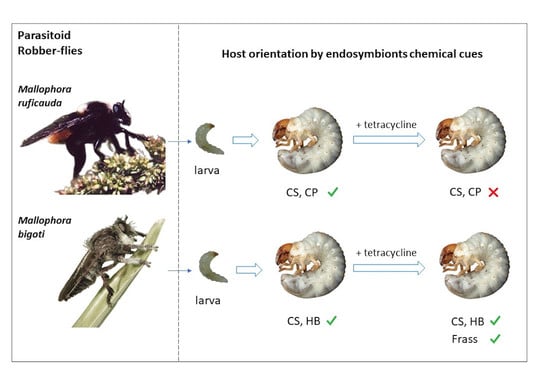
Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Experimental Procedures
Host Orientation
2.3. Symbiont Based Cues and Host Specificity during Host Orientation
2.4. Interspecific Competence during Host Orientation
2.5. Statistical Analyses
3. Results
3.1. Symbiont Based Cues and Host Specificity during Host Orientation
3.2. Interspecific Competence during Host Orientation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hosokawa, T.; Fukatsu, T. Relevance of Microbial Symbiosis to Insect Behavior. Curr. Opin. Insect Sci. 2020, 39, 91–100. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.-W.; Zhang, H.-Y.; Marshall, S.; Jackson, T.A. The Scarab Gut: A Potential Bioreactor for Bio-Fuel Production. Insect Sci. 2010, 17, 175–183. [Google Scholar] [CrossRef]
- Johnson, S.N.; Rasmann, S. Root-Feeding Insects and Their Interactions with Organisms in the Rhizosphere. Annu. Rev. Entomol. 2015, 60, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Cortés, N.; Quesada, M.; Watanabe, H.; Cano-Camacho, H.; Oyama, K. Endogenous Plant Cell Wall Digestion: A Key Mechanism in Insect Evolution. Annu. Rev. Ecol. Evol. Syst. 2012, 41, 45–71. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects-Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. The Microbial Dimension in Insect Nutritional Ecology. Funct. Ecol. 2009, 23, 38–47. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The Gut Bacteria of Insects: Nonpathogenic Interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Mazorra-Alonso, M.; Tomás, G.; Soler, J.J. Microbially mediated chemical ecology of animals: A review of its role in conspecific communication, parasitism and predation. Biology 2021, 10, 274. [Google Scholar] [CrossRef]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Ezenwa, V.O.; Gerardo, N.M.; Inouye, D.W.; Medina, M.; Xavier, J.B. Animal Behavior and the Microbiome. Science 2012, 338, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Godfray, H.C.J. Parasitoids: Behavioral and Evolutionary Ecology; Princeton University Press: Princeton, NJ, USA, 1994; ISBN 0-691-00047-6. [Google Scholar]
- Vinson, S.B. Host Selection by Insect Parasitoids. Annu. Rev. Entomol. 1976, 21, 109–133. [Google Scholar] [CrossRef]
- Feener Jr, D.H.; Brown, B.V. Diptera as Parasitoids. Annu. Rev. Entomol. 1997, 42, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Colazza, S.; Wajnberg, E. Chemical Ecology of Insect Parasitoids: Towards a New Era. Chem. Ecol. Insect Parasit. 2013, 1–8. [Google Scholar]
- Dicke, M.; Cusumano, A.; Poelman, E.H. Microbial Symbionts of Parasitoids. Annu. Rev. Entomol. 2020, 65, 171–190. [Google Scholar] [CrossRef]
- Rogers, M.E.; Potter, D.A. Kairomones from Scarabaeid Grubs and Their Frass as Cues in Below-Ground Host Location by the Parasitoids Tiphia Vernalis and Tiphia Pygidialis. Entomol. Exp. Et Appl. 2002, 102, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Villani, M.G.; Allee, L.L.; Díaz, A.; Robbins, P.S. Adaptive Strategies of Edaphic Arthropods. Annu. Rev. Entomol. 1999, 44, 233–256. [Google Scholar] [CrossRef]
- Jackson, T.A.; Klein, M.G. Scarabs as Pests: A Continuing Problem. Coleopt. Bull. 2006, 60, 102–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Ali, M.W.; Peng, T.; Peng, W.; Raza, M.F.; Zhao, Y.; Zhang, H. Cultivable Anaerobic and Aerobic Bacterial Communities in the Fermentation Chambers of Holotrichia Parallela (Coleoptera: Scarabaeidae) Larvae. PLoS ONE 2018, 13, e0190663. [Google Scholar] [CrossRef]
- Hull, F.M. Robber Flies of the World: The Genera of the Family Asilidae (United States National Museum. Bulletin 224 Parts 1 & 2). Two Volumes; U.S. Government Printing Office: Washington, DC, USA, 1962.
