Functionally Antagonistic Transcription Factors IRF1 and IRF2 Regulate the Transcription of the Dopamine Receptor D2 Gene Associated with Aggressive Behavior of Weaned Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Aggressive Behavior Analysis
2.2. Potential SNP Identified
2.3. Bioinformatics Analysis
2.4. Plasmid Construction
2.5. Cell Culture, Plasmid Transfection, and Luciferase Reporter Assay
2.6. RNA Isolation and RT-qPCR
2.7. Western Blotting
2.8. Chromatin Immunoprecipitation (ChIP) Assay
2.9. Immunofluorescence Assay
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
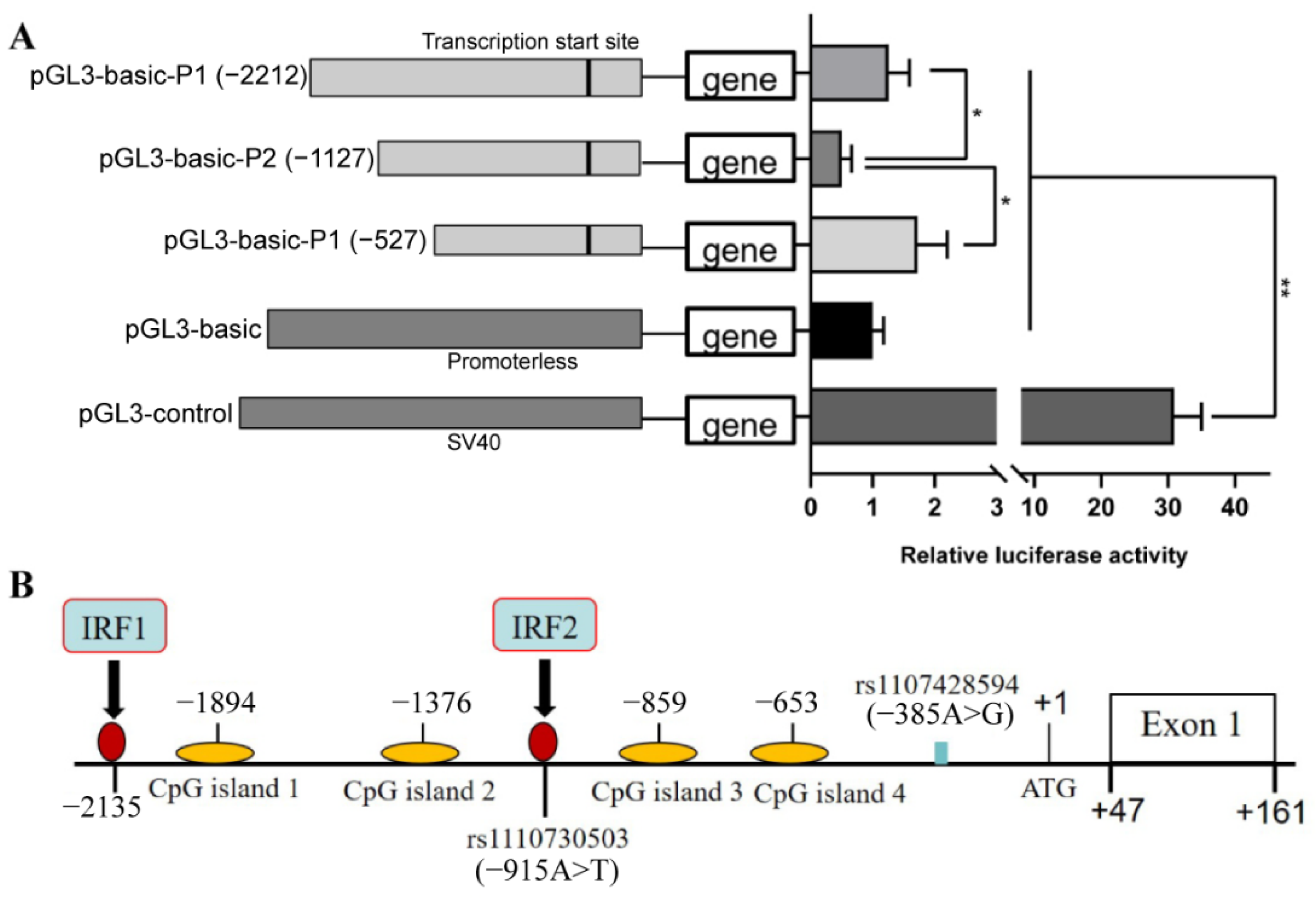
3.1. Identification of the Core Promoter Region of the Porcine DRD2 Gene
3.2. Identification of Functional SNPs Related to Aggressive Behavior in the Upstream Region of Porcine DRD2 Gene
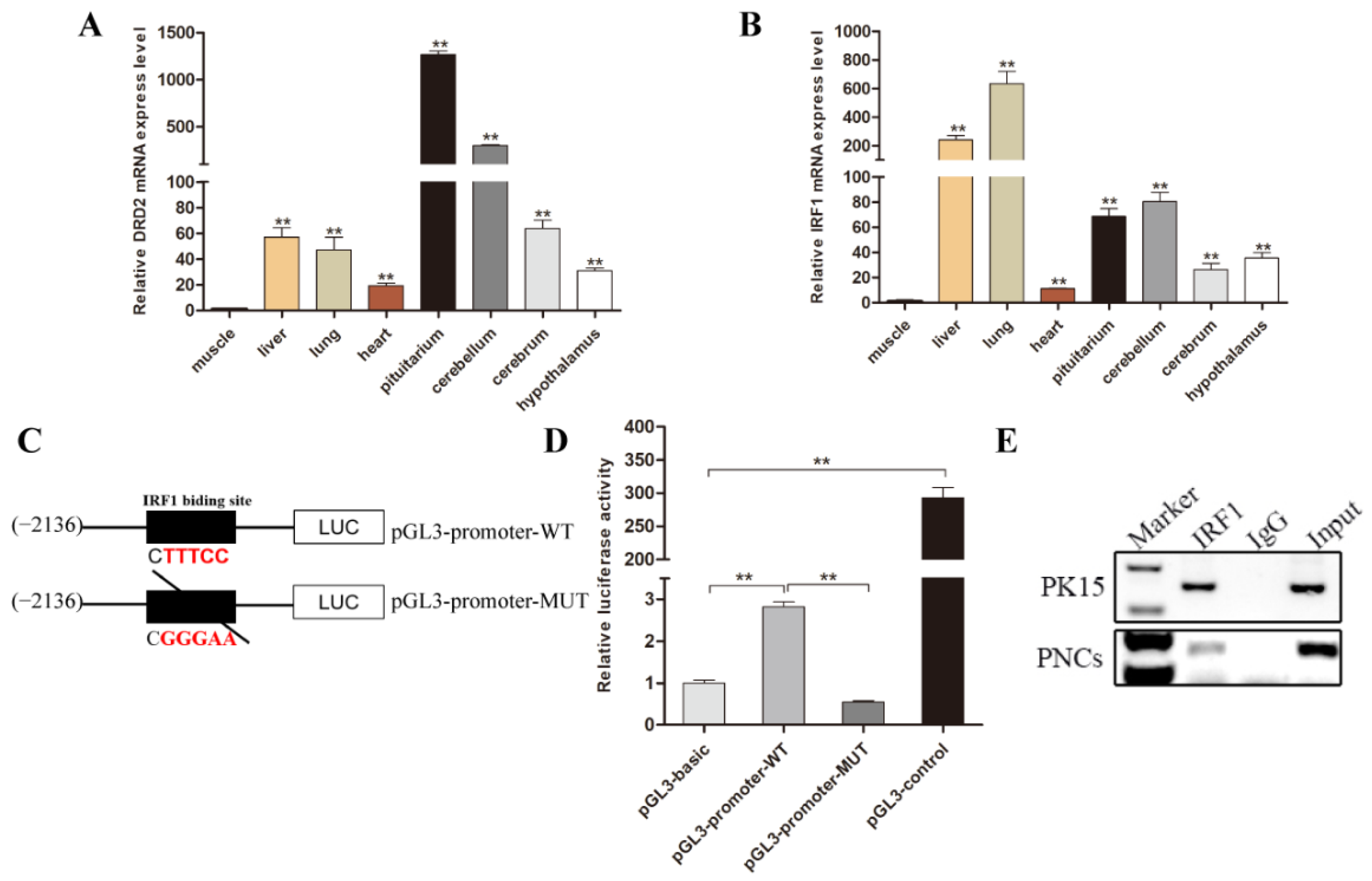
3.3. Transcription Factor IRF1 Binds to the DRD2 Promoter
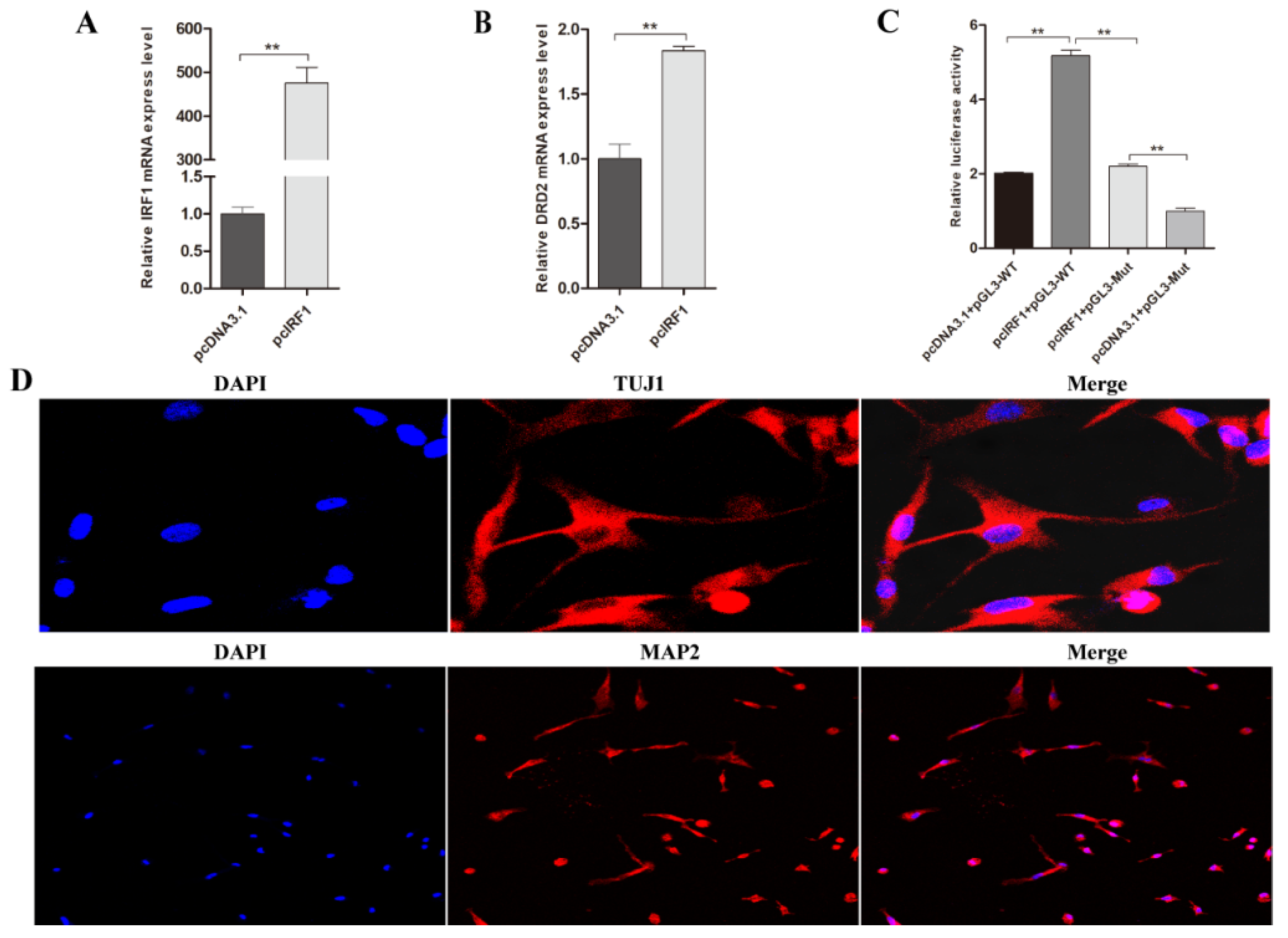
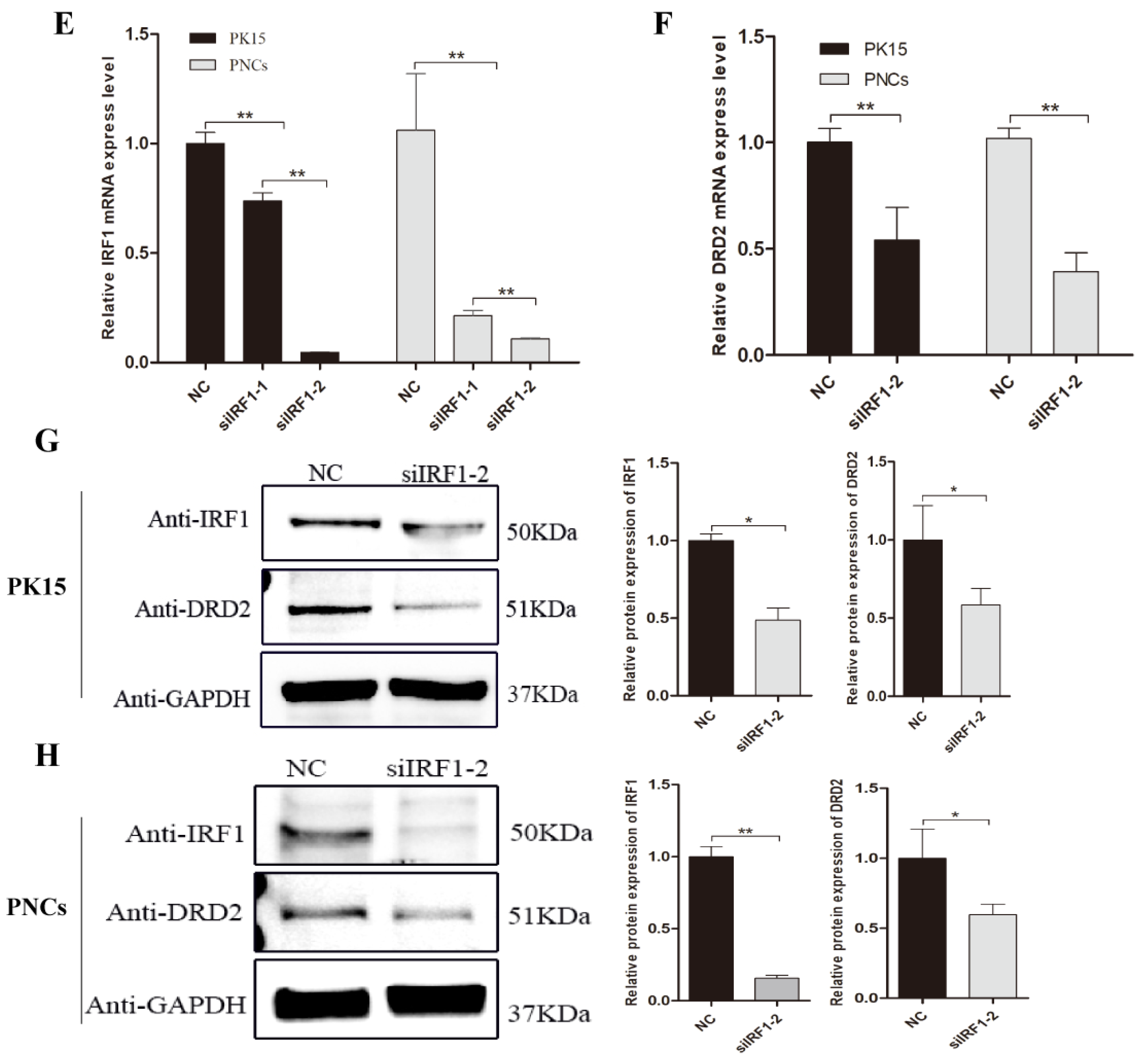
3.4. Transcription Factor IRF1 Upregulated the Expression Level of Porcine DRD2 Gene by Binding to Its Promoter Region
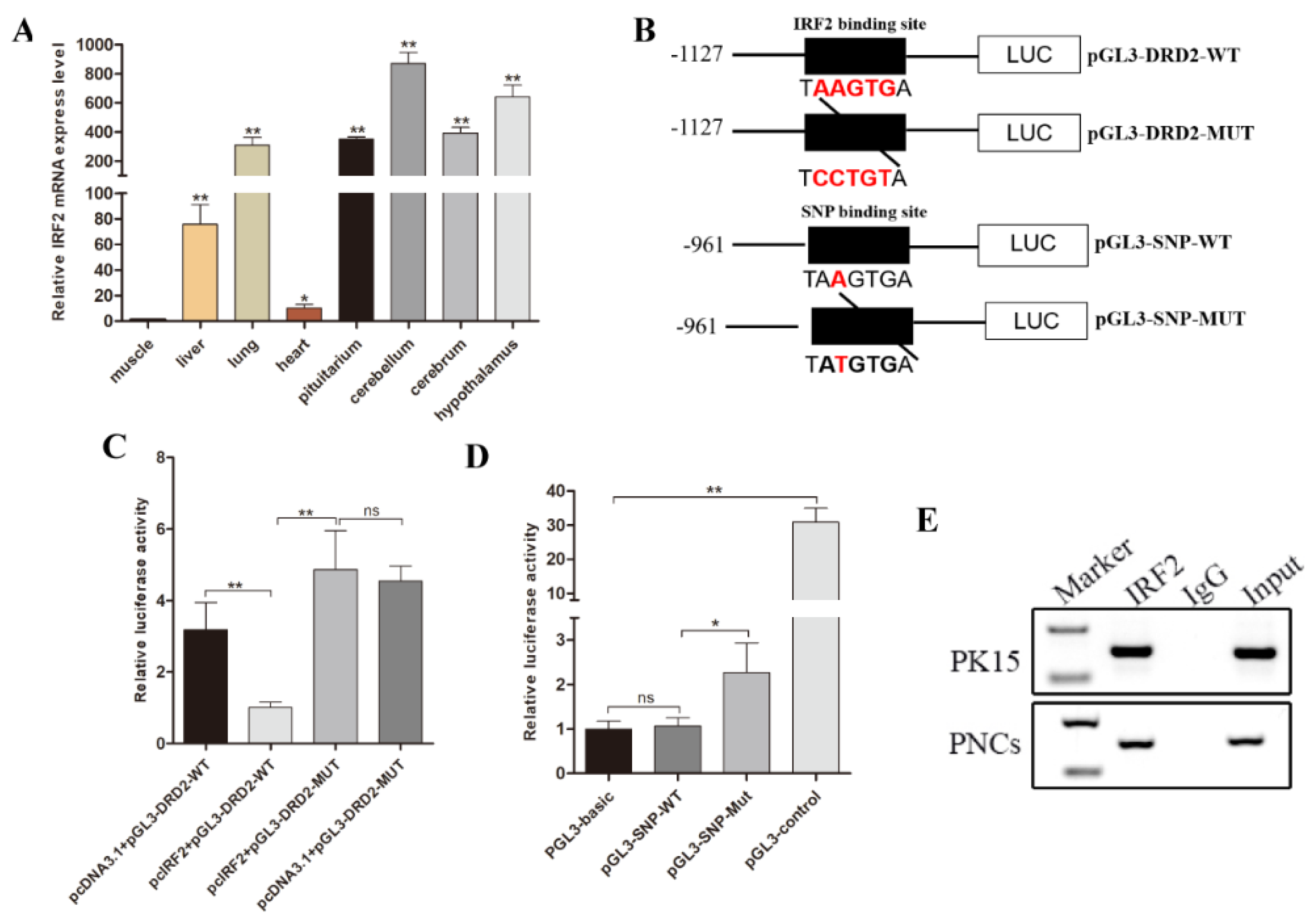
3.5. Transcription Factor IRF2 Downregulated the Expression Level of Luciferase Gene by Binding to the Promoter Region
3.6. IRF2 as a Transcription Repressor Downregulated the Expression Level of Porcine DRD2 Gene
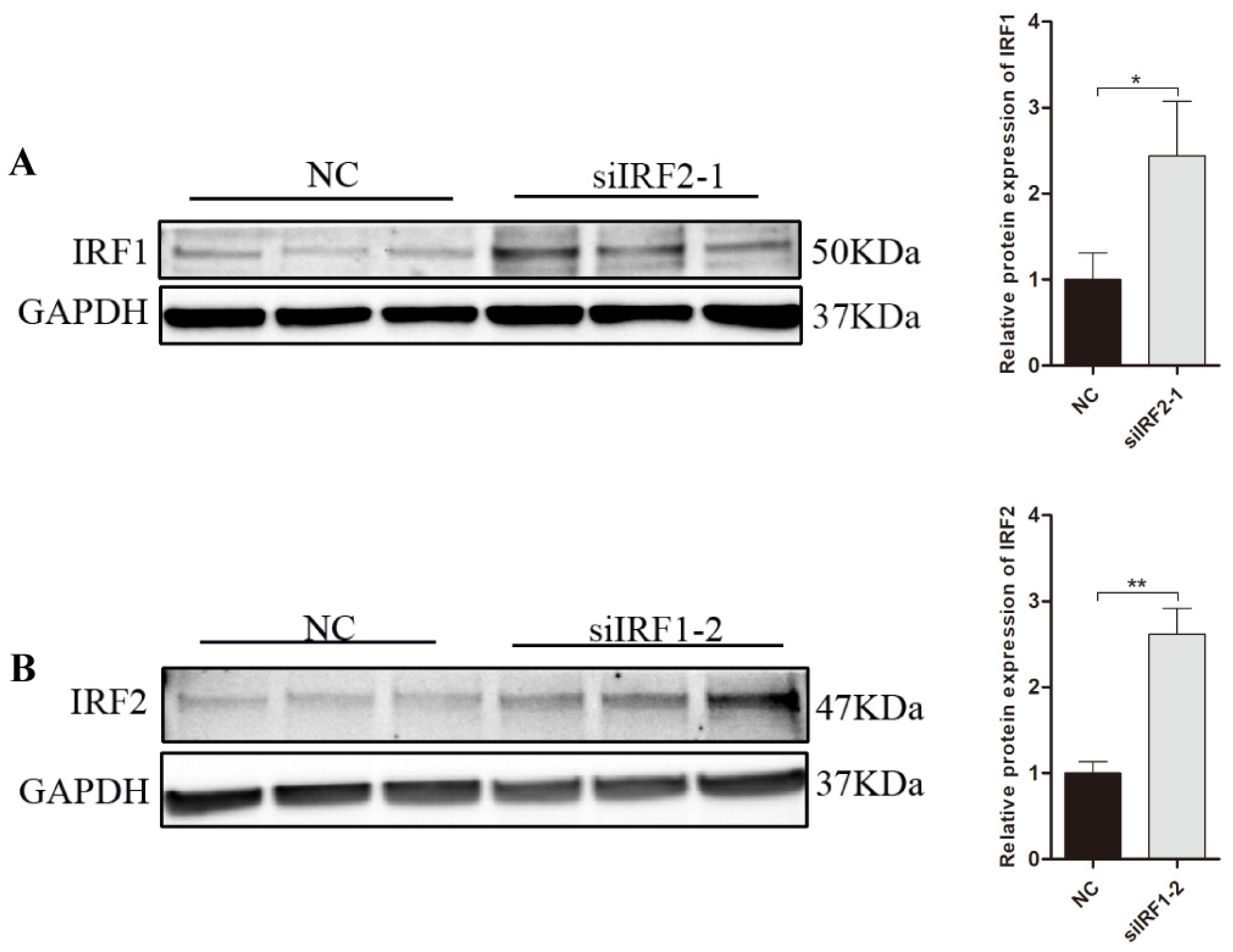
3.7. Transcription Factor IRF1 and IRF2 Are Functionally Antagonistic to Each Other in PNCs
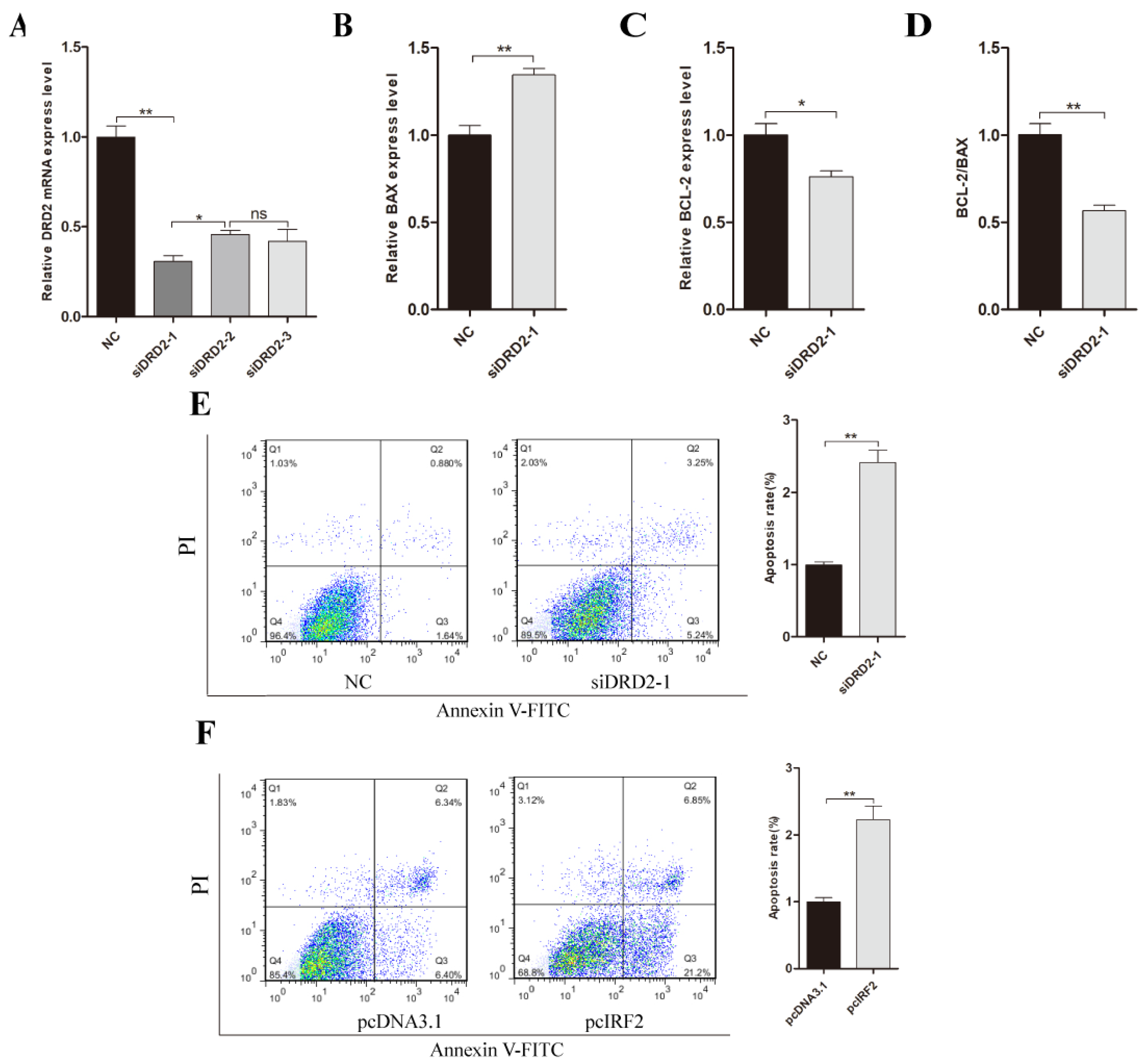
3.8. Effect of DRD2 Gene on Apoptosis of Porcine Neuroglial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smulders, D.; Verbeke, G.; Mormede, P.; Geers, R. Validation of a behavioral observation tool to assess pig welfare. Physiol. Behav. 2006, 89, 438–447. [Google Scholar] [CrossRef]
- Velie, B.D.; Maltecca, C.; Cassady, J.P. Genetic relationships among pig behavior, growth, backfat, and loin muscle area. J. Anim. Sci. 2009, 87, 2767–2773. [Google Scholar] [CrossRef] [Green Version]
- Canario, L.; Turner, S.P.; Roehe, R.; Lundeheim, N.; D’Eath, R.B.; Lawrence, A.B.; Knol, E.; Bergsma, R.; Rydhmer, L. Genetic associations between behavioral traits and direct-social effects of growth rate in pigs. J. Anim. Sci. 2012, 90, 4706–4715. [Google Scholar] [CrossRef] [Green Version]
