Perturbations of Adjuvant Chemotherapy on Cardiovascular Responses and Exercise Tolerance in Patients with Early-Stage Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
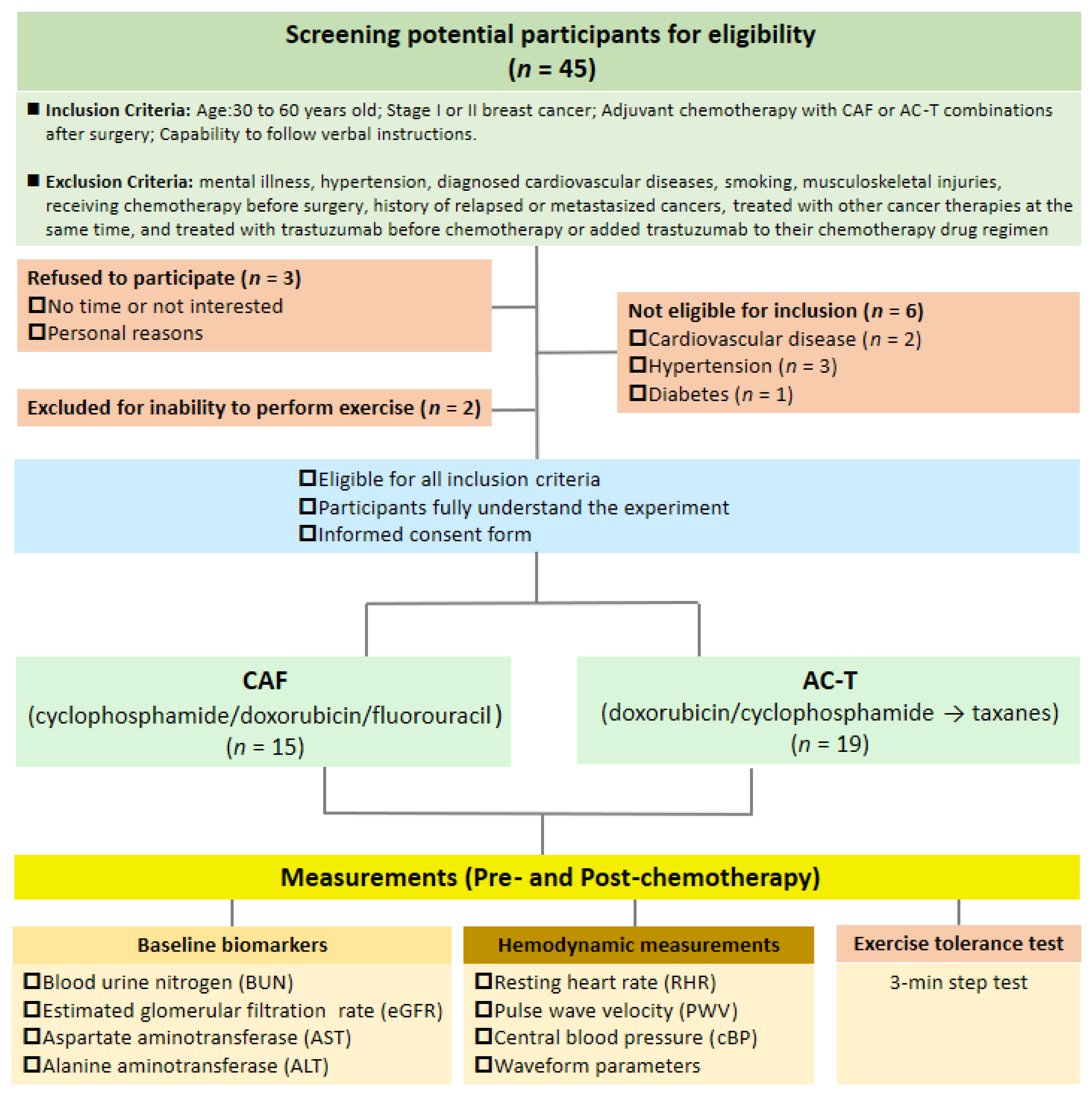
2.1. Participants
2.2. Study Design
2.3. Adjuvant Chemotherapy Intervention
2.4. Function Measurements
2.4.1. Blood Pressure and Pulse-Wave Velocity (PWV)
2.4.2. Waveform Analysis
2.5. Step-Exercise Tolerance Assessment
2.6. Statistical Analyses
3. Results
3.1. Characteristics of Participants
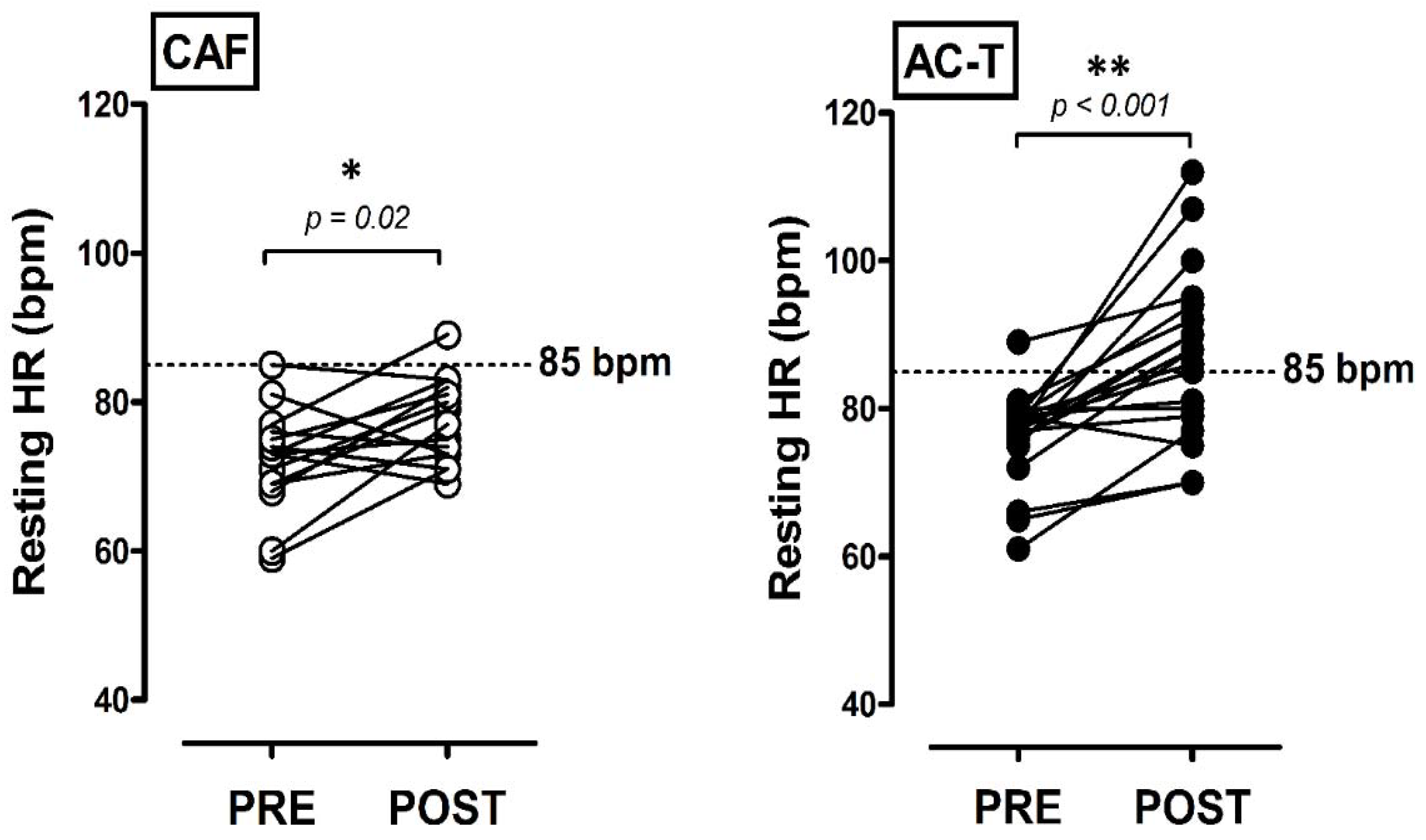
3.2. Central Hemodynamic Changes before and after Chemotherapies
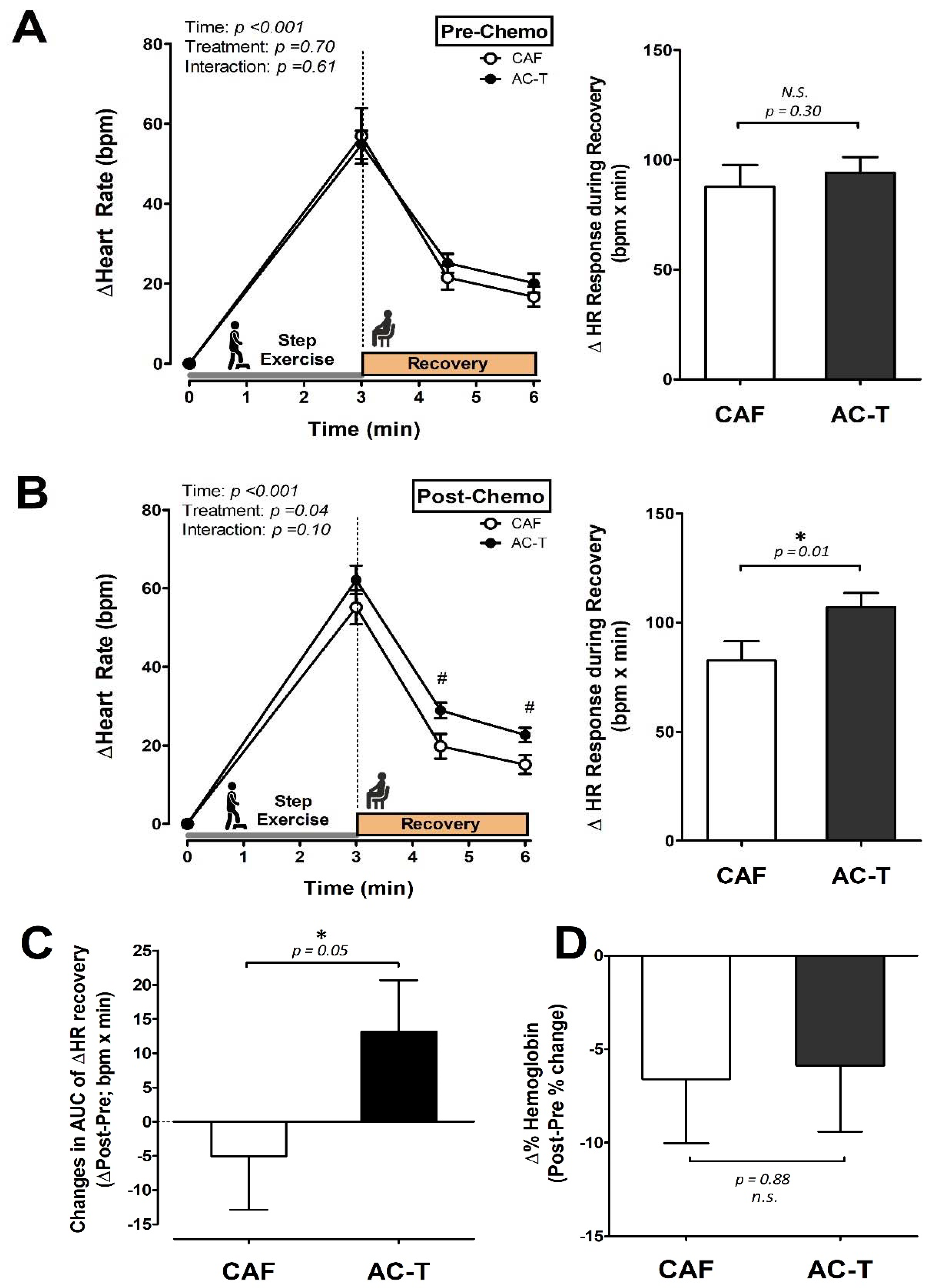
3.3. Heart Rate Responses to a Step-Exercise Tolerance Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Gogia, A.; Raina, V.; Deo, S.V.; Shukla, N.K.; Mohanti, B.K.; Sharma, D.N. Taxane and anthracycline based neoadjuvant chemotherapy for locally advanced breast cancer: Institutional experience. Asian Pac. J. Cancer Prev. 2014, 15, 1989–1992. [Google Scholar] [CrossRef]
- Ravdin, P.M.; Siminoff, L.A.; Davis, G.J.; Mercer, M.B.; Hewlett, J.; Gerson, N.; Parker, H.L. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J. Clin. Oncol. 2001, 19, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, D.; Lee, J.; Cresti, N.; Jackson, R.; Griffin, M.; Sludden, J.; Verrill, M.; Boddy, A.V. Pharmacogenetics of adjuvant breast cancer treatment with cyclophosphamide, epirubicin and 5-fluorouracil. Cancer Chemother. Pharm. 2014, 74, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Peto, R.; Davies, C.; Godwin, J.; Gray, R.; Pan, H.C.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Taylor, C.; et al. Comparisons between different polychemotherapy regimens for early breast cancer: Meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012, 379, 432–444. [Google Scholar]
