Antitumor Effect of Cabozantinib in Bone Metastatic Models of Renal Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Human Osteoblasts
2.2. RCC Cell Lines
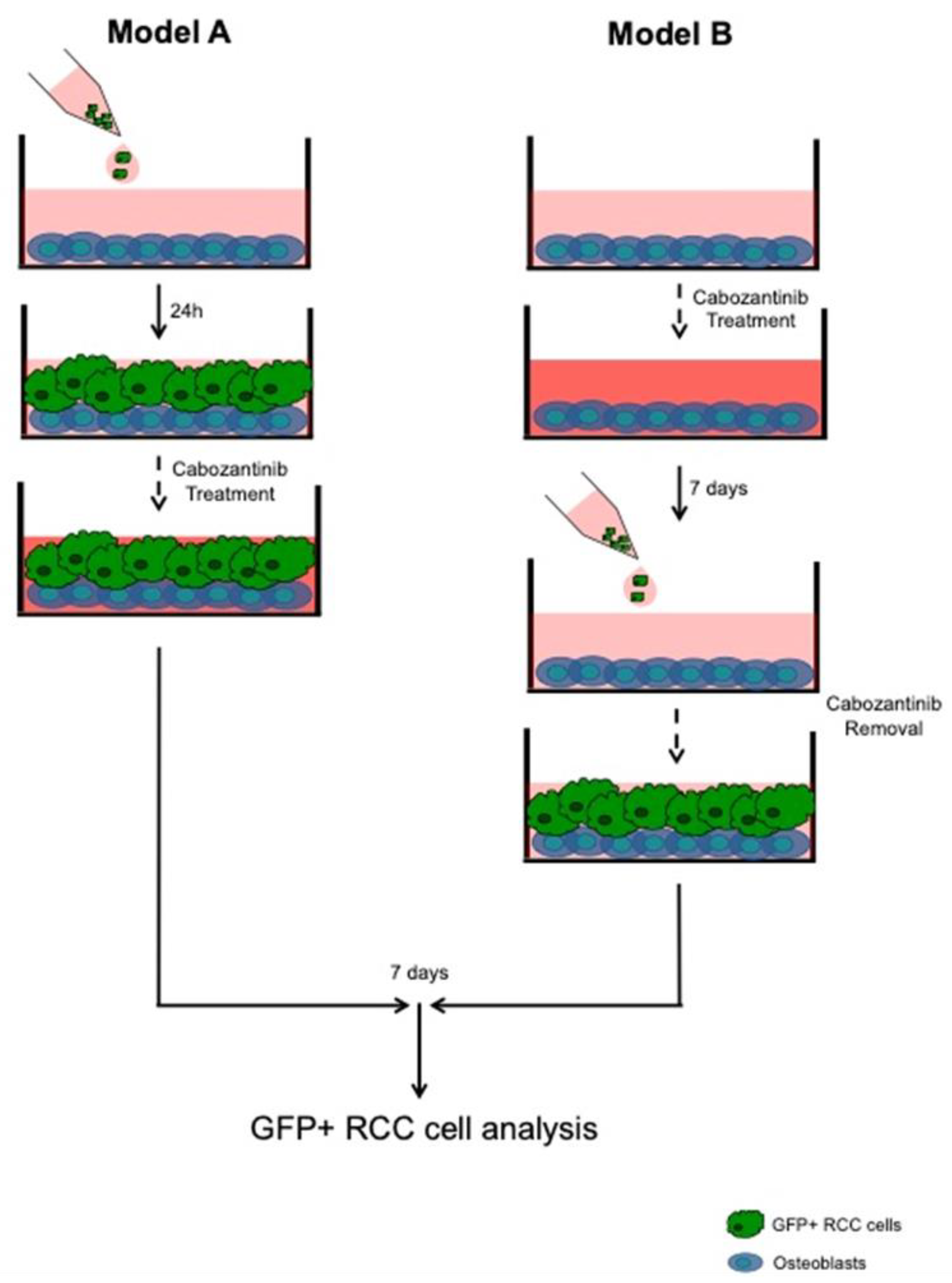
2.3. OBs–RCC Cells “Indirect” Coculture
2.4. OBs–RCC Cells “Direct” Coculture
2.5. Gene Expression Assay
2.6. Protein Expression Assay
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
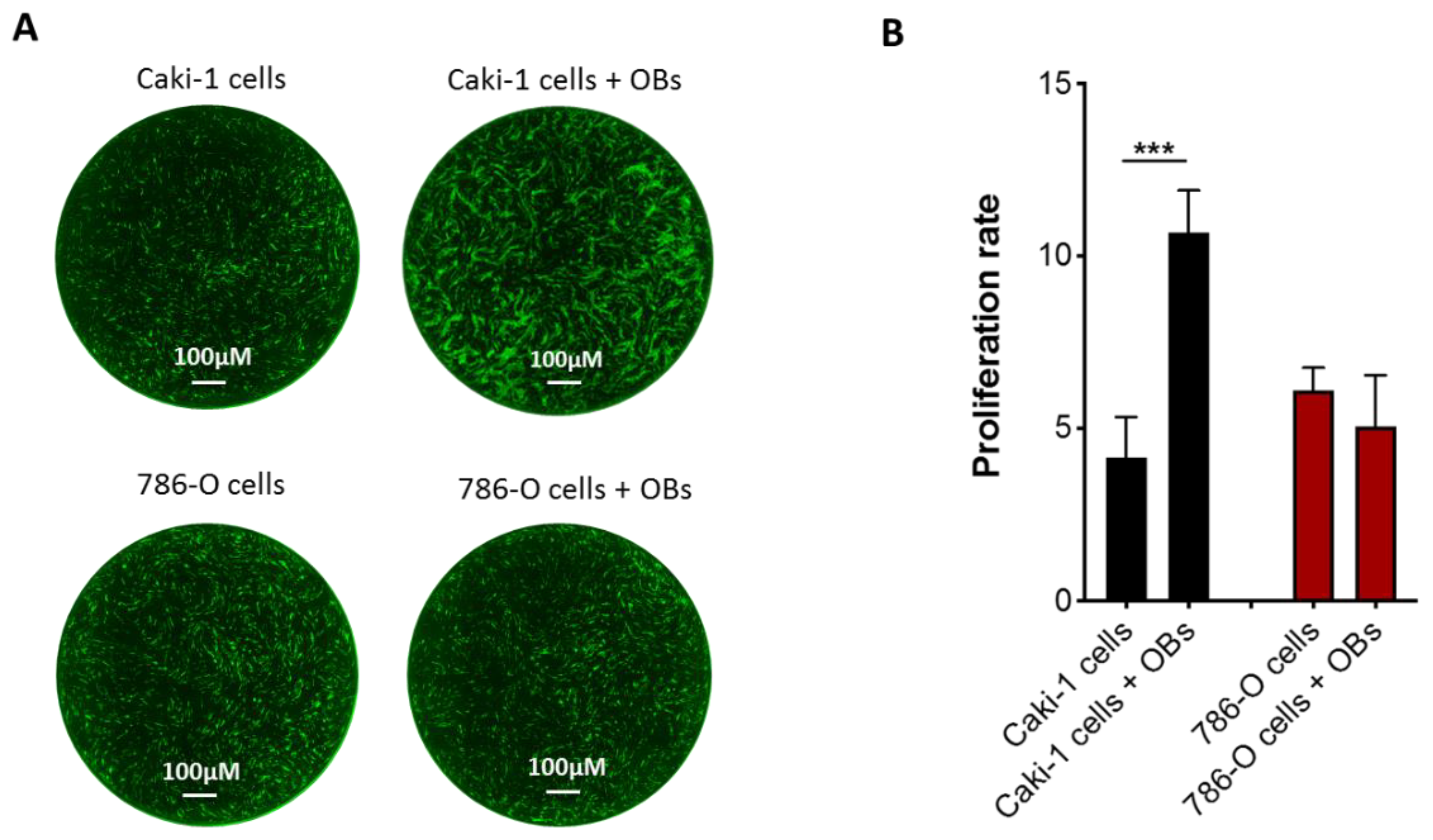
3.1. Effect of Osteoblasts on Metastatic RCC Cell Proliferation
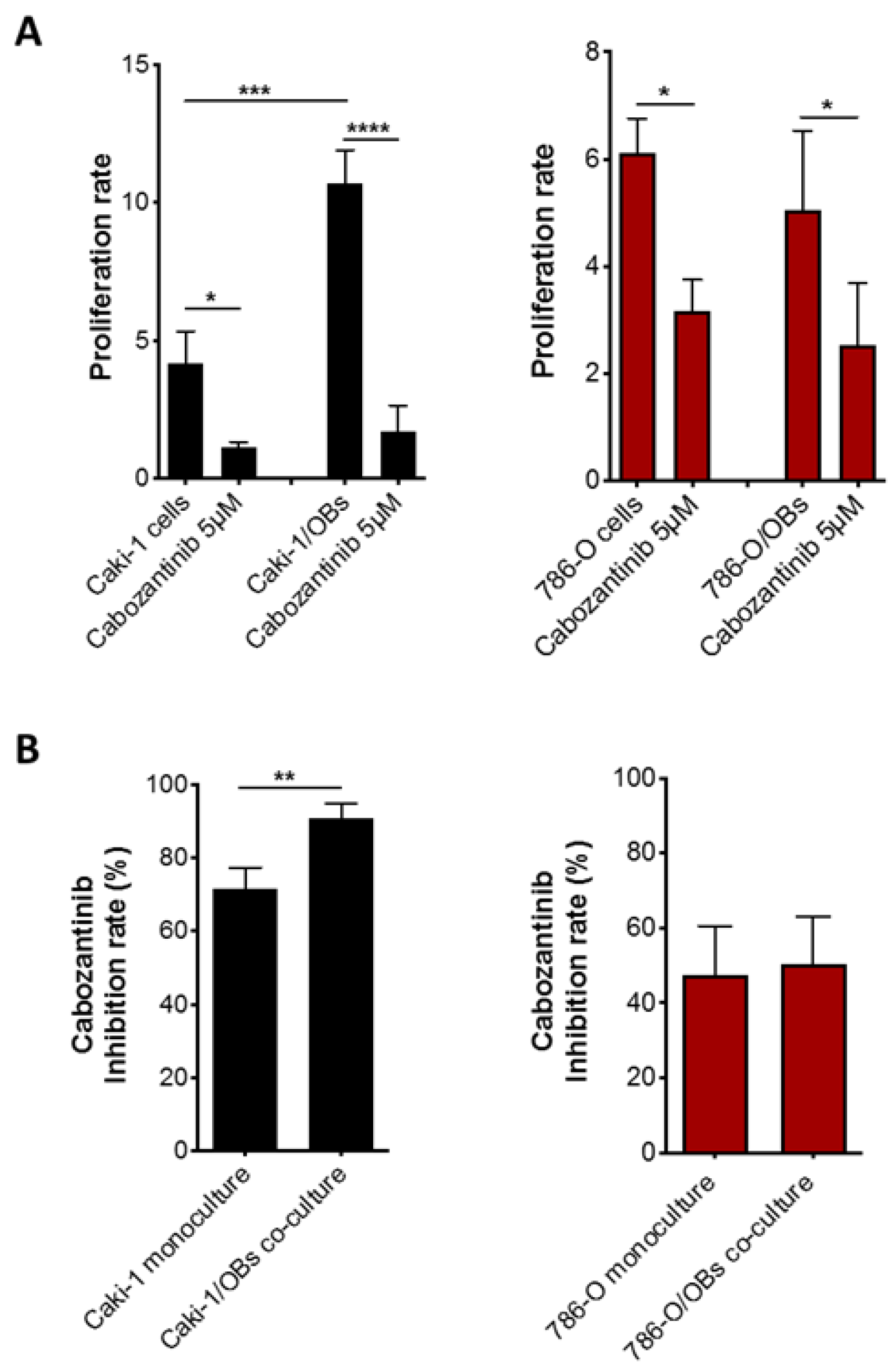
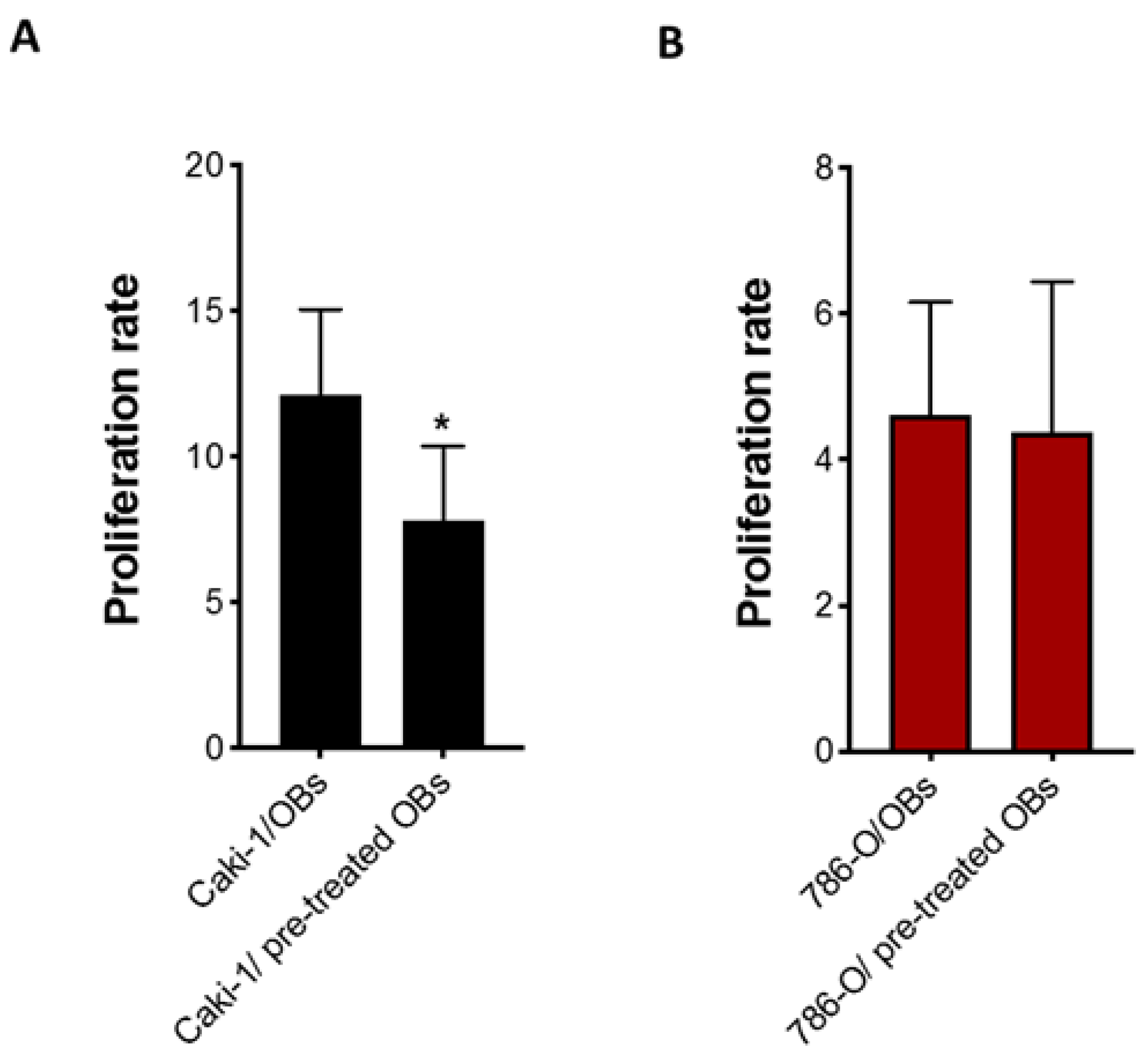
3.2. Antitumor Effect of Cabozantinib in RCC Bone Metastatic Models
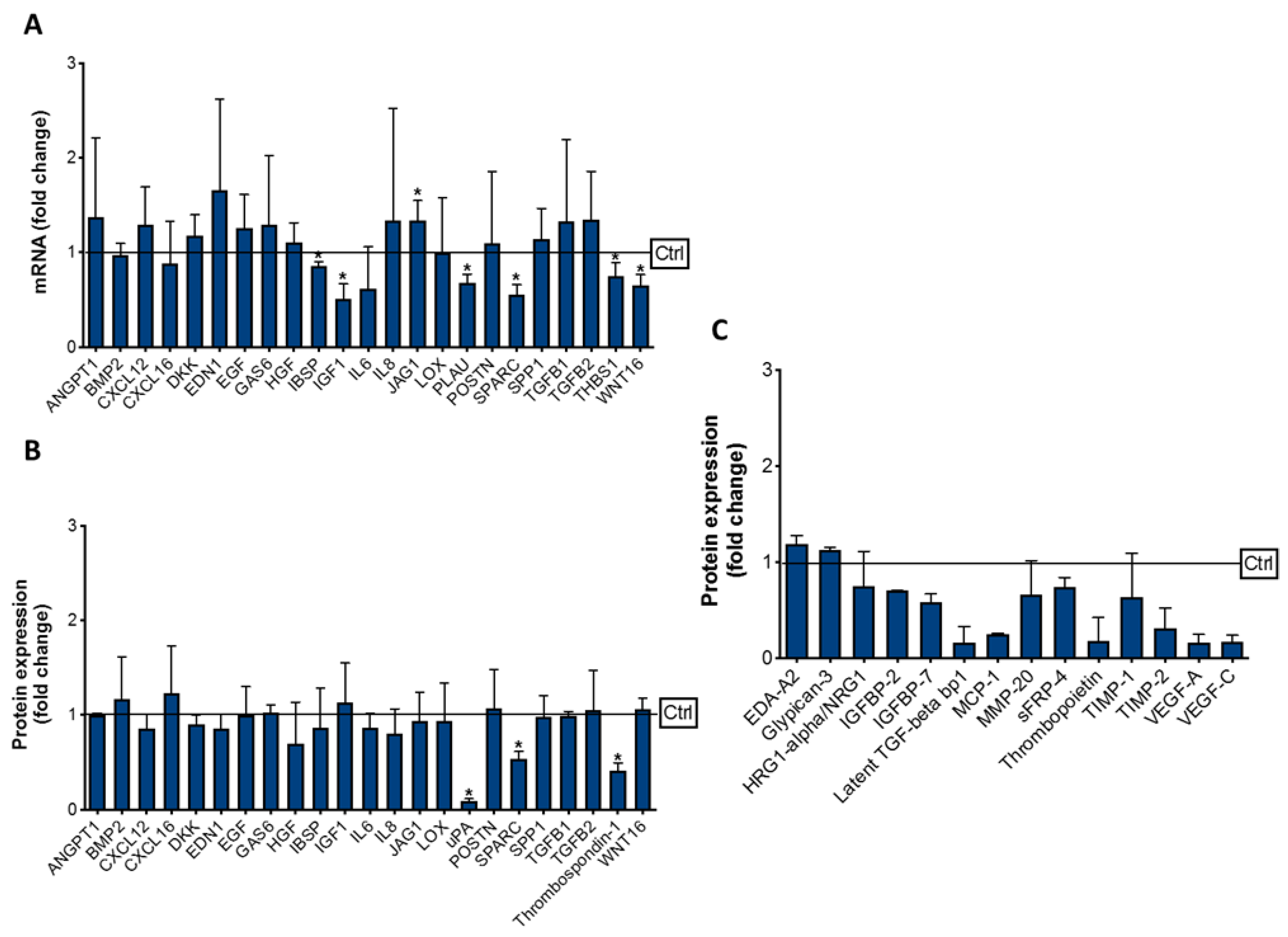
3.3. Effect of Cabozantinib on Osteoblast Gene and Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kozlowski, J.M. Management of distant solitary recurrence in the patient with renal cancer. Contralateral kidney and other sites. Urol. Clin. N. Am. 1994, 21, 601–624. [Google Scholar] [CrossRef]
- Lavu, H.; Yeo, C.J. Metastatic renal cell carcinoma to the pancreas. Gastroenterol. Hepatol. 2011, 7, 699–700. [Google Scholar]
- Santoni, M.; Conti, A.