LC-MS/MS Proteomic Study of MCF-7 Cell Treated with Dox and Dox-Loaded Calcium Carbonate Nanoparticles Revealed Changes in Proteins Related to Glycolysis, Actin Signalling, and Energy Metabolism
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of the Ar-CC-NPs, Encapsulation of DOX-Ar-CC-NPs, and In Vitro Release Study
2.3. Cell Culture
2.4. Cellular Uptake Assay
2.5. High-Resolution Microscopy of Treated MCF-7 Cancer Cells
2.6. Annexin FITC-V/Propidium Iodide Staining for Apoptosis Determination
2.7. Protein Extraction and Quantification
2.7.1. Radioimmunoprecipitation Assay (RIPA) Method
2.7.2. Bicinchoninic Acid (BCA) Protein Assay
2.8. 1-D SDS-Gel (One-Dimensional Polyacrylamide Gel Electrophoresis)
2.9. Trypsin Digestion
2.10. Proteomic Analysis Using Orbitrap MS and Statistical Analysis
2.11. Bioinformatics Analyses
3. Results
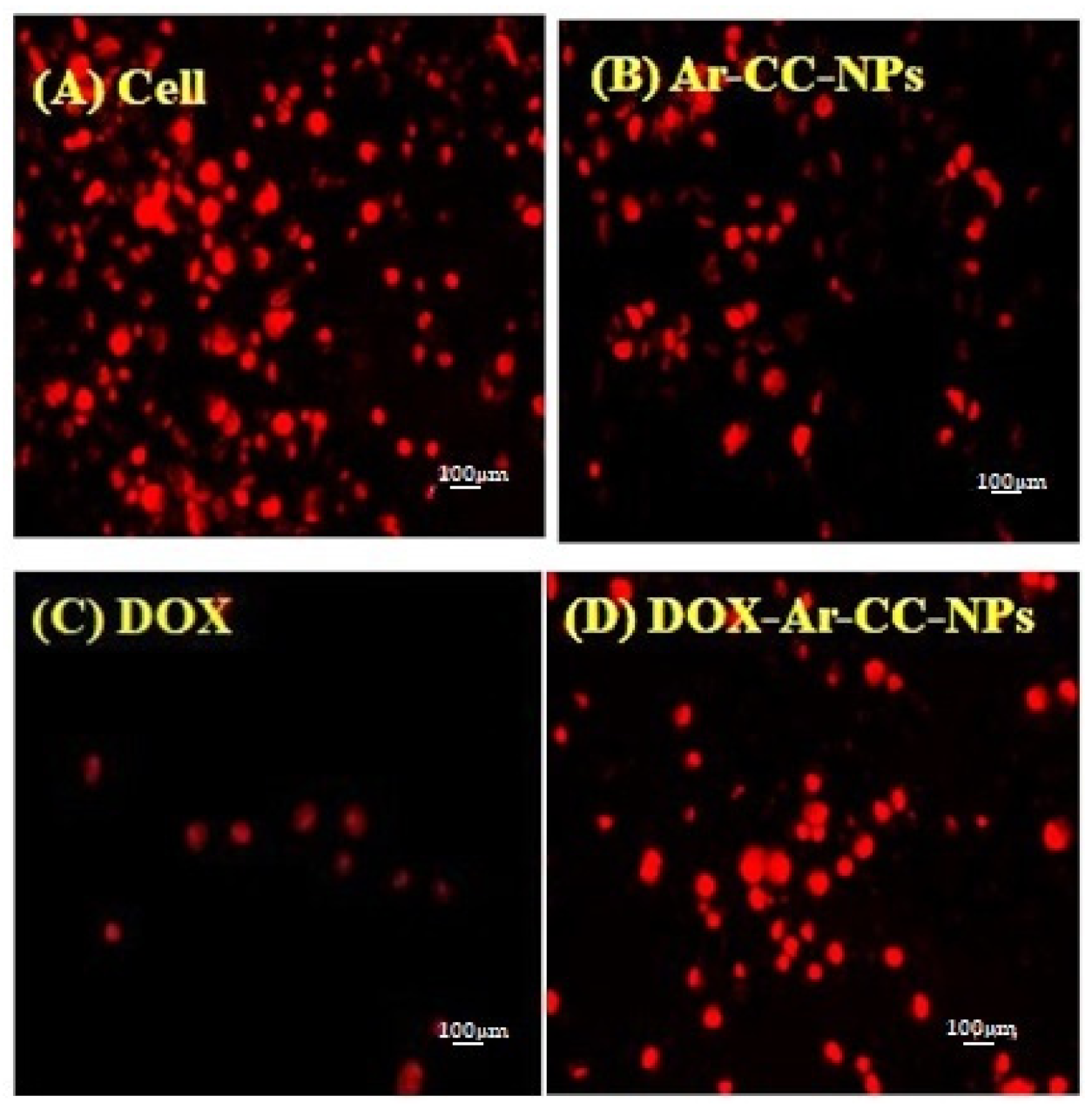
3.1. DOX and DOX-Ar-CC-NPs Cellular Uptake
3.2. Ultrastructural Observation of MCF-7 Cells Using TEM
3.3. Annexin-V FITC Assay of MCF-7 Cancer Cells
3.4. Annexin V and PI Binding Assay
3.5. Assessment of Protein Extract Using 1-D-Gel SDS-PAGE
3.6. Top Canonical Pathways Correlated with Biological Functions Affected by Changes in Protein Abundance in DOX-Ar-CC-NPs- Compared to DOX-Treated Cells
4. Discussion
4.1. Heat Shock Proteins
4.2. Structural Proteins
4.3. Proteins Associated with Carbohydrate and Energy Metabolism
4.4. Other Proteins
4.5. Bioinformatics Analyses Revealed That the Abundance of Proteins Affected the Migration of Cells and Glycolysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Abbass, A.K.; Al-Saffar, A.Z.; Al-Obaidi, J.R. An in vitro cytotoxicity of a novel pH-Sensitive lectin loaded-cockle shell-derived calcium carbonate nanoparticles against MCF-7 breast tumour cell. J. Drug Deliv. Sci. Technol. 2021, 61, 102230. [Google Scholar] [CrossRef]
- Haruta, S.; Hanafusa, T.; Fukase, H.; Miyajima, H.; Oki, T. An effective absorption behavior of insulin for diabetic treatment following intranasal delivery using porous spherical calcium carbonate in monkeys and healthy human volunteers. Diabetes Technol. Ther. 2003, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Futagawa, H.; Takagi, Y.; Ueno, A.; Mizushima, Y. Drug-incorporating calcium carbonate nanoparticles for a new delivery system. J. Control. Release 2005, 103, 93–98. [Google Scholar] [CrossRef]
- Qian, K.; Shi, T.; Tang, T.; Zhang, S.; Liu, X.; Cao, Y. Preparation and characterization of nano-sized calcium carbonate as controlled release pesticide carrier for validamycin against Rhizoctonia solani. Microchim. Acta 2011, 173, 51–57. [Google Scholar] [CrossRef]
- Dizaj, M.S.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K.; Lotfipour, F. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin. Drug Deliv. 2015, 12, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Kamba, S.A.; Ismail, M.; Hussein-Al-Ali, S.H.; Ibrahim, T.A.T.; Zakaria, Z.A.B. In vitro delivery and controlled release of doxorubicin for targeting osteosarcoma bone cancer. Molecules 2013, 18, 10580–10598. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Singh, S.; Lillard, J.W., Jr.; Singh, R. Drug-delivery-approaches-for-breast-cancer. Int. J. Nanomed. 2017, 12, 6205–6218. [Google Scholar] [CrossRef] [Green Version]
