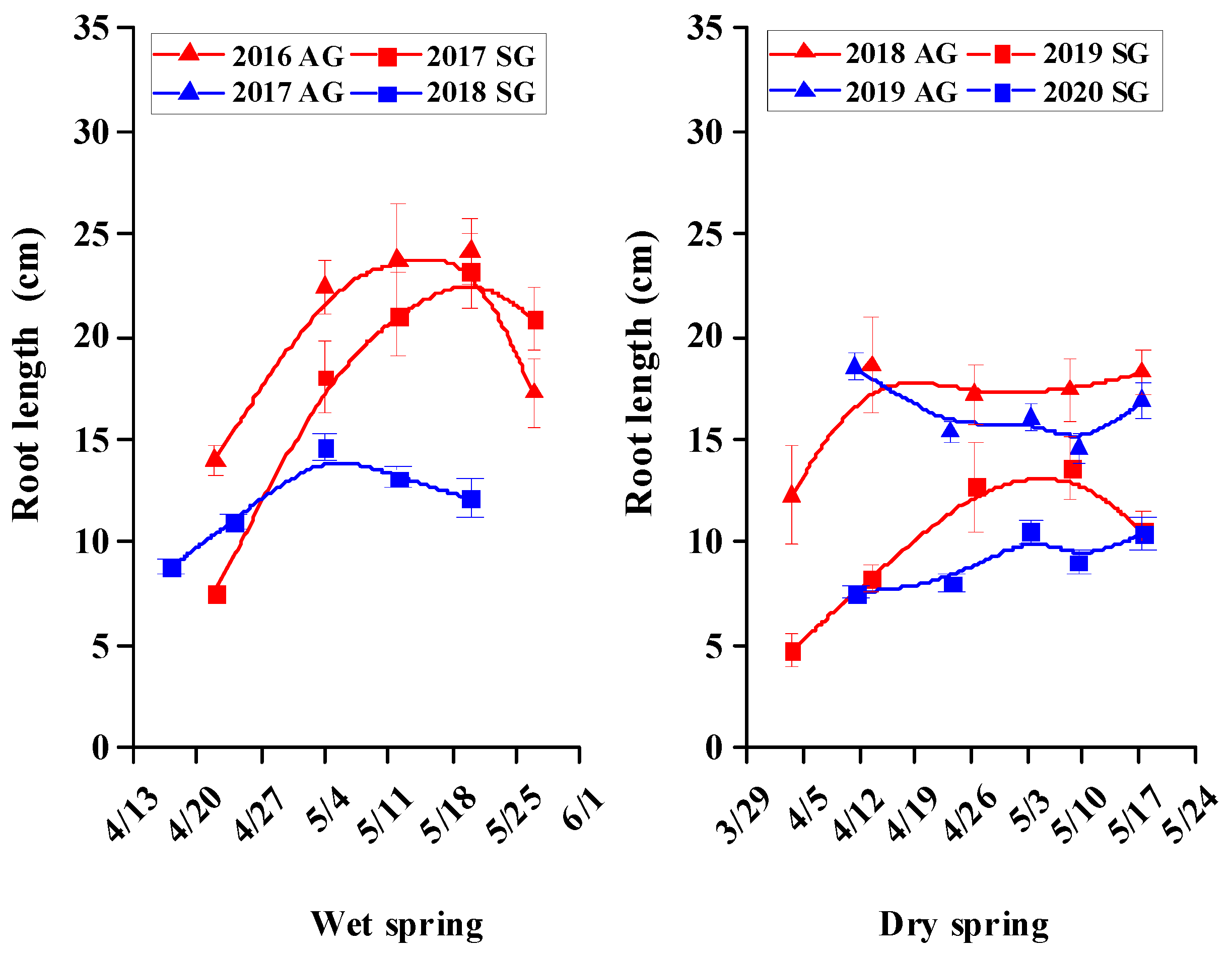
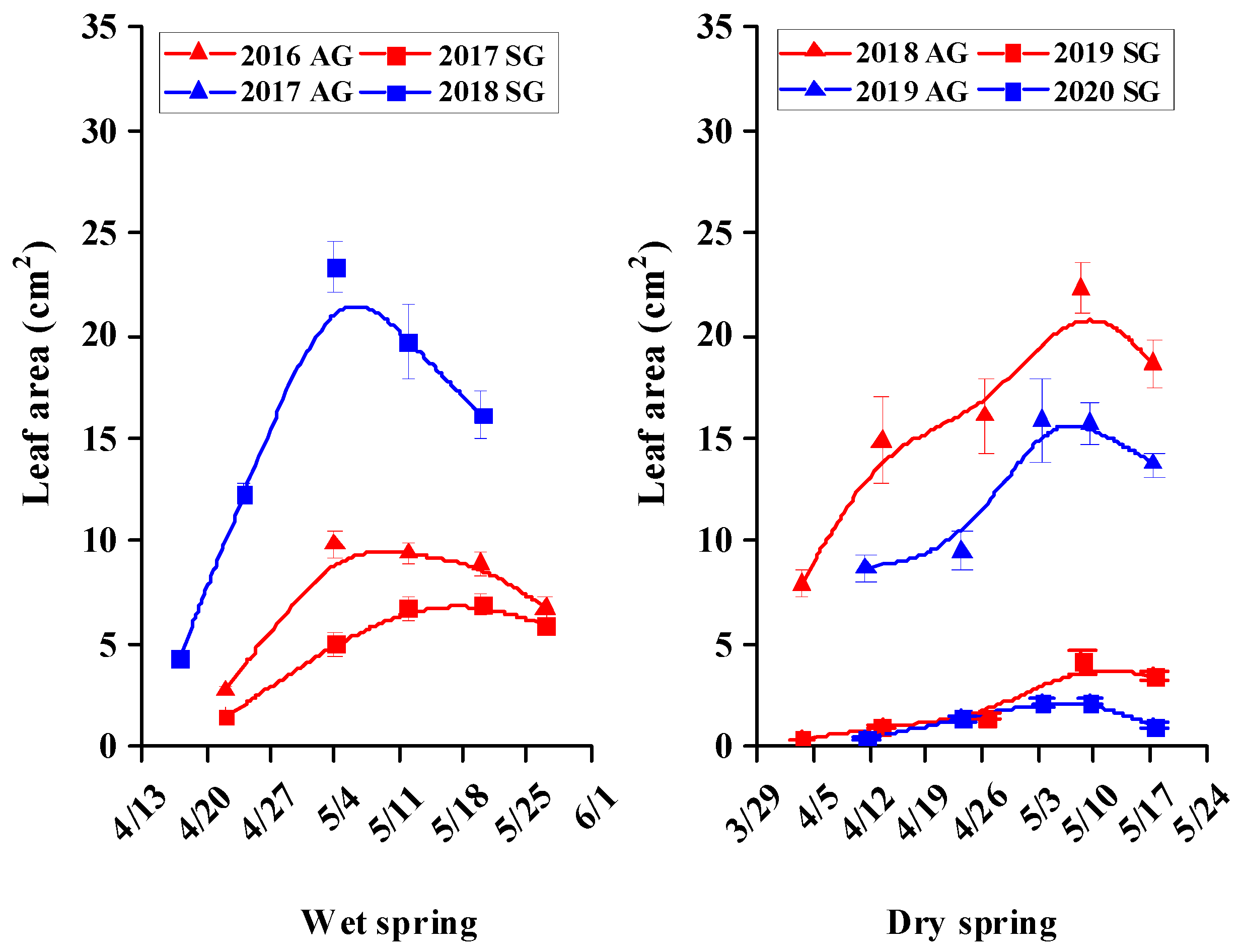
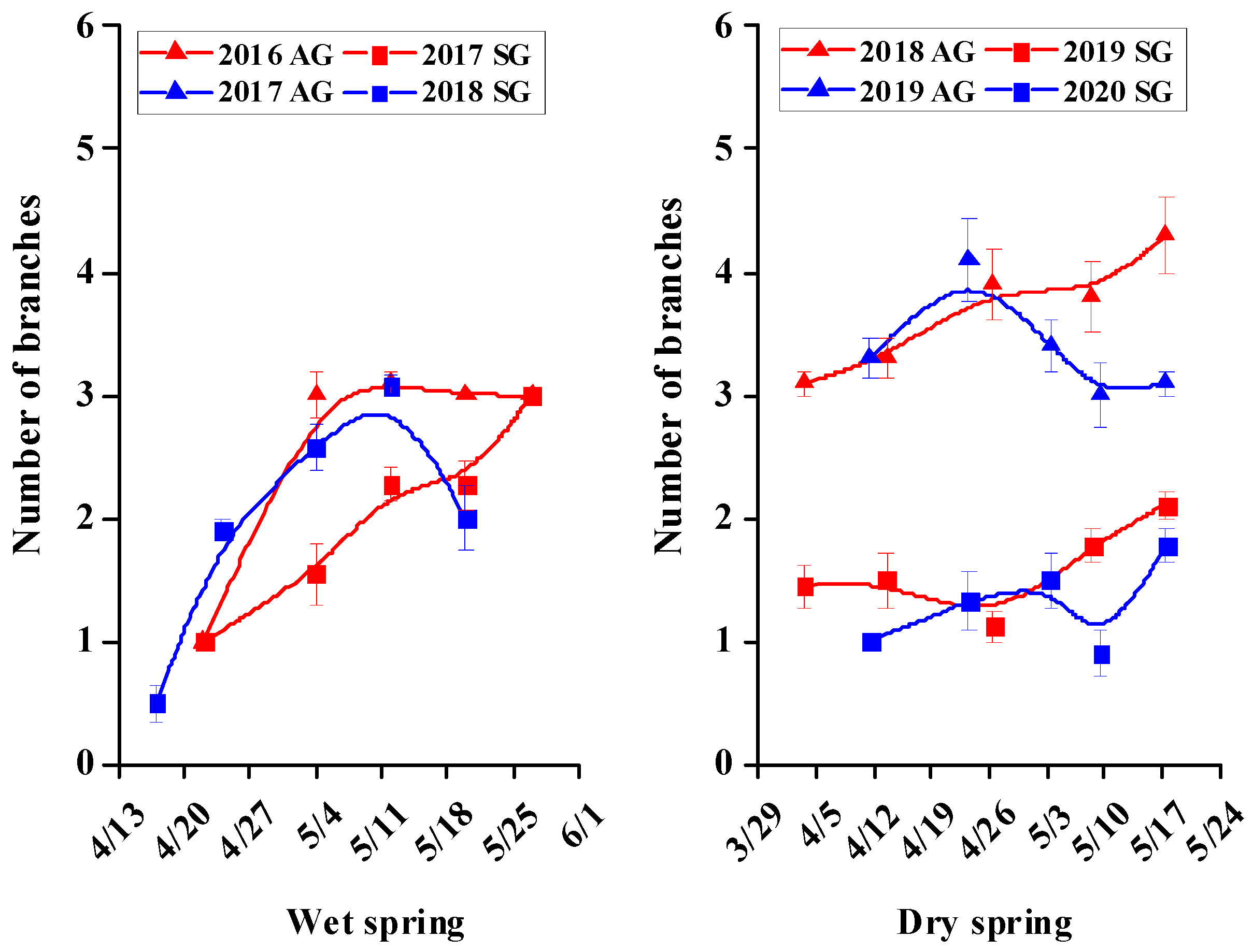
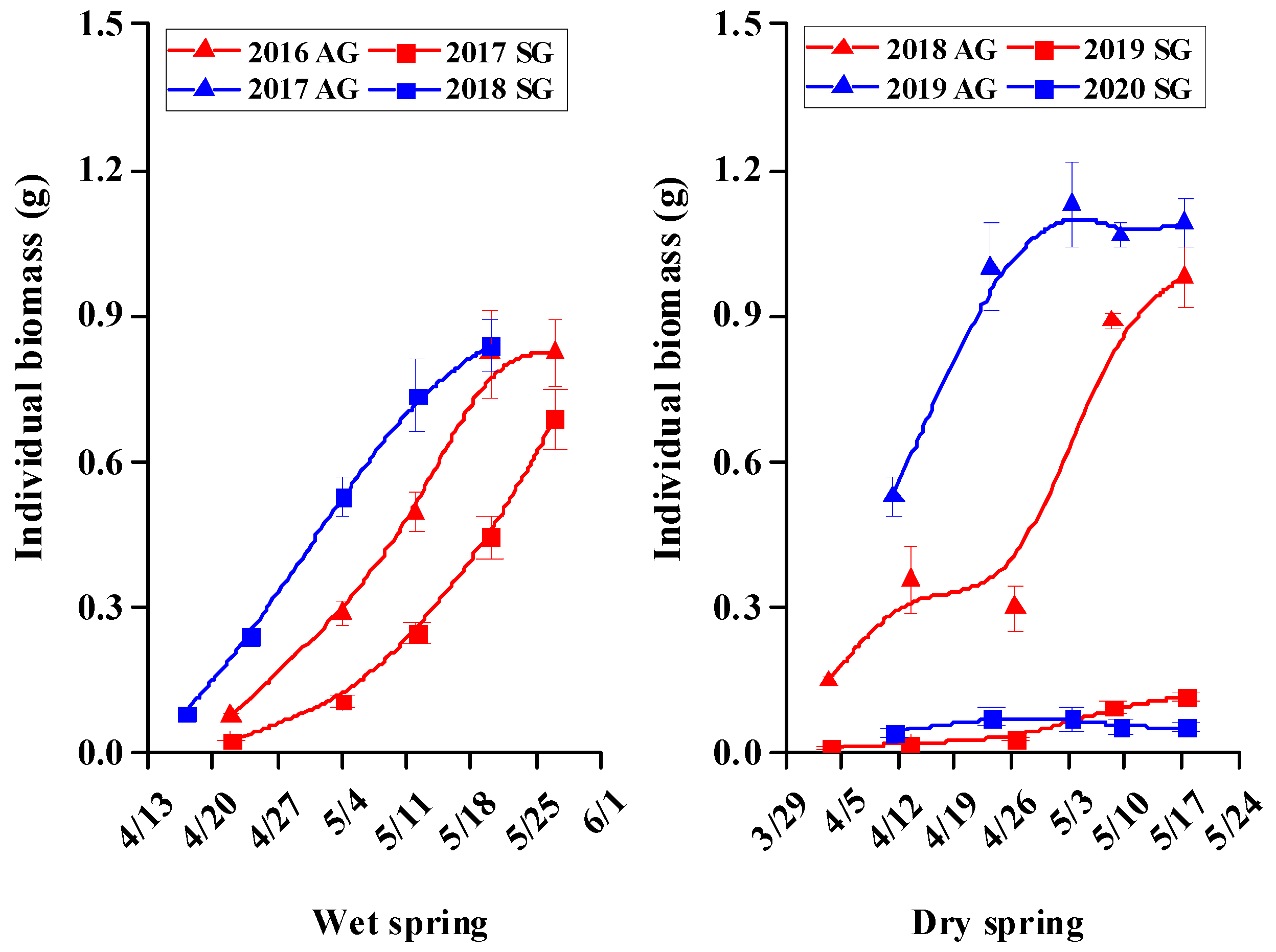
3.5. Morphological Characters of SG and AG Plants
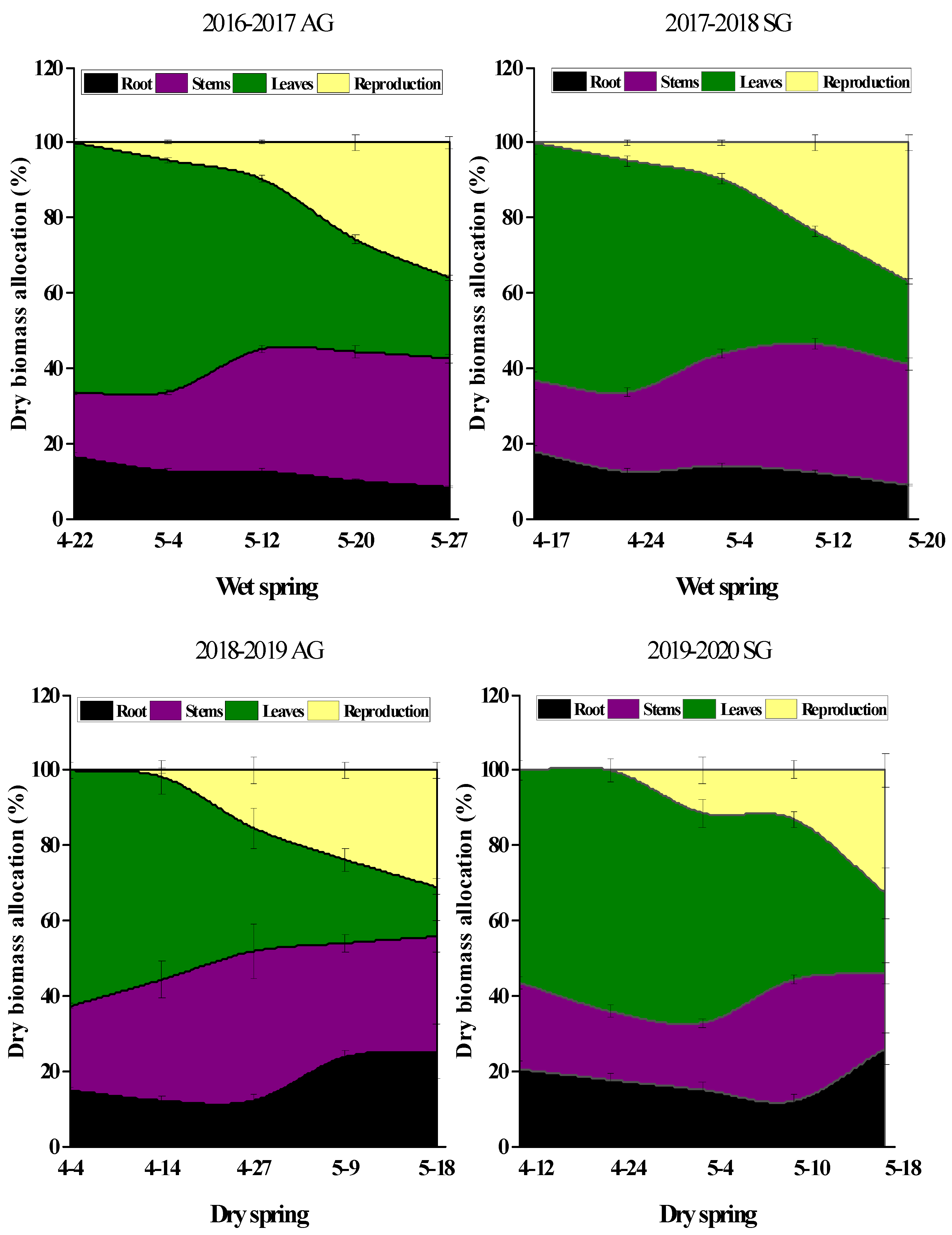
3.6. Dry Mass Accumulation and Allocation in SG and AG Plants
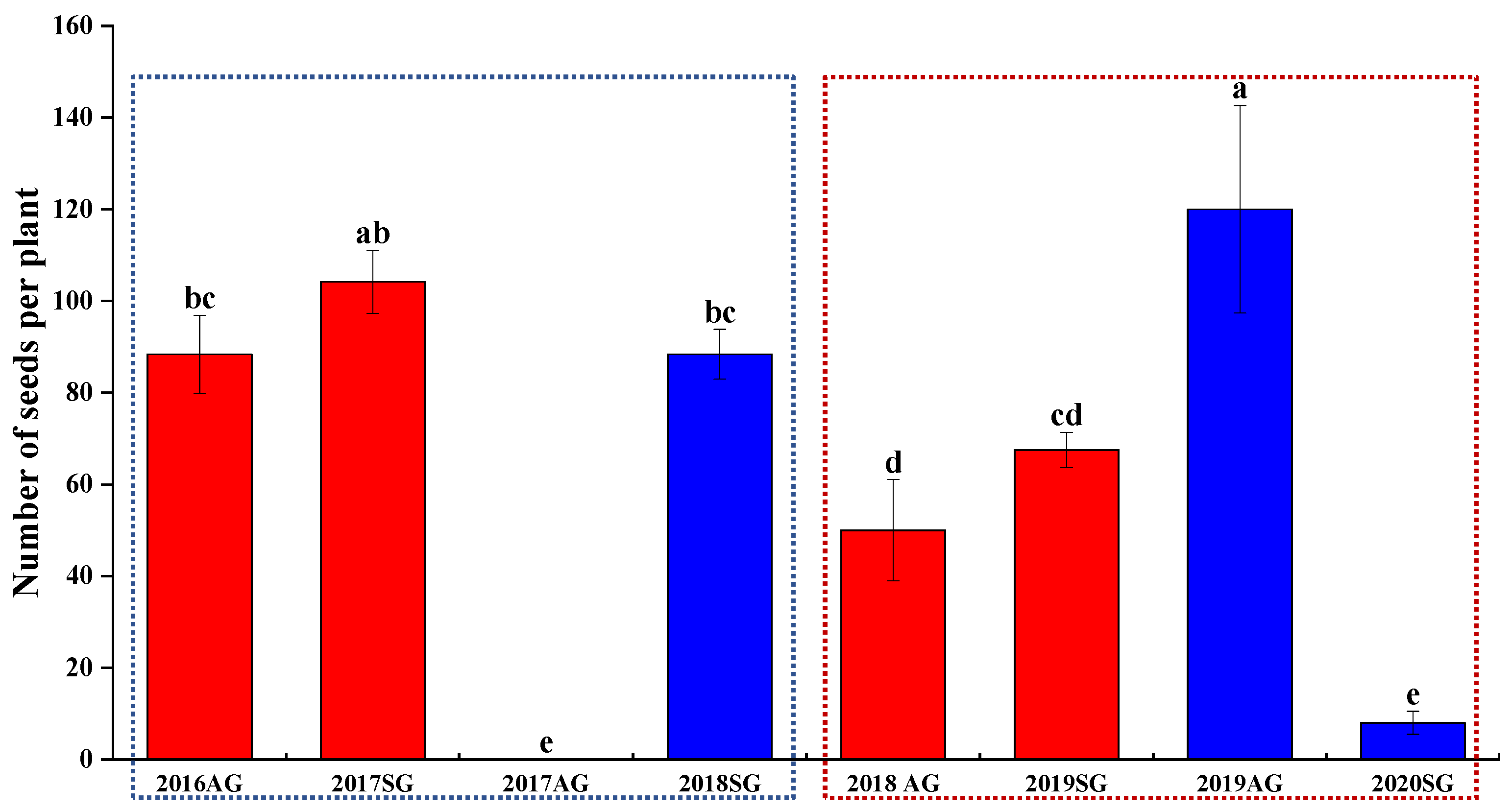
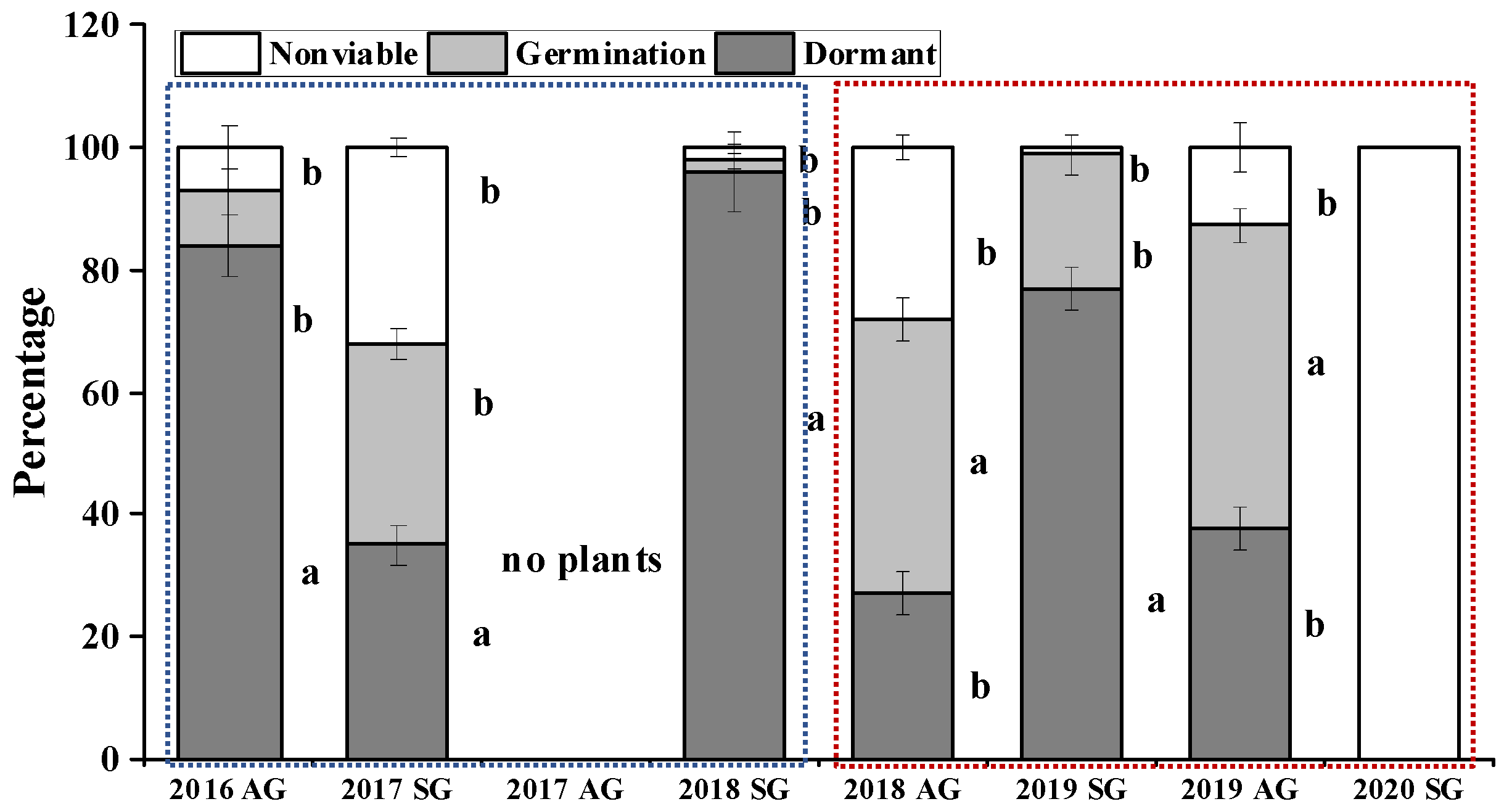
3.7. F2 Seed Dormancy and Germination of AG and SG Plants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knapp, A.K.; Smith, M.D. Variation among biomes in temporal dynamics of aboveground primary production. Science 2001, 291, 481–484. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.F.; Wu, J.; Xing, Q.; Pan, Q.; Huang, J.; Yang, D.; Han, X.G. Primary production and rain use efficiency across a precipitation gradient on the Mongolian plateau. Ecology 2008, 89, 2140–2153. [Google Scholar] [CrossRef] [PubMed]
- Salguero-Gómez, R.; Siewert, W.; Casper, B.B.; Tielbörger, K. A demographic approach to study effects of climate change in desert plants. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3100–3114. [Google Scholar] [CrossRef] [Green Version]
- Reichmann, L.G.; Sala, O.E. Differential sensitivities of grassland structural components to changes in precipitation mediate productivity response in a desert ecosystem. Funct. Ecol. 2014, 28, 1292–1298. [Google Scholar] [CrossRef] [Green Version]
- Giljohann, K.M.; McCarthy, M.A.; Keith, D.A.; Kelly, L.T.; Tozer, M.G.; Regan, T.J. Interactions between rainfall, fire and herbivory drive resprouter vital rates in a semi-arid ecosystem. J. Ecol. 2017, 105, 1562–1570. [Google Scholar] [CrossRef] [Green Version]