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Guida, F.M.; Fontana, A.; Berruti, A.; Berardi, R.; et al. Bone metastases in patients with metastatic renal cell carcinoma: Are they always associated with poor prognosis? J. Exp. Clin. Cancer Res. 2015, 34, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzato, R.L.; Buy, X.; Grasso, R.F.; Luppi, G.; Faiella, E.; Quattrocchi, C.C.; Pantano, F.; Zobel, B.B.; Tonini, G.; Santini, D.; et al. Interventional Radiologist’s perspective on the management of bone metastatic disease. Eur. J. Surg. Oncol. 2015, 41, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Beuselinck, B.; Oudard, S.; Rixe, O.; Wolter, P.; Blesius, A.; Ayllon, J.; Elaidi, R.; Schöffski, P.; Barrascout, E.; Morel, A.; et al. Negative impact of bone metastasis on outcome in clearcell renal cell carcinoma treated with sunitinib. Ann. Oncol. 2010, 22, 794–800. [Google Scholar] [CrossRef]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef] [Green Version]
- Huillard, O.; Alexandre, J.; Goldwasser, F.; Motzer, R.J.; Escudier, B.; Choueiri, T.K.; Santini, D.; Tonini, G. Treatment of Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 888–890. [Google Scholar]
- Shah, S.; Karathanasi, A.; Revythis, A.; Ioannidou, E.; Boussios, S. Cancer-Associated Thrombosis: A New Light on an Old Story. Diseases 2021, 9, 34. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donskov, F.; Hammers, H.; Hutson, T.E.; Lee, J.L.; Peltola, K.; et al. Faculty Opinions recommendation of Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2016, 373, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients with Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Fioramonti, M.; Santini, D.; Iuliani, M.; Ribelli, G.; Manca, P.; Papapietro, N.; Spiezia, F.; Vincenzi, B.; Denaro, V.; Russo, A.; et al. Cabozantinib targets bone microenvironment modulating human osteoclast and osteoblast functions. Oncotarget 2017, 8, 20113–20121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioramonti, M.; Fausti, V.; Pantano, F.; Iuliani, M.; Ribelli, G.; Lotti, F.; Pignochino, Y.; Grignani, G.; Santini, D.; Tonini, G.; et al. Cabozantinib Affects Osteosarcoma Growth Through A Direct Effect On Tumor Cells and Modifications In Bone Microenvironment. Sci. Rep. 2018, 8, 4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicione, C.; Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Blanco, F.J. Molecular profile and cellular characterization of human bone marrow mesenchymal stem cells: Donor influence on chondrogenesis. Differentiation 2010, 80, 155–165. [Google Scholar] [CrossRef] [Green Version]
- Lacy, S.A.; Miles, D.R.; Nguyen, L.T. Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin. Pharmacokinet. 2017, 56, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Ruppender, N.; Zhang, X.; Brown, L.G.; Gross, T.S.; Morrissey, C.; Gulati, R.; Vessella, R.L.; Schimmoller, F.; Aftab, D.T.; et al. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PLoS ONE 2013, 8, e78881. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Zhang, H.; Karatsinides, A.; Keller, J.M.; Kozloff, K.M.; Aftab, D.T.; Schimmoller, F.; Keller, E.T. Cabozantinib inhibits prostate cancer growth and prevents tumor-induced bone lesions. Clin. Cancer Res. 2014, 20, 617–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Sun, L.; Yuan, X.; Qiu, H. Thrombospondin-1 is a multifaceted player in tumor progression. Oncotarget 2017, 8, 84546–84558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, N.; Mihalcioiu, C.; Rabbani, S.A. Multifaceted Role of the Urokinase-Type Plasminogen Activator (uPA) and Its Receptor (uPAR): Diagnostic, Prognostic, and Therapeutic Applications. Front. Oncol. 2018, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Bao, J.M.; Dang, Q.; Lin, C.J.; Lo, U.G.; Feldkoren, B.; Dang, A.; Hernandez, E.; Li, F.; Panwar, V.; Lee, C.F.; et al. SPARC is a key mediator of TGF-β-induced renal cancer metastasis. J. Cell Physiol. 2020, 236, 1926. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef] [Green Version]