- Rhim, S.J.; Son, S.H.; Hwang, H.S.; Lee, J.K.; Hong, J.K. Effects of Mixing on the Aggressive Behavior of Commercially Housed Pigs. Asian-Australas J. Anim. Sci. 2015, 28, 1038–1043. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, G.A.; Brown-Brandl, T.; Rempel, L.A.; Schneider, J.F.; Holl, J. Genetic analysis of behavior traits in swine production. Livest. Sci. 2013, 157, 28–37. [Google Scholar] [CrossRef]
- Murani, E.; Ponsuksili, S.; D’Eath, R.B.; Turner, S.P.; Kurt, E.; Evans, G.; Tholking, L.; Klont, R.; Foury, A.; Mormede, P.; et al. Association of HPA axis-related genetic variation with stress reactivity and aggressive behaviour in pigs. BMC Genet. 2010, 11, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Choi, G.H.; Ko, K.H.; Kim, J.O.; Oh, S.H.; Park, Y.S.; Kim, O.J.; Kim, N.K. Association of the Single Nucleotide Polymorphisms in microRNAs 130b, 200b, and 495 with Ischemic Stroke Susceptibility and Post-Stroke Mortality. PLoS ONE 2016, 11, e0162519. [Google Scholar] [CrossRef]
- Huang, Q.; Whitington, T.; Gao, P.; Lindberg, J.F.; Yang, Y.; Sun, J.; Vaisanen, M.R.; Szulkin, R.; Annala, M.; Yan, J.; et al. A prostate cancer susceptibility allele at 6q22 increases RFX6 expression by modulating HOXB13 chromatin binding. Nat. Genet. 2014, 46, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Cowper-Sal, R.; Zhang, X.; Wright, J.B.; Bailey, S.D.; Cole, M.D.; Eeckhoute, J.; Moore, J.H.; Lupien, M. Breast cancer risk-associated SNPs modulate the affinity of chromatin for FOXA1 and alter gene expression. Nat. Genet. 2012, 44, 1191–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schodel, J.; Bardella, C.; Sciesielski, L.K.; Brown, J.M.; Pugh, C.W.; Buckle, V.; Tomlinson, I.P.; Ratcliffe, P.J.; Mole, D.R. Common genetic variants at the 11q13.3 renal cancer susceptibility locus influence binding of HIF to an enhancer of cyclin D1 expression. Nat. Genet. 2012, 44, 420–425. [Google Scholar] [CrossRef]
- Funke, B.; Malhotra, A.K.; Finn, C.T.; Plocik, A.M.; Lake, S.L.; Lencz, T.; DeRosse, P.; Kane, J.M.; Kucherlapati, R. COMT genetic variation confers risk for psychotic and affective disorders: A case control study. Behav. Brain Funct. 2005, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Getting formal with dopamine and reward. Neuron 2002, 36, 241–263. [Google Scholar] [CrossRef] [Green Version]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, K.A.; Seamans, J.K.; Trantham-Davidson, H. Dopamine receptor signaling. J. Recept. Signal Transduct. 2004, 24, 165–205. [Google Scholar] [CrossRef]