- Bialic-Murphy, L.; Gaoue, O.G. Low interannual precipitation has a greater negative effect than seedling herbivory on the population dynamics of a short-lived shrub, Schiedea obovate. Ecol. Evol. 2018, 8, 176–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwinning, S.; Sala, O.E.; Loik, M.E.; Ehleringer, J.R. Thresholds, memory, and seasonality: Understanding pulse dynamics in arid/semi-arid ecosystems. Oecologia 2004, 141, 191–193. [Google Scholar] [CrossRef] [PubMed]
- Mulhouse, J.M.; Hallett, L.M.; Collins, S.L. The influence of seasonal precipitation and grass competition on 20 years of forb dynamics in northern Chihuahuan Desert grassland. J. Veg. Sci. 2017, 28, 250–259. [Google Scholar] [CrossRef]
- Wang, A.B.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Effect of seed position in spikelet on life history of Eremopyrum distans (Poaceae) from the cold desert of north-west China. Ann. Bot. 2010, 106, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Hui, R.; Zhao, R.M.; Liu, L.C.; Li, Y.X.; Yang, H.T.; Wang, Y.L.; Xie, M.; Wang, X.Q. Changes in winter snow depth affects photosynthesis and physiological characteristics of biological soil crusts in the Tengger Desert. Photosynthetica 2018, 56, 1304–1312. [Google Scholar] [CrossRef]
- Tenhumberg, B.; Crone, E.E.; Ramula, S.; Tyre, A.J. Time-lagged effects of weather on plant demography: Drought and Astragalus scaphoides. Ecology 2018, 99, 915–925. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Zhang, L.W.; Shi, X.; Baskin, J.M.; Baskin, C.C.; Liu, H.L.; Zhang, D.Y. Effects of increased precipitation on the life history of spring- and autumn- germinated plants of the cold desert annual Erodium oxyrhinchum (Geraniaceae). AOB Plants 2019, 11, plz004. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.H.; Huang, W.; Jin, L.Y.; Chen, J.H.; Wang, J.S. Spatiotemporal precipitation variations in the arid Central Asia in the context of global warming. Earth Sci. 2011, 54, 1812–1821. [Google Scholar] [CrossRef]