- Musso, J.-J. Nutritive and Ecological Requirements of Robber Flies (Diptera: Brachycera: Asilidae). Entomol. Gen. 1983, 9, 35–50. [Google Scholar] [CrossRef]
- Crespo, J.E.; Martínez, G.A.; Castelo, M.K. Exposure to Competitors Influences Parasitism Decisions in Ectoparasitoid Fly Larvae. Anim. Behav. 2015, 100, 38–43. [Google Scholar] [CrossRef]
- Potter, D.A. Destructive Turfgrass Insects: Biology, Diagnosis, and Control, 1st ed.; Wiley: Chelsea, MI, USA, 1998; ISBN 978-1-57504-023-3. [Google Scholar]
- Groba, H.F.; Castelo, M.K. Chemical Interaction between the Larva of a Dipteran Parasitoid and Its Coleopteran Host: A Case of Exploitation of the Communication System during the Searching Behaviour? Bull. Entomol. Res. 2012, 102, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Groba, H.F.; Castelo, M.K. Host Gut Microorganisms’ Cues Mediate Orientation Behaviour in the Larva of the Parasitoid Mallophora Ruficauda. Bull. Entomol. Res. 2016, 106, 81–90. [Google Scholar] [CrossRef]
- Lucía, M.; Abrahamovich, A.; Diaz, N. Artrópodos Asociados a Colmenas de Apis Mellifera Del Partido de Mercedes (Bs. As.), Argentina. Cienc. Abejas 2006, 9, 8–13. [Google Scholar]
- Rabinovich, M.; Corley, J. An Important New Predator of Honey Bees-the Robber Fly Mallophora Ruficauda Wiedemann (Diptera, Asilidae) in Argentina. Am. Bee J. 1997, 137, 303–306. [Google Scholar]
- MAGyP Síntesis Apícola 2020. Available online: http://www.alimentosargentinos.gob.ar/HomeAlimentos/Apicultura/documentos/Sintesis-Apicola-Julio2020.pdf (accessed on 20 October 2021).
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI: Wallingford, UK, 2000; ISBN 978-0-85199-448-2. [Google Scholar]
- Crespo, J.E.; Castelo, M.K. The Ontogeny of Host-Seeking Behaviour in a Parasitoid Dipteran. J. Insect Physiol. 2008, 54, 842–847. [Google Scholar] [CrossRef]
- Alvarado, L. Sistemática Bionomía Coleópteros Estados Inmaduros Viven Suelo; Universidad Nacional de La Plata: La Plata, Argentina, 1980. [Google Scholar]
- Crespo, J.E.; Castelo, M.K. Barometric Pressure Influences Host-Orientation Behavior in the Larva of a Dipteran Ectoparasitoid. J. Insect Physiol. 2012, 58, 1562–1567. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; The European Environment Agency: Vienna, Austria, 2020. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. GlmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, J.; Bates, D.; Debroy, S.; Sarkar, D.; R Core, T. Nlme: Linear and Nonlinear Mixed Effects Models; 2021. [Google Scholar]
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; 2021. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Use R! Springer International Publishing: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Lenth, R.; Singmann, H.; Love, J.; Buerkner, P.; Herve, M. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; 2020. [Google Scholar]
- Chopra, I.; Roberts, M. Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance. Microbiol. Mol Biol Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egert, M.; Stingl, U.; Bruun, L.D.; Pommerenke, B.; Brune, A.; Friedrich, M.W. Structure and Topology of Microbial Communities in the Major Gut Compartments of Melolontha Melolontha Larvae (Coleoptera: Scarabaeidae). Appl. Environ. Microbiol. 2005, 71, 4556–4566. [Google Scholar] [CrossRef] [Green Version]
- Castelo, M.K.; Crespo, J.E. Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids-Supl. FigShare, 2022; 11, 129. [Google Scholar] [CrossRef]



| Parasitoid Species | Stimulus | Host Species | Host Treatment | N (Host orientation) |
|---|---|---|---|---|
| MR | empty | 62 | ||