- Yamaguchi, N.; Fujii, T.; Aoi, S.; Kozuch, P.S.; Hortobagyi, G.N.; Blum, R.H. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: A Bayesian network meta-analysis. Eur. J. Cancer 2015, 51, 2314–2320. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs 2017, 31, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, A.A.; Lloyd, M.G.; Claydon, V.E.; Gelmon, K.A.; McKenzie, D.C.; Campbell, K.L. A Longitudinal Study of the Association of Clinical Indices of Cardiovascular Autonomic Function with Breast Cancer Treatment and Exercise Training. Oncologist 2019, 24, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Rivera, E.; Cianfrocca, M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother. Pharm. 2015, 75, 659–670. [Google Scholar] [CrossRef] [Green Version]
- De Iuliis, F.; Taglieri, L.; Salerno, G.; Lanza, R.; Scarpa, S. Taxane induced neuropathy in patients affected by breast cancer: Literature review. Crit. Rev. Oncol. Hematol. 2015, 96, 34–45. [Google Scholar] [CrossRef]
- Cramp, F.; Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2008, 11, Cd006145. [Google Scholar]
- Markes, M.; Brockow, T.; Resch, K.L. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2006, 4, Cd005001. [Google Scholar]
- Speck, R.M.; Courneya, K.S.; Masse, L.C.; Duval, S.; Schmitz, K.H. An update of controlled physical activity trials in cancer survivors: A systematic review and meta-analysis. J. Cancer Surviv. 2010, 4, 87–100. [Google Scholar] [CrossRef]
- Courneya, K.S.; Friedenreich, C.M. Physical exercise and quality of life following cancer diagnosis: A literature review. Ann. Behav. Med. 1999, 21, 171–179. [Google Scholar] [CrossRef]
- Courneya, K.S. Exercise in cancer survivors: An overview of research. Med. Sci. Sports Exerc. 2003, 35, 1846–1852. [Google Scholar] [CrossRef]
- Mercurio, V.; Agnetti, G.; Pagliaro, P.; Tocchetti, C.G. Mechanisms of Cardiovascular Damage Induced by Traditional Chemotherapy. In Cardiovascular Complications in Cancer Therapy; Springer: New York, NY, USA, 2019; pp. 3–14. [Google Scholar]
- Boerma, M. Cardiovascular Side Effects of Breast Cancer Therapy. In Gender Differences in the Pathogenesis and Management of Heart Disease; Springer: New York, NY, USA, 2018; pp. 303–316. [Google Scholar]