- Zoccoli, A.; Iuliani, M.; Pantano, F.; Imperatori, M.; Intagliata, S.; Vincenzi, B.; Marchetti, P.; Papapietro, N.; Denaro, V.; Tonini, G.; et al. Premetastatic niche: Ready for new therapeutic interventions? Expert Opin. Ther. Targets 2012, 16 (Suppl. 2), S119–S129. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.F.; Weng, X.F.; Huang, X.C.; Peng, Y.H.; Guo, H.P.; Xu, Y.W. IGFBP2 in cancer: Pathological role and clinical significance. Oncol. Rep. 2021, 45, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, S.M.; Celestino, J.; Wang, H.; Jiang, R.; Holland, E.C.; Fuller, G.N.; Zhang, W. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc. Natl. Acad. Sci. USA 2007, 104, 11736–11741. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Zhang, X.; Song, Q.; Liu, L.; Forbes, E.; Tian, W.; Zhang, Z.; Kang, Y.A.; Wang, H.; Fleming, J.B.; et al. IGFBP2 promotes tumor progression by inducing alternative polarization of macrophages in pancreatic ductal adenocarcinoma through the STAT3 pathway. Cancer Lett. 2021, 500, 132–146. [Google Scholar] [CrossRef]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin Growth Factor Binding Protein 7 (IGFBP7)-Related Cancer and IGFBP3 and IGFBP7 Crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Dias, F.; Teixeira, A.L.; Nogueira, I.; Morais, M.; Maia, J.; Bodo, C.; Ferreira, M.; Vieira, I.; Silva, J.; Lobo, J.; et al. Plasma Extracellular Vesicle-Derived TIMP-1 mRNA as a Prognostic Biomarker in Clear Cell Renal Cell Carcinoma: A Pilot Study. Int. J. Mol. Sci. 2020, 21, 4624. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Sun, X.; Li, S.; Sun, Y.; Zhai, H. Tumor Suppressor Gene XEDAR Promotes Differentiation and Suppresses Proliferation and Migration of Gastric Cancer Cells Through Upregulating the RELA/LXRα Axis and Deactivating the Wnt/β-Catenin Pathway. Cell Transpl. 2021, 30, 963689721996346. [Google Scholar] [CrossRef]
- Tanikawa, C.; Ri, C.; Kumar, V.; Nakamura, Y.; Matsuda, K. Crosstalk of EDA-A2/XEDAR in the p53 Signaling Pathway. Mol. Cancer Res. 2010, 8, 855–863. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Anti-androgen Resistance in Prostate Cancer. Cancer Cell 2020, 38, 279–296.e9. [Google Scholar] [CrossRef]
- Pan, T.; Martinez, M.; Hubka, K.M.; Song, J.H.; Lin, S.C.; Yu, G.; Lee, Y.C.; Gallick, G.E.; Tu, S.M.; Harrington, D.A.; et al. Cabozantinib Reverses Renal Cell Carcinoma-mediated Osteoblast Inhibition in Three-dimensional Coculture In Vitro and Reduces Bone Osteolysis In Vivo. Mol. Cancer Ther. 2020, 19, 1266–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Mondal, K.; Panda, P.K.; Kaushik, A.; Abolhassani, R.; Ahuja, R.; Rubahn, H.