- Pan, D.D.; Zhou, T.J.; Zhang, L.X. Moisture sources associated with precipitation during dry and wet seasons over Central Asia. J. Clim. 2020, 33, 10755. [Google Scholar]
- Wang, X.Q.; Jin, J.; Lei, J.Q.; Zhang, W.M.; Qian, Y.B. The distribution of ephemeral vegetation on the longitudinal dune surface and its stabilization significance in the Gurbantunggut Desert. Acta Geog. Sin. 2004, 15, 556–560, (In Chinese with English abstract). [Google Scholar]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.F.; Shen, Y.P.; Kang, E.; Li, D.; Ding, Y.; Zhang, G. Recent and future climate change in northwest China. Clim. Chang. 2007, 80, 379–393, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Hu, Z.Y.; Hu, Q.; Zhang, C.; Chen, X.; Li, Q.X. Evaluation of reanalysis, spatially interpolated and satellite remotely sensed precipitation data sets in central Asia. J. Geophys. Res. Atmos. 2016, 121, 5648–5663. [Google Scholar] [CrossRef] [Green Version]
- Shriver, R.K.; Campbell, E.; Dailey, C.; Gaya, H.; Hill, A.; Kuzminski, S.; Miller-Bartley, M.; Moen, K.; Moettus, R.; Oschrin, E.; et al. Local landscape position impacts demographic rates in a widespread North American steppe bunchgrass. Ecosphere 2021, 12, e03351. [Google Scholar] [CrossRef]
- Lu, J.J.; Tan, D.Y.; Baskin, J.M.; Baskin, C.C. Germination season and watering regime, but not seed morph, affect life history traits in a cold desert diaspore-heteromorphic annual. PLoS ONE 2014, 9, e102018. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.F.; Zhang, L.W.; Shi, X.; Ban, Y.; Liu, H.L.; Zhang, D.Y. Life history responses of two ephemeral plant species to increased precipitation and nitrogen in the Gurbantunggut Desert. PeerJ 2019, 7, e6158. [Google Scholar] [CrossRef]