- Dalfardi, B.; Kashy-Zonouzy, K.; Asvadi-Kermani, T. Chemotherapy-induced cardiomyopathy in breast cancer patients. Res. Cardiovasc. Med. 2014, 3, e19096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, E.T.; Tong, A.T.; Lenihan, D.J.; Yusuf, S.W.; Swafford, J.; Champion, C.; Durand, J.B.; Gibbs, H.; Zafarmand, A.A.; Ewer, M.S. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 2004, 109, 3122–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaosuwannakit, N.; D'Agostino, R., Jr.; Hamilton, C.A.; Lane, K.S.; Ntim, W.O.; Lawrence, J.; Melin, S.A.; Ellis, L.R.; Torti, F.M.; Little, W.C.; et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J. Clin. Oncol. 2010, 28, 166–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yersal, Ö.; Eryilmaz, U.; Akdam, H.; Meydan, N.; Barutca, S. Arterial Stiffness in Breast Cancer Patients Treated with Anthracycline and Trastuzumab-Based Regimens. Cardiol. Res. Pract. 2018, 2018, 5352914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, L.W.; Courneya, K.S.; Mackey, J.R.; Muss, H.B.; Pituskin, E.N.; Scott, J.M.; Hornsby, W.E.; Coan, A.D.; Herndon, J.E., 2nd; Douglas, P.S.; et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J. Clin. Oncol. 2012, 30, 2530–2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peel, J.B.; Sui, X.; Adams, S.A.; Hebert, J.R.; Hardin, J.W.; Blair, S.N. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc. 2009, 41, 742–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuragi, S.; Abhayaratna, W.P. Arterial stiffness: Methods of measurement, physiologic determinants and prediction of cardiovascular outcomes. Int. J. Cardiol. 2010, 138, 112–118. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chen, J.Y.; Wang, M.C.; Wu, H.T.; Chi, C.K.; Chen, Y.K.; Chen, J.H.; Lin, L.J. Association of risk factors with increased pulse wave velocity detected by a novel method using dual-channel photoplethysmography. Am. J. Hypertens. 2005, 18, 1118–1122. [Google Scholar] [CrossRef] [Green Version]