- Pavlov, K.A.; Chistiakov, D.A.; Chekhonin, V.P. Genetic determinants of aggression and impulsivity in humans. J. Appl. Genet. 2012, 53, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Babenko, V.N.; Galyamina, A.G.; Rogozin, I.B.; Smagin, D.A.; Kudryavtseva, N.N. Dopamine response gene pathways in dorsal striatum MSNs from a gene expression viewpoint: cAMP-mediated gene networks. BMC Neurosci. 2020, 21, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Yang, J.M.; Hu, T.T.; Xu, T.J.; Xu, W.P.; Wei, W. Elevated dopamine D2 receptor in prefrontal cortex of CUMS rats is associated with downregulated cAMP-independent signaling pathway. Can. J. Physiol. Pharmacol. 2013, 91, 750–758. [Google Scholar] [CrossRef]
- Fatima, M.; Ahmad, M.H.; Srivastav, S.; Rizvi, M.A.; Mondal, A.C. A selective D2 dopamine receptor agonist alleviates depression through up-regulation of tyrosine hydroxylase and increased neurogenesis in hippocampus of the prenatally stressed rats. Neurochem. Int. 2020, 136, 104730. [Google Scholar] [CrossRef]
- Amar, S.; Shaltiel, G.; Mann, L.; Shamir, A.; Dean, B.; Scarr, E.; Bersudsky, Y.; Belmaker, R.H.; Agam, G. Possible involvement of post-dopamine D2 receptor signalling components in the pathophysiology of schizophrenia. Int. J. Neuropsychopharmacol. 2008, 11, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Colzato, L.S.; van den Wildenberg, W.P.; Van der Does, A.J.; Hommel, B. Genetic markers of striatal dopamine predict individual differences in dysfunctional, but not functional impulsivity. Neuroscience 2010, 170, 782–788. [Google Scholar] [CrossRef]
- Hopkins, B.L.; Neumann, C.A. Redoxins as gatekeepers of the transcriptional oxidative stress response. Redox Biol. 2019, 21, 101104. [Google Scholar] [CrossRef]
- Kamanu, F.K.; Medvedeva, Y.A.; Schaefer, U.; Jankovic, B.R.; Archer, J.A.; Bajic, V.B. Mutations and binding sites of human transcription factors. Front. Genet. 2012, 3, 100. [Google Scholar] [CrossRef] [Green Version]
- Mamane, Y.; Heylbroeck, C.; Genin, P.; Algarte, M.; Servant, M.J.; LePage, C.; DeLuca, C.; Kwon, H.; Lin, R.; Hiscott, J. Interferon regulatory factors: The next generation. Gene 1999, 237, 1–14. [Google Scholar] [CrossRef]