- Baskin, Y. TIPTA TEKNOLOJİK GELİŞİMİN NEDEN OLDUĞU KAVRAM DEĞİŞİMLERİ: KİŞİSELLEŞTİRİLMİŞ TIP Changes in Concepts of the Technological Progress: Personalized Medicine. Turk Hij. Den. Biyol. Derg. 2007, 64, 54–59. [Google Scholar]
- Gromov, P.S.; Østergaard, M.; Gromova, I.; Celis, J.E. Human proteomic databases: A powerful resource for functional genomics in health and disease. Prog. Biophys. Mol. Biol. 2002, 80, 3–22. [Google Scholar] [CrossRef]
- Baak, J.P.A.; Path, F.R.C.; Hermsen, M.A.J.A.; Meijer, G.; Schmidt, J.; Janssen, E.A.M. Genomics and proteomics in cancer. Eur. J. Cancer 2003, 39, 1199–1215. [Google Scholar] [CrossRef]
- Bleicher, K.H.; Böhm, H.J.; Müller, K.; Alanine, A.I. Hit and lead generation: Beyond high-throughput screening. Nat. Rev. Drug Discov. 2003, 2, 369–378. [Google Scholar] [CrossRef]
- Burbaum, J.; Tobal, G.M. Proteomics in drug discovery. Curr. Opin. Chem. Biol. 2002, 6, 427–433. [Google Scholar] [CrossRef]
- Beretov, J.; Wasinger, V.C.; Millar, E.K.A.; Schwartz, P.; Graham, P.H.; Li, Y. Proteomic analysis of urine to identify breast cancer biomarker candidates using a label-free LC-MS/MS approach. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, R.I.; Abbass, A.K.; Razali, N.; Al-Saffar, A.Z.; Al-Obaidi, J.R. Protein profile of MCF-7 breast cancer cell line treated with lectin delivered by CaCO3NPs revealed changes in molecular chaperones, cytoskeleton, and membrane-associated proteins. Int. J. Biol. Macromol. 2021, 184, 636–647. [Google Scholar] [CrossRef]
- Steiner, C.; Ducret, A.; Tille, J.C.; Thomas, M.; Mckee, T.A.; Rubbia-Brandt, L.; Scherl, A.; Lescuyer, P.; Cutler, P. Applications of mass spectrometry for quantitative protein analysis in formalin-fixed paraffin-embedded tissues. Proteomics 2015, 14, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Savino, R.; Paduano, S.; Preianò, M.; Terracciano, R. The proteomics big challenge for biomarkers and new drug-targets discovery. Int. J. Mol. Sci. 2012, 13, 13926–13948. [Google Scholar] [CrossRef] [Green Version]
- Hamidu, A.; Mokrish, A.; Mansor, R.; Razak, I.S.A.; Danmaigoro, A.; Jaji, A.Z.; Bakar, Z.A. Modified methods of nanoparticles synthesis in ph-sensitive nano-carriers production for doxorubicin delivery on MCF-7 breast cancer cell line. Int. J. Nanomed. 2019, 14, 3615–3627. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.T.; Zhang, Y.L.; Chen, S.X.; Yin, G.H.; Yang, Z.Z.; Lee, S.; Liu, C.G.; Zhao, D.D.; Ma, Y.K.; Song, F.Q.; et al. Proteomics of methyl jasmonate induced defense response in maize leaves against Asian corn borer. BMC Genom. 2015, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 2009, 32, 5–8. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Al-Obaidi, J.R.; Razali, N.; Junit, S.M.; Ajang, M.J.; Hussin, S.N.I.S.; Hamid, S.S.; Hanafi, N.M.; Roni, A.N.H.M.; Saleh, N.M. Molecular investigation of carrageenan production in Kappaphycus alvarezii in different culture conditions: A proteomic approach. J. Appl. Phycol. 2017, 29, 1989–2001. [Google Scholar] [CrossRef]