- Lieber, A.; Millasseau, S.; Bourhis, L.; Blacher, J.; Protogerou, A.; Levy, B.I.; Safar, M.E. Aortic wave reflection in women and men. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H236–H242. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.L.; Cheng, H.M.; Sung, S.H.; Chuang, S.Y.; Li, C.H.; Spurgeon, H.A.; Ting, C.T.; Najjar, S.S.; Lakatta, E.G.; Yin, F.C.; et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: A community-based study. Hypertension 2010, 55, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.S.; Levinson, G.E.; Schwartz, C.J.; Ettinger, P.O. Systolic Time Intervals as Measures of the Contractile State of the Left Ventricular Myocardium in Man. Circulation 1972, 46, 559–571. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.I.; Buckberg, G.D. The myocardial supply:demand ratio--a critical review. Am. J. Cardiol. 1978, 41, 327–332. [Google Scholar] [CrossRef]
- Tsai, M.W.; Wang, W.T.J.; Lee, H.C.; Hwang, S.Z.; Chen, J.J. A Field-Based Step Test on the Risk Assessment of the Metabolic Syndrome. J. Phys. Ther. 2012, 37, 146–156. [Google Scholar]
- Palatini, P.; Julius, S. Heart rate and the cardiovascular risk. J. Hypertens. 1997, 15, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Kristal-Boneh, E.; Silber, H.; Harari, G.; Froom, P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur. Heart J. 2000, 21, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Perret-Guillaume, C.; Joly, L.; Benetos, A. Heart rate as a risk factor for cardiovascular disease. Prog. Cardiovasc. Dis. 2009, 52, 6. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Heart rate in the pathophysiology of coronary blood flow and myocardial ischaemia: Benefit from selective bradycardic agents. Br. J. Pharm. 2008, 153, 1589–1601. [Google Scholar] [CrossRef]
- Reil, J.C.; Bohm, M. The role of heart rate in the development of cardiovascular disease. Clin. Res. Cardiol. 2007, 96, 585–592. [Google Scholar] [CrossRef]
- Hilkens, P.H.; Verweij, J.; Vecht, C.J.; Stoter, G.; van den Bent, M.J. Clinical characteristics of severe peripheral neuropathy induced by docetaxel (Taxotere). Ann. Oncol. 1997, 8, 187–190. [Google Scholar] [CrossRef]