- Ren, G.; Cui, K.; Zhang, Z.; Zhao, K. Division of labor between IRF1 and IRF2 in regulating different stages of transcriptional activation in cellular antiviral activities. Cell Biosci. 2015, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Choo, A.; Palladinetti, P.; Passioura, T.; Shen, S.; Lock, R.; Symonds, G.; Dolnikov, A. The role of IRF1 and IRF2 transcription factors in leukaemogenesis. Curr. Gene Ther. 2006, 6, 543–550. [Google Scholar] [CrossRef]
- Taniguchi, T.; Ogasawara, K.; Takaoka, A.; Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 2001, 19, 623–655. [Google Scholar] [CrossRef] [PubMed]
- Treadway, M.T.; Cooper, J.A.; Miller, A.H. Can’t or Won’t? Immunometabolic Constraints on Dopaminergic Drive. Trends Cogn. Sci. 2019, 23, 435–448. [Google Scholar] [CrossRef]
- Momtazmanesh, S.; Zare-Shahabadi, A.; Rezaei, N. Cytokine Alterations in Schizophrenia: An Updated Review. Front. Psychiatry 2019, 10, 892. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.Y.; Tong, X.; Chen, R.N.; Gao, S.Y.; Liu, X.P.; Schinckel, A.P.; Li, Y.; Xu, F.L.; Zhou, B. Identifying blood-based biomarkers associated with aggression in weaned pigs after mixing. Appl. Anim. Behav. Sci. 2020, 224, 104927. [Google Scholar] [CrossRef]
- Knudsen, S. Promoter2.0: For the recognition of PolII promoter sequences. Bioinformatics 1999, 15, 356–361. [Google Scholar] [CrossRef]
- Reese, M.G. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput. Chem. 2001, 26, 51–56. [Google Scholar] [CrossRef]
- Farre, D.; Roset, R.; Huerta, M.; Adsuara, J.E.; Rosello, L.; Alba, M.M.; Messeguer, X. Identification of patterns in biological sequences at the ALGGEN server: PROMO and MALGEN. Nucleic Acids Res. 2003, 31, 3651–3653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messeguer, X.; Escudero, R.; Farre, D.; Nunez, O.; Martinez, J.; Alba, M.M. PROMO: Detection of known transcription regulatory elements using species-tailored searches. Bioinformatics 2002, 18, 333–334. [Google Scholar] [CrossRef]