- Liu, K.; Tang, Z.; Huang, A.; Chen, P.; Liu, P.; Yang, J.; Lu, W.; Liao, J.; Sun, Y.; Wen, S.; et al. Glyceraldehyde-3-phosphate dehydrogenase promotes cancer growth and metastasis through upregulation of SNAIL expression. Int. J. Oncol. 2017, 50, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Aziz, A.A.; Lim, C.Y.; Junit, S.M. Investigation into the effects of antioxidant-rich extract of Tamarindus indica leaf on antioxidant enzyme activities, oxidative stress and gene expression profiles in HepG2 cells. PeerJ 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kooijmans, S.A.A.; Gitz-Francois, J.J.J.M.; Schiffelers, R.M.; Vader, P. Recombinant phosphatidylserine-binding nanobodies for targeting of extracellular vesicles to tumor cells: A plug-and-play approach. Nanoscale 2018, 10, 2413–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Hicks, D.G.; Kulkarni, S. HER2+ breast cancer: Review of biologic relevance and optimal use of diagnostic tools. Am. J. Clin. Pathol. 2008, 129, 263–273. [Google Scholar] [CrossRef] [Green Version]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Workman, P.; Powers, M.V. Chaperoning cell death: A critical dual role for Hsp90 in small-cell lung cancer. Nat. Chem. Biol. 2007, 3, 455–457. [Google Scholar] [CrossRef]
- Pick, E.; Kluger, Y.; Giltnane, J.M.; Moeder, C.; Camp, R.L.; Rimm, D.L.; Kluger, H.M. High HSP90 expression is associated with decreased survival in breast cancer. Cancer Res. 2007, 67, 2932–2937. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.X.; Ju, H.Q.; Liu, K.S.; Zhang, J.X.; Wang, X.; Xiang, Y.F.; Wang, R.; Liu, J.Y.; Liu, Q.Y.; Xia, M.; et al. SNX-2112, a novel Hsp90 inhibitor, induces G2/M cell cycle arrest and apoptosis in MCF-7 cells. Biosci. Biotechnol. Biochem. 2011, 75, 1540–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karantza, V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011, 30, 1127–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.D.; Kenny, L.; Perry, C.; Frazer, I.; Punyadeera, C. The overexpression of salivary cytokeratins as potential diagnostic biomarkers in head and neck squamous cell carcinomas. Oncotarget 2017, 8, 72272–72280. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Keratins as biochemical markers of epithelial differentiation. Trends Genet. 1988, 4, 277–281. [Google Scholar] [CrossRef]
- Janke, C. The tubulin code: Molecular components, readout mechanisms, functions. J. Cell Biol. 2014, 206, 461–472. [Google Scholar] [CrossRef] [Green Version]
- Chakraborti, S.; Natarajan, K.; Curiel, J.; Janke, C.; Liu, J. The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease. Cytoskeleton 2016, 73, 521–550. [Google Scholar] [CrossRef]