- Jerian, S.M.; Sarosy, G.A.; Link, C.J., Jr.; Fingert, H.J.; Reed, E.; Kohn, E.C. Incapacitating autonomic neuropathy precipitated by taxol. Gynecol. Oncol. 1993, 51, 277–280. [Google Scholar] [CrossRef]
- Groopman, J.E.; Itri, L.M. Chemotherapy-induced anemia in adults: Incidence and treatment. J. Natl. Cancer Inst. 1999, 91, 1616–1634. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, S.E.; Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Gordon, B.; Pena, M.M. Effects of 5-fluorouracil chemotherapy on fatigue: Role of MCP-1. Brain Behav. Immun. 2013, 27, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Cella, D. Quality of life and clinical decisions in chemotherapy-induced anemia. Oncology 2006, 20 (Suppl. 6), 25–28. [Google Scholar]
- Chaumard, N.; Limat, S.; Villanueva, C.; Nerich, V.; Fagnoni, P.; Bazan, F.; Chaigneau, L.; Dobi, E.; Cals, L.; Pivot, X. Incidence and risk factors of anemia in patients with early breast cancer treated by adjuvant chemotherapy. Breast 2012, 21, 464–467. [Google Scholar] [CrossRef]
- Franchitto, N.; Despas, F.; Labrunee, M.; Roncalli, J.; Boveda, S.; Galinier, M.; Senard, J.M.; Pathak, A. Tonic chemoreflex activation contributes to increased sympathetic nerve activity in heart failure-related anemia. Hypertension 2010, 55, 1012–1017. [Google Scholar] [CrossRef] [Green Version]
- Sturgeon, K.; Schadler, K.; Muthukumaran, G.; Ding, D.; Bajulaiye, A.; Thomas, N.J.; Ferrari, V.; Ryeom, S.; Libonati, J.R. Concomitant low-dose doxorubicin treatment and exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R685–R692. [Google Scholar] [CrossRef] [Green Version]
- Floyd, J.D.; Nguyen, D.T.; Lobins, R.L.; Bashir, Q.; Doll, D.C.; Perry, M.C. Cardiotoxicity of cancer therapy. J. Clin. Oncol. 2005, 23, 7685–7696. [Google Scholar] [CrossRef]