- Li, L.C.; Dahiya, R. MethPrimer: Designing primers for methylation PCRs. Bioinformatics 2002, 18, 1427–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stojanovic, T.; Orlova, M.; Sialana, F.J.; Hoger, H.; Stuchlik, S.; Milenkovic, I.; Aradska, J.; Lubec, G. Validation of dopamine receptor DRD1 and DRD2 antibodies using receptor deficient mice. Amino Acids 2017, 49, 1101–1109. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Li, Z.; Zheng, M.; Abdalla, B.A.; Zhang, Z.; Xu, Z.; Ye, Q.; Xu, H.; Luo, W.; Nie, Q.; Zhang, X. Genome-wide association study of aggressive behaviour in chicken. Sci. Rep. 2016, 6, 30981. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef]
- Bidwell, L.C.; Karoly, H.C.; Thayer, R.E.; Claus, E.D.; Bryan, A.D.; Weiland, B.J.; YorkWilliams, S.; Hutchison, K.E. DRD2 promoter methylation and measures of alcohol reward: Functional activation of reward circuits and clinical severity. Addict. Biol. 2019, 24, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Boyle, M.P.; Dollar, P.; Lee, H.; Lein, E.S.; Perona, P.; Anderson, D.J. Functional identification of an aggression locus in the mouse hypothalamus. Nature 2011, 470, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Nelson, R.J.; Trainor, B.C. Neural mechanisms of aggression. Nat. Rev. Neurosci. 2007, 8, 536–546. [Google Scholar] [CrossRef]
- Takahashi, A.; Shimamoto, A.; Boyson, C.O.; DeBold, J.F.; Miczek, K.A. GABA(B) receptor modulation of serotonin neurons in the dorsal raphe nucleus and escalation of aggression in mice. J. Neurosci. 2010, 30, 11771–11780. [Google Scholar] [CrossRef]
- Bergan, J.F. Neural Computation and Neuromodulation Underlying Social Behavior. Integr. Comp. Biol. 2015, 55, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Zhou, N.; Masterson, S.P.; Damron, J.K.; Guido, W.; Bickford, M.E. The Mouse Pulvinar Nucleus Links the Lateral Extrastriate Cortex, Striatum, and Amygdala. J. Neurosci. 2018, 38, 347–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, X.L.; Fang, Q.; Yan, L.; Zhong, W.; Peng, B.; Li, H.; Wei, J.; Tao, H.W.; Zhang, L.I. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 2020, 9, e54157. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Chou, X.L.; Peng, B.; Zhong, W.; Zhang, L.I.; Tao, H.W. A Differential Circuit via Retino-Colliculo-Pulvinar Pathway Enhances Feature Selectivity in Visual Cortex through Surround Suppression. Neuron 2020, 105, 355–369. [Google Scholar] [CrossRef]