- Gadadhar, S.; Bodakuntla, S.; Natarajan, K.; Janke, C. The tubulin code at a glance. J. Cell Sci. 2017, 130, 1347–1353. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Vo, T.; Hajar, A.; Li, S.; Chen, X.; Parissenti, A.M.; Brindley, D.N.; Wang, Z. Multiple mechanisms underlying acquired resistance to taxanes in selected docetaxel-resistant MCF-7 breast cancer cells. BMC Cancer 2014, 14, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mariani, M.; Karki, R.; Spennato, M.; Pandya, D.; He, S.; Andreoli, M.; Fiedler, P.; Ferlini, C. Class III β-tubulin in Normal and Cancer tissues Marisa. Gene 2015, 563, 109–114. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Plasticity of cell migration: A multiscale tuning model. J. Cell Biol. 2010, 188, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Friedl, P.; Locker, J.; Sahai, E.; Segall, J.E. Classifying collective cancer cell invasion. Nat. Cell Biol. 2012, 14, 777–783. [Google Scholar] [CrossRef]
- Hsu, K.S.; Kao, H.Y. Alpha-Actinin 4 and Tumorigenesis of Breast Cancer. Vitam. Horm. 2013, 93, 323–351. [Google Scholar] [CrossRef] [Green Version]
- Garrett, R.H.; Grisham, C.M. Biochemistry; Brooks/Cole: Boston, MA, USA, 2013; ISBN 978-0-495-10935-8. [Google Scholar]
- Song, S.; Finkel, T. GAPDH and the search for alternative energy. Nat. Cell Biol. 2007, 9, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Oka, T.; Sato, H.; Shinnou, Y.; Washio, K. Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development. Int. J. Oncol. 2009, 35, 547–557. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Wang, L.; Chen, S. The sweet trap in tumors: Aerobic glycolysis and potential targets for therapy. Oncotarget 2016, 7, 38908–38926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Shelton, L.M. Cancer as a metabolic disease. Nutr. Metab. 2010, 7, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, N. Self-dual Leonard pairs ATP Synthase: Structure, Function and Inhibition. Biomol. Concepts 2019, 10, 1–10. [Google Scholar]
- España, L.; Martín, B.; Aragüés, R.; Chiva, C.; Oliva, B.; Andreu, D.; Sierra, A. Bcl-xL-mediated changes in metabolic pathways of breast cancer cells: From survival in the blood stream to organ-specific metastasis. Am. J. Pathol. 2005, 167, 1125–1137. [Google Scholar] [CrossRef]
- Dowling, P.; Meleady, P.; Dowd, A.; Henry, M.; Glynn, S.; Clynes, M. Proteomic analysis of isolated membrane fractions from superinvasive cancer cells. Biochim. Biophys. Acta Proteins Proteom. 2007, 1774, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Mori, R.; Wang, Q.; Danenberg, K.D.; Pinski, J.K.; Danenberg, P.V. Both β-actin and GAPDH are useful reference genes for normalization of quantitative RT-PCR in human FFPE tissue samples of prostate cancer. Prostate 2008, 68, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Révillion, F.; Pawlowski, V.; Hornez, L.; Peyrat, J.P. Glyceraldehyde-3-phosphate dehydrogenase gene expression in human breast cancer. Eur. J. Cancer 2000, 36, 1038–1042. [Google Scholar] [CrossRef]
- Palmieri, F. Mitochondrial transporters of the SLC25 family and associated diseases: A review. J. Inherit. Metab. Dis. 2014, 37, 565–575. [Google Scholar] [CrossRef]
- Palmieri, F.; Monné, M. Discoveries, metabolic roles and diseases of mitochondrial carriers: A review. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2362–2378. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Ke, Z.; Song, Y.; Yao, Y.; Hu, X.; Zhang, M.; Li, H.; Yin, J. Annexin A3 is associated with a poor prognosis in breast cancer and participates in the modulation of apoptosis in vitro by affecting the Bcl-2/Bax balance. Exp. Mol. Pathol. 2013, 95, 23–31. [Google Scholar] [CrossRef]