- Slordal, L.; Spigset, O. Heart failure induced by non-cardiac drugs. Drug Saf. 2006, 29, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Gilbert, M.R.; McGuire, W.P.; Noe, D.A.; Grochow, L.B.; Forastiere, A.A.; Ettinger, D.S.; Lubejko, B.G.; Clark, B.; Sartorius, S.E.; et al. Sequences of taxol and cisplatin: A phase I and pharmacologic study. J. Clin. Oncol. 1991, 9, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, K.J.; Richel, D.J.; van den Brink, R.B.; Guchelaar, H.J. Cardiotoxicity of cytotoxic drugs. Cancer Treat. Rev. 2004, 30, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, A.Y.; Yeh, E.T. Therapy insight: Management of cardiovascular disease in patients with cancer and cardiac complications of cancer therapy. Nat. Clin. Pract. Oncol. 2008, 5, 655–667. [Google Scholar] [CrossRef]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef]
- Souza, C.A.; Simões, R.; Borges, K.B.G.; Oliveira, A.N.; Zogeib, J.B.; Alves, B.; Malachias, M.V.B.; Drummond-Lage, A.P.; Rezende, B.A. Arterial Stiffness Use for Early Monitoring of Cardiovascular Adverse Events due to Anthracycline Chemotherapy in Breast Cancer Patients. A Pilot Study. Arq. Bras. Cardiol. 2018, 111, 721–728. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, B.H. Aging and arterial stiffness. Circ. J. 2010, 74, 2257–2262. [Google Scholar] [CrossRef] [Green Version]
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [Green Version]
- Saif, M.W.; Shah, M.M.; Shah, A.R. Fluoropyrimidine-associated cardiotoxicity: Revisited. Expert Opin. Drug Saf. 2009, 8, 191–202. [Google Scholar] [CrossRef]
- Jurcut, R.; Wildiers, H.; Ganame, J.; D'Hooge, J.; Paridaens, R.; Voigt, J.U. Detection and monitoring of cardiotoxicity-what does modern cardiology offer? Support. Care Cancer 2008, 16, 437–445. [Google Scholar] [CrossRef]
- Jones, L.W.; Haykowsky, M.J.; Swartz, J.J.; Douglas, P.S.; Mackey, J.R. Early Breast Cancer Therapy and Cardiovascular Injury. J. Am. Coll. Cardiol. 2007, 50, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Choe, Y.-R.; Song, M.-K.; Choi, I.-S.; Han, J.