- Wang, X.Q.; Jiang, J.; Wang, Y.C.; Luo, W.L.; Song, C.W.; Chen, J.J. Responses of ephemeral plant germination and growth to water and heat conditions in the southern part of Gurbantunggut Desert. Chin. Sci. Bull. 2006, 51, 110–116, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, T.; Sun, Y.; Tian, C.Y.; Feng, G. Ecological and biological differences between spring and autumn plants of two desert ephemerals. J. Plant Ecol. 2007, 31, 1174–1180, (In Chinese with English abstract). [Google Scholar]
- Zhou, Y.M.; Lu, J.J.; Tan, D.Y.; Baskin, J.M.; Baskin, J.M. Seed germination ecology of the cold desert annual Isatis violascens (Brassicaceae): Two levels of physiological dormancy and role of the pericarp. PLoS ONE 2015, 10, e0140983. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ramos, I.; Cambrolle, J.; Hidalgo-Galvez, M.D.; Matias, L.; Montero-Ramirez, A.; Santolaya, S.; Godoy, O. Phenological responses to climate change in communities of plants species with contrasting functional strategies. Environ. Exp. Bot. 2020, 170, 103852. [Google Scholar] [CrossRef]
- Evers, S.M.; Knight, T.M.; Inouye, D.W.; Miller, T.E.X.; Salguero-Gomez, R.; Iler, A.M.; Compagnoni, A. Lagged and dormant season climate better predict plant vital rates than climate during the growing season. Glob. Chang. Biol. 2021, 27, 1027–1941. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Tong, L.; Shen, X.; Niu, P. Environment-dependence of seed germination in autumn in Grurbantonggut Desert. Chin. J. Ecol. 2011, 30, 1604–1611, (In Chinese with English abstract). [Google Scholar]
- Fan, L.L.; Tang, L.S.; Wu, L.F.; Ma, J.Y.; Li, Y.; Güsewell, S. The limited role of snow water in the growth and development of ephemeral plants in a cold desert. J. Veg. Sci. 2014, 25, 681–690. [Google Scholar] [CrossRef]
- Jiang, F.Q.; Hu, R.J.; Wang, S.P.; Zhang, Y.W.; Wang, S.P.; Zhang, Y.W.; Tong, L. Trends of precipitation extremes during 1960–2008 in Xinjiang, the Northwest China. Theor. Appl. Climatol. 2013, 111, 133–148. [Google Scholar] [CrossRef]