- Bingle, L.; Singleton, V.; Bingle, C.D. The putative ovarian tumour marker gene HE4 (WFDC2), is expressed in normal tissues and undergoes complex alternative splicing to yield multiple protein isoforms. Oncogene 2002, 21, 2768–2773. [Google Scholar] [CrossRef]
- Bingle, L.; Cross, S.S.; High, A.S.; Wallace, W.A.; Rassl, D.; Yuan, G.; Hellstrom, I.; Campos, M.A.; Bingle, C.D. WFDC2 (HE4): A potential role in the innate immunity of the oral cavity and respiratory tract and the development of adenocarcinomas of the lung. Respir. Res. 2006, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.G.; McMeekin, D.S.; Brown, A.K.; DiSilvestro, P.; Miller, M.C.; Allard, W.J.; Gajewski, W.; Kurman, R.; Bast, R.C.; Skates, S.J. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol. Oncol. 2009, 112, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Piovano, E.; Attamante, L.; Macchi, C.; Cavallero, C.; Romagnolo, C.; Maggino, T.; Landoni, F.; Gadducci, A.; Sartori, E.; Gion, M.; et al. The role of HE4 in ovarian cancer follow-up: A review. Int. J. Gynecol. Cancer 2014, 24, 1359–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamei, M.; Yamashita, S.I.; Tokuishi, K.; Hashioto, T.; Moroga, T.; Suehiro, S.; Ono, K.; Miyawaki, M.; Takeno, S.; Yamamoto, S.; et al. HE4 expression can be associated with lymph node metastases and disease-free survival in breast cancer. Anticancer Res. 2010, 30, 4779–4783. [Google Scholar] [PubMed]
- Gündüz, U.R.; Gunaldi, M.; Isiksacan, N.; Gündüz, S.; Okuturlar, Y.; Kocoglu, H. A new marker for breast cancer diagnosis, human epididymis protein 4: A preliminary study. Mol. Clin. Oncol. 2016, 5, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 642–652. [Google Scholar] [CrossRef] [Green Version]
- Troussard, A.A.; McDonald, P.C.; Wederell, E.D.; Mawji, N.M.; Filipenko, N.R.; Gelmon, K.A.; Kucab, J.E.; Dunn, S.E.; Emerman, J.T.; Bally, M.B.; et al. Preferential Dependence of Breast Cancer Cells versus Normal Cells on Integrin-Linked Kinase for Protein Kinase B/Akt Activation and Cell Survival. Cancer Res. 2006, 66, 393LP–403LP. [Google Scholar] [CrossRef] [Green Version]
- Kiso, M.; Tanaka, S.; Saji, S.; Toi, M.; Sato, F. Long isoform of VEGF stimulates cell migration of breast cancer by filopodia formation via NRP1/ARHGAP17/Cdc42 regulatory network. Int. J. Cancer 2018, 143, 2905–2918. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-M.; Bae, E.; Ahn, S.G.; Pang, K.; Park, Y.; Park, J.; Lee, J.; Ooshima, A.; Park, B.; Kim, J.; et al. Co-chaperone BAG2 Determines the Pro-oncogenic Role of Cathepsin B in Triple-Negative Breast Cancer Cells. Cell Rep. 2017, 21, 2952–2964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shortrede, J.E.; Uzair, I.D.; Neira, F.J.; Flamini, M.I.; Sanchez, A.M. Paxillin, a novel controller in the signaling of estrogen to FAK/N-WASP/Arp2/3 complex in breast cancer cells. Mol. Cell. Endocrinol. 2016, 430, 56–67. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, J.; Zhao, Q.; Fu, S.; Jin, J. Emerging roles of aerobic glycolysis in breast cancer. Clin. Transl. Oncol. 2020, 22, 631–646. [Google Scholar] [CrossRef] [PubMed]