-Y. Relationship Between Post-exercise Heart Rate Recovery and Changing Ratio of Cardiopulmonary Exercise Capacity. Ann. Rehabil. Med. 2017, 41, 1039–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.D.; Dewland, T.A.; Wencker, D.; Katz, S.D. Post-exercise heart rate recovery independently predicts mortality risk in patients with chronic heart failure. J. Card. Fail. 2009, 15, 850–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olshansky, B.; Sabbah, H.N.; Hauptman, P.J.; Colucci, W.S. Parasympathetic nervous system and heart failure: Pathophysiology and potential implications for therapy. Circulation 2008, 118, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Baldini, E.; Prochilo, T.; Salvadori, B.; Bolognesi, A.; Aldrighetti, D.; Venturini, M.; Rosso, R.; Carnino, F.; Gallo, L.; Giannessi, P.; et al. Multicenter randomized phase III trial of Epirubicin plus Paclitaxel vs Epirubicin followed by Paclitaxel in metastatic breast cancer patients: Focus on cardiac safety. Br. J. Cancer 2004, 91, 45–49. [Google Scholar] [CrossRef]
- Singal, P.K.; Iliskovic, N. Doxorubicin-Induced Cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Mustacchi, G.; De Laurentiis, M. The role of taxanes in triple-negative breast cancer: Literature review. Drug Des. Dev. Ther. 2015, 9, 4303–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| CAF (n = 15) | AC-T (n = 19) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Age (y) | 45 ± 2 | - | 42 ± 2 | - |
| Height (cm) | 162 ± 6 | - | 159 ± 7 | - |
| Weight (kg) | 56 ± 9 | 59 ± 10 | 53 ± 7 | 57 ± 7 |
| RHR (bpm) | 72 ± 7 | 77 ± 6 * | 76 ± 6 | 87 ± 11 *# |
| BMI (kg/m2) | 21.3 ± 3.3 | 22.5 ± 3.3 | 21.0 ± 2.2 | 22.6 ± 2.3 |
| Body fat (%) | 30.5 ± 5.2 | 31.4 ± 5.4 | 28.1 ± 4.1 | 28.6 ± 3.9 |
| Muscle mass (%) | 25.3 ± 2.5 | 25.1 ± 2.4 | 26.2 ± 2.0 | 26.5 ± 2.9 |
| Waist (cm) | 75.7 ± 5.9 | 79.5 ± 5.1 | 73.3 ± 5.8 | 77.6 ± 6.3 |
| Hip (cm) | 92.0 ± 1.5 | 95.1 ± 1.4 | 91.2 ± 1.0 | 94.8 ± 1.0 |
| Waist-to-hip ratio (WHR) | 0.82 ± 0.04 | 0.83 ± 0.03 | 0.80 ± 0.05 | 0.82 ± 0.06 |
| bSBP (mmHg) | 115 ± 3 | 113 ± 4 | 113 ± 4 | 112 ± 4 |
| bDBP (mmHg) | 89 ± 2 | 88 ± 3 | 91 ± 4 | 88 ± 3 |
| Hemoglobin (g/dL) | 12.79 ± 0.43 | 11.79 ± 0.28 * | 12.77 ± 0.39 | 11.80 ± 0.17 * |