-G.; Mishra, Y.K. Core–shell nanostructures: Perspectives towards drug delivery applications. J. Mater. Chem. B 2020, 8, 8992–9027. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iuliani, M.; Simonetti, S.; Pantano, F.; Ribelli, G.; Di Martino, A.; Denaro, V.; Vincenzi, B.; Russo, A.; Tonini, G.; Santini, D. Antitumor Effect of Cabozantinib in Bone Metastatic Models of Renal Cell Carcinoma. Biology 2021, 10, 781. https://doi.org/10.3390/biology10080781
Iuliani M, Simonetti S, Pantano F, Ribelli G, Di Martino A, Denaro V, Vincenzi B, Russo A, Tonini G, Santini D. Antitumor Effect of Cabozantinib in Bone Metastatic Models of Renal Cell Carcinoma. Biology. 2021; 10(8):781. https://doi.org/10.3390/biology10080781
Chicago/Turabian StyleIuliani, Michele, Sonia Simonetti, Francesco Pantano, Giulia Ribelli, Alberto Di Martino, Vincenzo Denaro, Bruno Vincenzi, Antonio Russo, Giuseppe Tonini, and Daniele Santini. 2021. "Antitumor Effect of Cabozantinib in Bone Metastatic Models of Renal Cell Carcinoma" Biology 10, no. 8: 781. https://doi.org/10.3390/biology10080781
APA StyleIuliani, M., Simonetti, S., Pantano, F., Ribelli, G., Di Martino, A., Denaro, V., Vincenzi, B., Russo, A., Tonini, G., & Santini, D. (2021). Antitumor Effect of Cabozantinib in Bone Metastatic Models of Renal Cell Carcinoma. Biology, 10(8), 781. https://doi.org/10.3390/biology10080781