| Time | Control | Ar-CC-NPs | DOX | DOX-Ar-CC-NPs | |
|---|---|---|---|---|---|
| LL | 98.46 ± 0.18 | 98.54 ± 0.04 | 52.71 ± 4.78 | 39.22 ± 1.94 | |
| 24 h | LR | 0.83 ± 0.08 | 0.68 ± 0.07 | 40.30 ± 5.08 | 56.69 ± 1.67 |
| UR | 0.48 ± 0.05 | 0.45 ± 0.04 | 6.95 ± 0.32 | 3.98 ± 0.30 | |
| UL | 0.24 ± 0.04 | 0.34 ± 0.06 | 0.05 ± 0.01 | 0.14 ± 0.06 | |
| LL | 93.38 ± 0.92 | 91.63 ± 1.25 | 22.19 ± 2.83 | 0.87 ± 0.14 | |
| 48 h | LR | 0.71 ± 0.14 | 0.65 ± 0.03 | 8.36 ± 2.05 | 96.91 ± 0.36 |
| UR | 5.74 ± 0.77 | 7.57 ± 1.15 | 67.92 ± 4.18 | 2.22 ± 0.24 | |
| UL | 0.17 ± 0.06 | 0.21 ± 0.10 | 1.53 ± 0.67 | 0.00 ± 0.00 | |
| LL | 88.83 ± 0.61 | 92.86 ± 0.65 | 8.90 ± 2.24 | 8.61 ± 0.42 | |
| LR | 2.11 ± 0.32 | 3.99 ± 1.14 | 3.55 ± 0.66 | 50.03 ± 0.46 | |
| 72 h | UR | 8.53 ± 0.69 | 2.05 ± 0.40 | 85.70 ± 2.80 | 40.34 ± 0.01 |
| UL | 0.71 ± 0.21 | 0.37 ± 0.06 | 1.86 ± 0.08 | 1.03 ± 0.03 |
| No. | Protein Name | Taxonomy | Accession No. a | Score b | Matches c | Coverage % d | MW e | PI f | Th. MW g | Th. PI h |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Actin, cytoplasmic 1 | Homo sapiens (Human) | P60709 | 315.1 | 88 | 71.2 | 41.71 | 5.48 | 1841.25 | 5.54 |
| 2. | Glucose-regulated protein | Homo sapiens (Human) | V9HWB4 | 277.8 | 80 | 50.3 | 72.288 | 5.16 | 5088.28 | 4.33 |
| 3. | Chaperonin | Homo sapiens (Human) | A0A024R3X4 | 247.1 | 69 | 40.1 | 61.016 | 5.87 | 4747.86 | 4.33 |
| 4. | Annexin A2 | Homo sapiens (Human) | P07355 | 335.7 | 88 | 57.5 | 38.58 | 7.75 | 856.09 | 4.00 |
| 5. | Vimentin OS = Homo sapiens | Homo sapiens (Human) | P08670 | 235.6 | 76 | 40.9 | 53.619 | 5.12 | 2699.14 | 4.51 |
| 6. | Actinin, alpha 1, isoform CRA_a | Homo sapiens (Human) | A0A024R694 | 134.3 | 43 | 30.3 | 102.993 | 5.41 | 2699.14 | 4.51 |
| 7. | Epididymis luminal protein 33 | Homo sapiens (Human) | V9HW22 | 144.4 | 44 | 41.1 | 70.854 | 5.52 | 2949.33 | 3.92 |
| 8. | ATP synthase | Homo sapiens (Human) | V9HW31 | 172.7 | 51 | 48.2 | 56.525 | 5.4 | 2423.32 | 4.51 |
| 9. | Myosin, isoform CRA_a | Homo sapiens (Human) | A0A024R1N1 | 124 | 41 | 12.5 | 226.392 | 5.6 | 5546.36 | 4.57 |
| 10. | Pyruvate kinase | Homo sapiens (Human) | V9HWB8 | 171 | 56 | 33.1 | 57.9 | 7.84 | 1627.68 | 6.56 |
| 11. | Actinin alpha 4 isoform 1 (Fragment) | Homo sapiens (Human) | A0A0S2Z3G9 | 115.4 | 41 | 30.8 | 104.788 | 5.44 | 3253.85 | 8.30 |
| 12. | Elongation factor 1-alpha (Fragment) | Homo sapiens (Human) | Q53G85 | 121.8 | 42 | 48.9 | 50.081 | 9.01 | 3518.07 | 6.84 |
| 13. | HSPA9 protein (Fragment) | Homo sapiens (Human) | Q8N1C8 | 138.3 | 44 | 31.1 | 73.808 | 6.37 | 2265.53 | 6.76 |
| 14. | Tubulin alpha-1B chain | Homo sapiens (Human) | P68363 | 110.5 | 31 | 39.6 | 50.12 | 5.06 | 2017.12 | 6.61 |
| 15. | Actin, alpha cardiac muscle 1 | Homo sapiens (Human) | P68032 | 153.1 | 50 | 36.8 | 41.992 | 5.39 | 2456.77 | 5.32 |
| 16. | ATP synthase subunit alpha, mitochondrial | Homo sapiens (Human) | P25705 | 147.4 | 39 | 30.3 | 59.714 | 9.13 | 4240.06 | 6.27 |