- Ibrahim, L.A.; Mesik, L.; Ji, X.Y.; Fang, Q.; Li, H.F.; Li, Y.T.; Zingg, B.; Zhang, L.I.; Tao, H.W. Cross-Modality Sharpening of Visual Cortical Processing through Layer-1-Mediated Inhibition and Disinhibition. Neuron 2016, 89, 1031–1045. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Horta, S.; Sampedro, F.; Horta-Barba, A.; Perez-Perez, J.; Pagonabarraga, J.; Gomez-Anson, B.; Kulisevsky, J. Structural brain correlates of irritability and aggression in early manifest Huntington’s disease. Brain Imaging Behav. 2021, 15, 107–113. [Google Scholar] [CrossRef] [PubMed]
- McTeague, L.M.; Rosenberg, B.M.; Lopez, J.W.; Carreon, D.M.; Huemer, J.; Jiang, Y.; Chick, C.F.; Eickhoff, S.B.; Etkin, A. Identification of Common Neural Circuit Disruptions in Emotional Processing Across Psychiatric Disorders. Am. J. Psychiatry 2020, 177, 411–421. [Google Scholar] [CrossRef]
- Chen, R.; Chu, Q.; Shen, C.; Tong, X.; Gao, S.; Liu, X.; Zhou, B.; Schinckel, A.P. Identification of Single Nucleotide Polymorphisms in Porcine MAOA Gene Associated with Aggressive Behavior of Weaned Pigs after Group Mixing. Animals 2019, 9, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Oberley, L.W. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996, 21, 335–348. [Google Scholar] [CrossRef]
- Thiel, G.; Lietz, M.; Hohl, M. How mammalian transcriptional repressors work. Eur. J. Biochem. 2004, 271, 2855–2862. [Google Scholar] [CrossRef]
- Hill, S.Y.; Jones, B.L.; Haas, G.L. Suicidal ideation and aggression in childhood, genetic variation and young adult depression. J. Affect. Disord. 2020, 276, 954–962. [Google Scholar] [CrossRef]
- Park, C.I.; Kim, H.W.; Hwang, S.S.; Kang, J.I.; Kim, S.J. Influence of dopamine-related genes on craving, impulsivity, and aggressiveness in Korean males with alcohol use disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Zai, C.C.; Ehtesham, S.; Choi, E.; Nowrouzi, B.; de Luca, V.; Stankovich, L.; Davidge, K.; Freeman, N.; King, N.; Kennedy, J.L.; et al. Dopaminergic system genes in childhood aggression: Possible role for DRD2. World J. Biol. Psychiatry 2012, 13, 65–74. [Google Scholar] [CrossRef]
- Skretting, G.; Andersen, E.; Myklebust, C.F.; Sandset, P.M.; Tinholt, M.; Iversen, N.; Stavik, B. Transcription factor FOXP3: A repressor of the TFPI gene? J. Cell. Biochem. 2019, 120, 12924–12936. [Google Scholar] [CrossRef]