- Jilili, A.; Ma, L.; Chen, X. Overview of Central Asian Environments; Meterological Press: Beijing, China, 2013. [Google Scholar]
- Qian, Y.; Wu, Z.; Zhao, R.; Zhang, L. Vegetation patterns and species—Environment relationships in the Gurbantunggut Desert of China. J. Geogr. Sci. 2008, 18, 400–414, (In Chinese with English abstract). [Google Scholar] [CrossRef]
- Huang, G.; Su, Y.G.; Zhu, L.; Li, Y. The role of spring ephemerals and soil microbes in soil nutrient retention in a temperate desert. Plant Soil 2016, 406, 43–54. [Google Scholar] [CrossRef]
- Jia, F.Q.; Ren, J.J.; Zhang, Y.M. Effect of slope aspect and terrain of sand dune on herbaceous diversity in Gurbantunggut desert. Chin. J. Ecol. 2018, 37, 26–34, (In Chinese with English abstract). [Google Scholar]
- Zang, Y.X.; Min, X.J.; de Dios, V.R.; Ma, J.Y.; Sun, W. Extreme drought affects the productivity, but not the composition, of a desert plant community in Central Asia differentially across microtopographies. Sci. Total Environ. 2020, 717, 137251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Zhang, L.W.; Shi, X.; Ban, Y.; Liu, H.L.; Zhang, D.Y. Life history responses of spring-and autumn-germinated ephemeral plants to increased nitrogen and precipitation in the Gurbantunggut Desert. Sci. Total Environ. 2019, 659, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.H.; Huang, G.; Li, Y.; Zheng, X.J.; Xu, G.Q.; Liu, Y. Population dynamics and life history response to precipitation changes for a desert ephemeral plant with biseasonal germination. Front. Plant Sci. 2021, 12, 625475. [Google Scholar] [CrossRef]
- Li, D.X.; Zhang, D.Y.; Zhang, L.W.; Zhang, Q.G.; Liu, H.L. Biological seed characteristics of spring-emergence and autumn-emergence Erodium oxyrrhynchum. Arid Zone Res. 2020, 37, 1562–1568, (In Chinese with English abstract). [Google Scholar]