| Alive host | CM | untreated | 30 | |
| tetracycline | 26 | |||
| CP | untreated | 25 | ||
| tetracycline | 30 | |||
| CS | untreated | 29 | ||
| tetracycline | 29 | |||
| MB | empty | 34 | ||
| Alive host | CS | untreated | 34 | |
| tetracycline | 24 | |||
| HB | untreated | 23 | ||
| tetracycline | 32 | |||
| PB | untreated | 23 | ||
| tetracycline | 24 | |||
| Frass | CS | untreated | 33 | |
| HB | untreated | 20 | ||
| Searching Parasitoid Species | Host Status | N |
|---|---|---|
| MR | healthy | 26 |
| parasitized with MR | 24 | |
| parasitized with MB | 25 | |
| MB | healthy | 29 |
| parasitized with MR | 29 | |
| parasitized with MB | 29 |
| Mallophora ruficauda | |||||
|---|---|---|---|---|---|
| Random Effects | Variance | Fixed Effects | Chisq | df | Pr (>Chisq) |
| olfactometer | 9.55−2 | host species | 2.3701 | 2 | 0.3057 |
| egg-cluster | 1.75−1 | host treatment | 4.3804 | 1 | 0.0364 |
| year | 7.42−10 | host species × host treatment | 4.1314 | 2 | 0.1267 |
| Mallophora bigoti | |||||
| olfactometer | 9.86−9 | host species | 2.3117 | 1 | 0.1284 |
| egg-cluster | 4.02−2 | host treatment | 5.6645 | 2 | 0.0589 |
| host species × host treatment | 0.6215 | 2 | 0.7329 | ||
| Mallophora ruficauda | ||||||||
| Host Species | Treatment with Tetracycline | Probability | Std. Error | df | LCI | UCI | p | OR (LCI;UCI) |
| CM | no | 0.534 | 0.112 | 161 | 0.321 | 0.730 | 0.7613 | 1.06 (0.298;3.78) |
| yes | 0.519 | 0.123 | 0.294 | 0.737 | 0.8741 | |||
| CP | no | 0.827 | 0.095 | 0.563 | 0.947 | 0.0196 | 4.33 (0.847;22.13) | |
| yes | 0.525 | 0.111 | 0.317 | 0.725 | 0.8214 | |||
| CS | no | 0.871 | 0.067 | 0.676 | 0.956 | 0.0016 | 6.62 (1.536;28.48) | |
| yes | 0.505 | 0.116 | 0.290 | 0.719 | 0.9639 | |||
| Mallophora bigoti | ||||||||
| Host Species | Treatment with Tetracycline | Probability | Std. Error | df | LCI | UCI | p | OR (LCI;UCI) |
| CS | no | 0.853 | 0.061 | 153 | 0.690 | 0.938 | <0.001 | 1.53 (0.384;6.672) |
| yes | 0.792 | 0.083 | 0.585 | 0911 | 0.009 | |||
| HB | no | 0.870 | 0.070 | 0.662 | 0.958 | 0.003 | 2.22 (0.513;9.627) | |
| yes | 0.750 | 0.077 | 0.573 | 0.870 | 0.008 | |||
| PB | no | 0.609 | 0.102 | 0.401 | 0.783 | 0.303 | 0.78 (0.234;2.593) | |
| yes | 0.667 | 0.096 | 0.460 | 0.825 | 0.112 | |||
| Species Parasitizing Host | Species Orienting to Host | Probability | Std. Error | df | LCI | UCI | p | OR (LCI;UCI) |
|---|---|---|---|---|---|---|---|---|
| M. ruficauda | M. bigoti | 0.759 | 0.080 | 166 | 0.572 | 0.881 | 0.0091 | 0.42 (0.11;1.63) |
| M. ruficauda | 0.882 | 0.055 | 0.724 | 0.955 | 0.0002 | |||
| M. bigoti | M. bigoti | 0.793 | 0.075 | 0.608 | 0.905 | 0.0039 | 0.96 (0.25;3.66) | |
| M. ruficauda | 0.800 | 0.080 | 0.598 | 0.915 | 0.0062 | |||
| None | M. bigoti | 0.828 | 0.070 | 0.645 | 0.927 | 0.0017 | 1.14 (0.29;4.55) | |
| M. ruficauda | 0.808 | 0.077 | 0.611 | 0.918 | 0.0044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castelo, M.K.; Crespo, J.E. Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids. Biology 2022, 11, 129. https://doi.org/10.3390/biology11010129
Castelo MK, Crespo JE. Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids. Biology. 2022; 11(1):129. https://doi.org/10.3390/biology11010129
Chicago/Turabian StyleCastelo, Marcela K., and José E. Crespo. 2022. "Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids" Biology 11, no. 1: 129. https://doi.org/10.3390/biology11010129
APA StyleCastelo, M. K., & Crespo, J. E. (2022). Microorganismal Cues Involved in Host-Location in Asilidae Parasitoids. Biology, 11(1), 129. https://doi.org/10.3390/biology11010129