- Golden, S.A.; Jin, M.; Heins, C.; Venniro, M.; Michaelides, M.; Shaham, Y. Nucleus Accumbens Drd1-Expressing Neurons Control Aggression Self-Administration and Aggression Seeking in Mice. J. Neurosci. 2019, 39, 2482–2496. [Google Scholar] [CrossRef] [Green Version]
- Haddad, S.K.; Reiss, D.; Spotts, E.L.; Ganiban, J.; Lichtenstein, P.; Neiderhiser, J.M. Depression and internally directed aggression: Genetic and environmental contributions. J. Am. Psychoanal. Assoc. 2008, 56, 515–550. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.H.; Yao, Z.W.; Zhao, C.B.; Li, Q.Y.; Shi, J.L.; Guo, J.Y. Nardosinone Alleviates Parkinson’s Disease Symptoms in Mice by Regulating Dopamine D2 Receptor. Evid.-Based Complementary Altern. Med. 2021, 2021, 6686965. [Google Scholar] [CrossRef]
- Suzuki, H.; Han, S.D.; Lucas, L.R. Chronic passive exposure to aggression decreases D2 and 5-HT 1B receptor densities. Physiol. Behav. 2010, 99, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Lin, G.; Lai, Q.; Zhong, B.; Liu, Y.; Mi, Y.; Chen, H.; Wang, B.; Fan, L.; Hu, C. Ctenopharyngodon idella IRF2 plays an antagonistic role to IRF1 in transcriptional regulation of IFN and ISG genes. Dev. Comp. Immunol. 2015, 49, 103–112. [Google Scholar] [CrossRef]
- Coccia, E.M.; Del Russo, N.; Stellacci, E.; Orsatti, R.; Benedetti, E.; Marziali, G.; Hiscott, J.; Battistini, A. Activation and repression of the 2-5A synthetase and p21 gene promoters by IRF-1 and IRF-2. Oncogene 1999, 18, 2129–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Zheng, L.; Du, Q.; Yazdani, H.; Dong, K.; Guo, Y.; Geller, D.A. Interferon regulatory factor 1(IRF-1) activates anti-tumor immunity via CXCL10/CXCR3 axis in hepatocellular carcinoma (HCC). Cancer Lett. 2021, 506, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Robinson, S.W.; Missale, C.; Caron, M.G. Dopamine receptors and brain function. Neuropharmacology 1996, 35, 1503–1519. [Google Scholar] [CrossRef]
- Collins, A.G.; Frank, M.J. Opponent actor learning (OpAL): Modeling interactive effects of striatal dopamine on reinforcement learning and choice incentive. Psychol. Rev. 2014, 121, 337–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ousman, S.S.; Tomooka, B.H.; van Noort, J.M.; Wawrousek, E.F.; O’Connor, K.C.; Hafler, D.A.; Sobel, R.A.; Robinson, W.H.; Steinman, L. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature 2007, 448, 474–479. [Google Scholar] [CrossRef]