- Wei, W.S.; He, Q.; Liu, M.Z.; Gao, W.D. Climate change and the desert environment in Junggar Basin, Xinjiang, China. J. Des. Res. 2003, 23, 101–105, (In Chinese with English abstract). [Google Scholar]
- Huang, G.; Cao, Y.F.; Wang, B.; Li, Y. Effects of nitrogen addition on soil microbes and their implications for soil C emission in the Gurbantunggut Desert, center of the Eurasian Continent. Sci. Total Environ. 2015, 515, 215–224. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.F.; Lowe, J.; et al. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef] [Green Version]
- van Vuuren, D.; Riahi, K.; Moss, R.; Edmonds, J.; Thomson, A.; Nakicenovic, N.; Kram, T.; Berkhout, F.; Swart, R.; Janetos, A.; et al. A proposal for a new scenario framework to support research and assessment in different climate research communities. Glob. Environ. Chang. 2011, 22, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Shi, X.; Wang, J.C.; Yin, L.K.; Huang, Z.Y.; Zhang, D.Y. Effects of sand burial, soil water content and distribution pattern of seeds in sand on seed germination and seedling survival of Eremosparton songoricum (Fabaceae), a rare species inhabiting the moving sand dunes of the Gurbantunggut Desert of China. Plant Soil 2011, 345, 69–87. [Google Scholar] [CrossRef]
- Guo, Q.F.; Brown, J.H. Interactions between winter and summer annuals in the Chihuahuan Desert. Oecologia 1997, 111, 123–128. [Google Scholar] [CrossRef]
- Veenendaal, E.M.; Ernst WH, O.; Modise, G.S. Effect of seasonal rainfall pattern on seedling emergence and establishment of grasses in a savanna in south-eastern Botswana. J. Arid Environ. 1996, 32, 305–317. [Google Scholar] [CrossRef]
- Zheng, M.Q.; Zheng, Y.R.; Zhou, G.S.; Wu, Y.Z.; Baskin, C.C.; Baskin, J.M. Effects of watering regime and depth of burial on seedling emergence of four dominant psammophytes in the Mu Us sandy land, Inner Mongolia, China, and relevance to revegetation of a desertified region. Ann. Appl. Biol. 2009, 154, 87–96. [Google Scholar] [CrossRef]