| 17. | Trypsin | Homo sapiens (Human) | P07477 | 167.5 | 62 | 31.6 | 24.394 | 7.18 | 3061.55 | 7.89 |
| 18. | Tubulin β chain | Homo sapiens (Human) | P68371 | 113 | 39 | 46.7 | 49.799 | 4.89 | 1943.50 | 5.21 |
| 19. | Tubulin β chain | Homo sapiens (Human) | P07437 | 113.2 | 39 | 46.3 | 49.639 | 4.89 | 1828.85 | 5.21 |
| 20. | α-enolase | Homo sapiens (Human) | A0A2R8Y6G6 | 94.8 | 24 | 23.9 | 47.297 | 6.99 | 1388.54 | 4.51 |
| 21. | Calreticulin, isoform CRA_b | Homo sapiens (Human) | V9HW88 | 79 | 25 | 31.8 | 48.112 | 4.44 | 4719.06 | 7.98 |
| 22. | Glyceraldehyde-3-phosphate dehydrogenase | Homo sapiens (Human) | P04406 | 72 | 20 | 35.2 | 36.03 | 8.46 | 4447.65 | 4.39 |
| 23. | HSP 90kDa α (Cytosolic), isoform CRA_a | Homo sapiens (Human) | A0A024RD80 | 74.6 | 26 | 22.3 | 83.212 | 5.03 | 7038.38 | 7.85 |
| 24. | cDNA FLJ53906, highly similar to Tubulin beta chain | Homo sapiens (Human) | B7Z4N1 | 62.8 | 24 | 40.3 | 39.869 | 4.97 | ||
| 25. | Histone H2A | Homo sapiens (Human) | A0A0U1RRH7 | 72.4 | 17 | 33.5 | 18.541 | 11.5 | 1145.24 | 5.98 |
| 26. | ADP, ATP carrier protein | Homo sapiens (Human) | Q59EI9 | 61.4 | 18 | 24.1 | 35.361 | 9.85 | 5857.86 | 11.16 |
| 27. | Beta-enolase | Homo sapiens (Human) | P13929 | 69.5 | 17 | 15.8 | 46.957 | 7.71 | 1314.92 | 3.67 |
| 28. | Epididymis luminal protein 4 | Homo sapiens (Human) | D0PNI1 | 62.1 | 19 | 34.6 | 27.728 | 4.79 | 2949.33 | 3.92 |
| 29. | Actin-like protein (Fragment) | Homo sapiens (Human) | Q562L5 | 61.7 | 20 | 85.4 | 11.518 | 6.35 | 2859.31 | 6.26 |
| 30. | cDNA FLJ54371, highly similar to Serum albumin | Homo sapiens (Human) | B4DPP6 | 63.6 | 20 | 12.4 | 70.317 | 6.09 | ||
| 31. | Keratin, type I cytoskeletal 10 | Homo sapiens (Human) | P13645 | 469.9 | 127 | 55.3 | 58.79 | 5.2 | 2911.32 | 4.95 |
| 32. | Keratin, type I cytoskeletal 9 | Homo sapiens (Human) | P35527 | 379.7 | 110 | 58.5 | 62.03 | 5.2 | 2911.32 | 4.95 |
| 33. | Keratin, type II cytoskeletal 2 epidermal | Homo sapiens (Human) | P35908 | 315.1 | 100 | 58.3 | 65.39 | 8 | 4079.69 | 4.55 |
| 34. | Keratin 1 | Homo sapiens (Human) | H6VRG1 | 389.1 | 115 | 44.8 | 66.09 | 8.1 | 830.94 | 8.75 |
| 35. | Keratin, type II cytoskeletal 5 | Homo sapiens (Human) | P13647 | 73.5 | 26 | 16.10 | 62.34 | 7.7 | 3024.48 | 4.95 |
| 36. | Keratin, type I cytoskeletal 14 | Homo sapiens (Human) | P02533 | 60.02 | 21 | 23.7 | 51.5 | 5.16 | 2911.32 | 4.95 |
| No. | Protein | Taxonomy | Accession No. a | Score b | Matches c | Coverage % d | MW e | PI f | Th. MW g | Th. PI h |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 78 kDa glucose-regulated protein | Homo sapiens (Human) | V9HWB4 | 64.7 | 23 | 20.4 | 72.288 | 5.16 | 3179.27 | 4.36 |
| 2. | Annexin A2 | Homo sapiens (Human) | P07355 | 96.7 | 30 | 32.1 | 38.58 | 7.75 | 856.09 | 4.00 |
| 3. | Putative keratin-87 protein | Homo sapiens (Human) | A6NCN2 | 76 | 28 | 41.9 | 29.099 | 5.8 | 2640.88 | 6.65 |
| 4. | Tubulin beta chain | Homo sapiens (Human) | P07437 | 69.2 | 25 | 36.9 | 49.639 | 4.89 | 1828.85 | 5.21 |
| 5. | IF rod domain-containing protein | Homo sapiens (Human) | A0A140TA62 | 122.9 | 44 | 19.2 | 49.38 | 5.06 | 3655.25 | 4.56 |