| Mutation Site Number | Mutation Site Position | Serial Number | Alleles | Genotype |
|---|---|---|---|---|
| 1 | 41134564 5′-UTR | rs1110730503 | A > T | AA/AT/TT |
| 2 | 41134034 5′-UTR | rs1107428594 | A > G | AA/AG/GG |
| SNP | Genotype | Number | Composite Aggressive Score (CAS) | Duration of Active Attack(s) | Duration of Bullying | Duration of Standoff(s) | Standoff Frequency |
|---|---|---|---|---|---|---|---|
| rs1110730503 (41134564) | AA | 26 | 3.9295 ± 0.1984 a | 6.0369 ± 0.2 a | 2.9482 ± 0.1978 | 2.7236 ± 0.2091 | 6.6762 ± 0.2013 |
| AT | 49 | 3.4457 ± 0.1501 b | 5.5254 ± 0.1514 b | 2.9498 ± 0.1487 | 2.3509 ± 0.1462 | 6.2924 ± 0.1461 | |
| TT | 107 | 3.3232 ± 0.105 b | 5.35 ± 0.1038 b | 2.9183 ± 0.1029 | 2.2571 ± 0.1087 | 6.5448 ± 0.1063 | |
| p-value | 0.0303 * | 0.011 * | 0.9816 | 0.1767 | 0.2262 | ||
| rs1107428594 (41134034) | AA | 13 | 3.6333 ± 0.2937 | 5.4633 ± 0.2931 | 5.1862 ± 0.2885 ab | 3.0320 ± 0.2985 a | 6.7209 ± 0.2986 |
| AG | 70 | 3.3233 ± 0.1256 | 5.3503 ± 0.1274 | 5.4742 ± 0.1241 a | 2.2823 ± 0.1257 b | 6.3155 ± 0.1256 | |
| GG | 70 | 3.3975 ± 0.1278 | 5.4442 ± 0.1288 | 5.1024 ± 0.1280 b | 2.2546 ± 0.1336 b | 6.5753 ± 0.1307 | |
| p-value | 0.6088 | 0.8577 | 0.1200 | 0.05 * | 0.2334 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Gao, S.; Guo, Y.; Xu, Q.; Liu, M.; Zhang, C.; Cheng, M.; Zhao, X.; Schinckel, A.P.; Zhou, B. Functionally Antagonistic Transcription Factors IRF1 and IRF2 Regulate the Transcription of the Dopamine Receptor D2 Gene Associated with Aggressive Behavior of Weaned Pigs. Biology 2022, 11, 135. https://doi.org/10.3390/biology11010135
Zhao J, Gao S, Guo Y, Xu Q, Liu M, Zhang C, Cheng M, Zhao X, Schinckel AP, Zhou B. Functionally Antagonistic Transcription Factors IRF1 and IRF2 Regulate the Transcription of the Dopamine Receptor D2 Gene Associated with Aggressive Behavior of Weaned Pigs. Biology. 2022; 11(1):135. https://doi.org/10.3390/biology11010135
Chicago/Turabian StyleZhao, Jing, Siyuan Gao, Yanli Guo, Qinglei Xu, Mingzheng Liu, Chunlei Zhang, Meng Cheng, Xianle Zhao, Allan P. Schinckel, and Bo Zhou. 2022. "Functionally Antagonistic Transcription Factors IRF1 and IRF2 Regulate the Transcription of the Dopamine Receptor D2 Gene Associated with Aggressive Behavior of Weaned Pigs" Biology 11, no. 1: 135. https://doi.org/10.3390/biology11010135