- Gutterman, Y.; Gozlan, S. Amounts of winter or summer rain triggering germination and “the point of no return” of seedling desiccation tolerance of Hordeum spontaneum local ecotypes in Israel. Plant Soil 1998, 204, 223–234. [Google Scholar] [CrossRef]
- Forrest, J.R.K. Plant—Pollinator interactions and phenological change: What can we learn about climate impacts from experiments and observations? Oikos 2015, 124, 4–13. [Google Scholar] [CrossRef]
- Laws, A.N. Climate change effects on predator-prey interactions. Curr. Opin. Insect Sci. 2017, 6, 28–34. [Google Scholar] [CrossRef]
- Sanderson, R.A.; Goffe, L.A.; Leifert, C. Time-series models to quantify short- term effects of meteorological conditions on bumblebee forager activity in agricultural landscapes. Agric. For. Entomol. 2015, 17, 270–276. [Google Scholar] [CrossRef]
- Herrera, C.M.; Medrano, M. Pollination consequences of mimicking intrafloral microbial warming in an early-blooming herb. Flora 2017, 232, 142–149. [Google Scholar] [CrossRef]
- Holzapfel, C.; Tielbörger, K.; Parag, H.A.; Kigel, J.; Sternberg, M. Annual plant-shrub interactions along an aridity gradient. Basic Appl. Ecol. 2006, 7, 268–279. [Google Scholar] [CrossRef]
- Tielbörger, K.; Petrů, M. An experimental test for effects of the maternal environment on delayed germination. J. Ecol. 2010, 98, 1216–1223. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Ehrlen, J.; Morris, W.F. Predicting changes in the distribution and abundance of species under environmental change. Ecol. Lett. 2015, 18, 303–314. [Google Scholar] [CrossRef]
- Williams, J.L.; Jacquemyn, H.; Ochocki, B.M.; Brys, R.; Miller, T.E.X. Life history evolution under climate change and its influence on the population dynamics of a long-lived plant. J. Ecol. 2015, 103, 798–808. [Google Scholar] [CrossRef]