| 6. | Tubulin alpha-1B chain | Homo sapiens (Human) | P68363 | 61.7 | 22 | 33.4 | 50.12 | 5.06 | 2017.12 | 6.61 |
| 7. | Actin, cytoplasmic 1 | Homo sapiens (Human) | P60709 | 71.8 | 27 | 34.9 | 41.71 | 5.48 | 1841.25 | 5.54 |
| Top Canonical Pathway | Treatment | |
|---|---|---|
| Actin cytoskeleton signalling | DOX-Ar-CC-NPs | DOX |
| p value | 2.95 × 10−5 | 6.68 × 10−2 |
| Activation z-score | 2.236 | N. A |
| Genes | ACTB, ACTC1, ACTN1, ACTN4, MYH9 | ACTB |
| Integrin-linked kinase (ILK) signalling | DOX-Ar-CC-NPs | DOX |
| p value | 4.19 × 10−7 | 5.4 × 10−2 |
| Activation z-score | 2.236 | N. A |
| Genes | ACTB, ACTC1, ACTN1, ACTN4, MYH9, VIM | ACTB |
| VEGF signalling | DOX-Ar-CC-NPs | DOX |
| p value | 1.4 × 10−5 | 2.74 × 10−2 |
| Activation z-score | 2 | N. A |
| Genes | ACTB, ACTC1, ACTN1, ACTN4, MYH9 | ACTB |
| BAG2 signalling | DOX-Ar-CC-NPs | DOX |
| p value | 6.85 × 10−6 | 2.19 × 10−4 |
| Activation z-score | 2 | N. A |
| Genes | ANXA2, HSPA5, HSPA8, HSPA9 | ANXA2, HSPA5 |
| Integrin signalling | DOX-Ar-CC-NPs | DOX |
| p value | 2.58 × 10−4 | 5.73 × 10−2 |
| Activation z-score | 2 | N. A |
| Genes | ACTB, ACTC1, ACTN1, ACTN4 | ACTB |
| Paxilin signalling | DOX-Ar-CC-NPs | DOX |
| p value | 1.99 × 10−5 | 3.00 × 10−2 |
| Activation z-score | 2 | N. A |
| Genes | ACTB, ACTC1, ACTN1, ACTN4 | ACTB |
| Glycolysis I | DOX-Ar-CC-NPs | DOX |
| p value | 1.99 × 10−5 | - |
| Activation z-score | 2 | - |
| Genes | GAPDH, ENO1, ENO3, PKM | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.; Ajat, M.; Mahmood, R.I.; Mansor, R.; Razak, I.S.A.; Al-Obaidi, J.R.; Razali, N.; Jaji, A.Z.; Danmaigoro, A.; Bakar, M.Z.A. LC-MS/MS Proteomic Study of MCF-7 Cell Treated with Dox and Dox-Loaded Calcium Carbonate Nanoparticles Revealed Changes in Proteins Related to Glycolysis, Actin Signalling, and Energy Metabolism. Biology 2021, 10, 909. https://doi.org/10.3390/biology10090909
Ahmed H, Ajat M, Mahmood RI, Mansor R, Razak ISA, Al-Obaidi JR, Razali N, Jaji AZ, Danmaigoro A, Bakar MZA. LC-MS/MS Proteomic Study of MCF-7 Cell Treated with Dox and Dox-Loaded Calcium Carbonate Nanoparticles Revealed Changes in Proteins Related to Glycolysis, Actin Signalling, and Energy Metabolism. Biology. 2021; 10(9):909. https://doi.org/10.3390/biology10090909
Chicago/Turabian StyleAhmed, Hamidu, Mokrish Ajat, Rana I. Mahmood, Rozaihan Mansor, Intan Shameha Abdul Razak, Jameel R. Al-Obaidi, Nurhanani Razali, Alhaji Zubair Jaji, Abubakar Danmaigoro, and Md Zuki Abu Bakar. 2021. "LC-MS/MS Proteomic Study of MCF-7 Cell Treated with Dox and Dox-Loaded Calcium Carbonate Nanoparticles Revealed Changes in Proteins Related to Glycolysis, Actin Signalling, and Energy Metabolism" Biology 10, no. 9: 909. https://doi.org/10.3390/biology10090909
APA StyleAhmed, H., Ajat, M., Mahmood, R. I., Mansor, R., Razak, I. S. A., Al-Obaidi, J. R., Razali, N., Jaji, A. Z., Danmaigoro, A., & Bakar, M. Z. A. (2021). LC-MS/MS Proteomic Study of MCF-7 Cell Treated with Dox and Dox-Loaded Calcium Carbonate Nanoparticles Revealed Changes in Proteins Related to Glycolysis, Actin Signalling, and Energy Metabolism. Biology, 10(9), 909. https://doi.org/10.3390/biology10090909