| BUN (mg/dL) | 10.64 ± 0.51 | - | 9.89 ± 0.63 | - |
| Creatinine (mg/dL) | 0.76 ± 0.02 | - | 0.79 ± 0.03 | - |
| eGFR (mL/min/1.732) | 88.27 ± 3.03 | - | 86.89 ± 3.87 | - |
| AST (U/L) | 18.21 ± 1.09 | - | 19.78 ± 1.60 | - |
| ALT (U/L) | 16.27 ± 2.77 | - | 17.72 ± 2.92 | - |
| CAF (n = 15) | AC-T (n = 19) | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| cSBP (mmHg) | 109 ± 3 | 107 ± 5 | 109 ± 5 | 105 ± 4 |
| cDBP (mmHg) | 65 ± 4 | 72 ± 2 | 77 ± 4 | 76 ± 3 |
| cPP (mmHg) | 34 ± 3 | 32 ± 3 | 32 ± 2 | 29 ± 2 |
| PWV (m/s) | 6.8 ± 0.4 | 7.4 ± 0.5 | 7.2 ± 0.5 | 6.4 ± 0.8 |
| AI (%) | 33 ± 5 | 34 ± 5 | 29 ± 4 | 30 ± 2 |
| Augmented pressure (mmHg) | 10 ± 1 | 8 ± 2 | 9 ± 1 | 7 ± 1 |
| Forward pressure (mmHg) | 21 ± 1 | 20 ± 2 | 22 ± 1 | 20 ± 1 |
| Backward pressure (mmHg) | 12 ± 1 | 11 ± 1 | 11 ± 1 | 9 ± 1 |
| Zc (dyne/s/cm5) | 94 ± 9 | 92 ± 11 | 109 ± 7 | 98 ± 8 |
| SEVR | 1.20 ± 0.06 | 1.11 ± 0.07 | 1.14 ± 0.06 | 0.94 ± 0.04 *# |
| Systolic time (ms) | 363.9 ± 9.3 | 370.1 ± 10.9 | 375.7 ± 18.6 | 351.1 ± 6.6 |
| Diastolic time (ms) | 502.4 ± 21.8 | 471.4 ± 23.1 * | 501.8 ± 39.8 | 379.7 ± 18.3 *# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-F.; Tseng, C.-Y.; Mündel, T.; Lin, Y.-Y.; Lin, C.-C.; Chen, C.-N.; Liao, Y.-H. Perturbations of Adjuvant Chemotherapy on Cardiovascular Responses and Exercise Tolerance in Patients with Early-Stage Breast Cancer. Biology 2021, 10, 910. https://doi.org/10.3390/biology10090910
Lin H-F, Tseng C-Y, Mündel T, Lin Y-Y, Lin C-C, Chen C-N, Liao Y-H. Perturbations of Adjuvant Chemotherapy on Cardiovascular Responses and Exercise Tolerance in Patients with Early-Stage Breast Cancer. Biology. 2021; 10(9):910. https://doi.org/10.3390/biology10090910
Chicago/Turabian StyleLin, Hsin-Fu, Ching-Ying Tseng, Toby Mündel, Yi-Yuan Lin, Chung-Chi Lin, Chiao-Nan Chen, and Yi-Hung Liao. 2021. "Perturbations of Adjuvant Chemotherapy on Cardiovascular Responses and Exercise Tolerance in Patients with Early-Stage Breast Cancer" Biology 10, no. 9: 910. https://doi.org/10.3390/biology10090910