- Tye, M.; Dahlgren, J.P.; Oien, D.I.; Moen, A.; Sletvold, N. Demographic responses to climate variation depend on spatial and life history-differentiation at multiple scales. Biol. Conserv. 2018, 228, 62–69. [Google Scholar] [CrossRef]
- Czachura, K.; Miller, T.E.X. Demographic back-casting reveals that subtle dimensions of climate change have strong effects on population viability. J. Ecol. 2020, 108, 2557–2570. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Chen, Y.; Zhang, L.; Baskin, J.M.; Baskin, C.C.; Zhang, L.; Liu, Y.; Zhang, D.; Zhang, Y. Is the Life History Flexibility of Cold Desert Annuals Broad Enough to Cope with Predicted Climate Change? The Case of Erodium oxyrhinchum in Central Asia. Biology 2021, 10, 780. https://doi.org/10.3390/biology10080780
Liu H, Chen Y, Zhang L, Baskin JM, Baskin CC, Zhang L, Liu Y, Zhang D, Zhang Y. Is the Life History Flexibility of Cold Desert Annuals Broad Enough to Cope with Predicted Climate Change? The Case of Erodium oxyrhinchum in Central Asia. Biology. 2021; 10(8):780. https://doi.org/10.3390/biology10080780
Chicago/Turabian StyleLiu, Huiliang, Yanfeng Chen, Lingwei Zhang, Jerry M. Baskin, Carol C. Baskin, Lan Zhang, Yan Liu, Daoyuan Zhang, and Yuanming Zhang. 2021. "Is the Life History Flexibility of Cold Desert Annuals Broad Enough to Cope with Predicted Climate Change? The Case of Erodium oxyrhinchum in Central Asia" Biology 10, no. 8: 780. https://doi.org/10.3390/biology10080780
APA StyleLiu, H., Chen, Y., Zhang, L., Baskin, J. M., Baskin, C. C., Zhang, L., Liu, Y., Zhang, D., & Zhang, Y. (2021). Is the Life History Flexibility of Cold Desert Annuals Broad Enough to Cope with Predicted Climate Change? The Case of Erodium oxyrhinchum in Central Asia. Biology, 10(8), 780. https://doi.org/10.3390/biology10080780





