Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
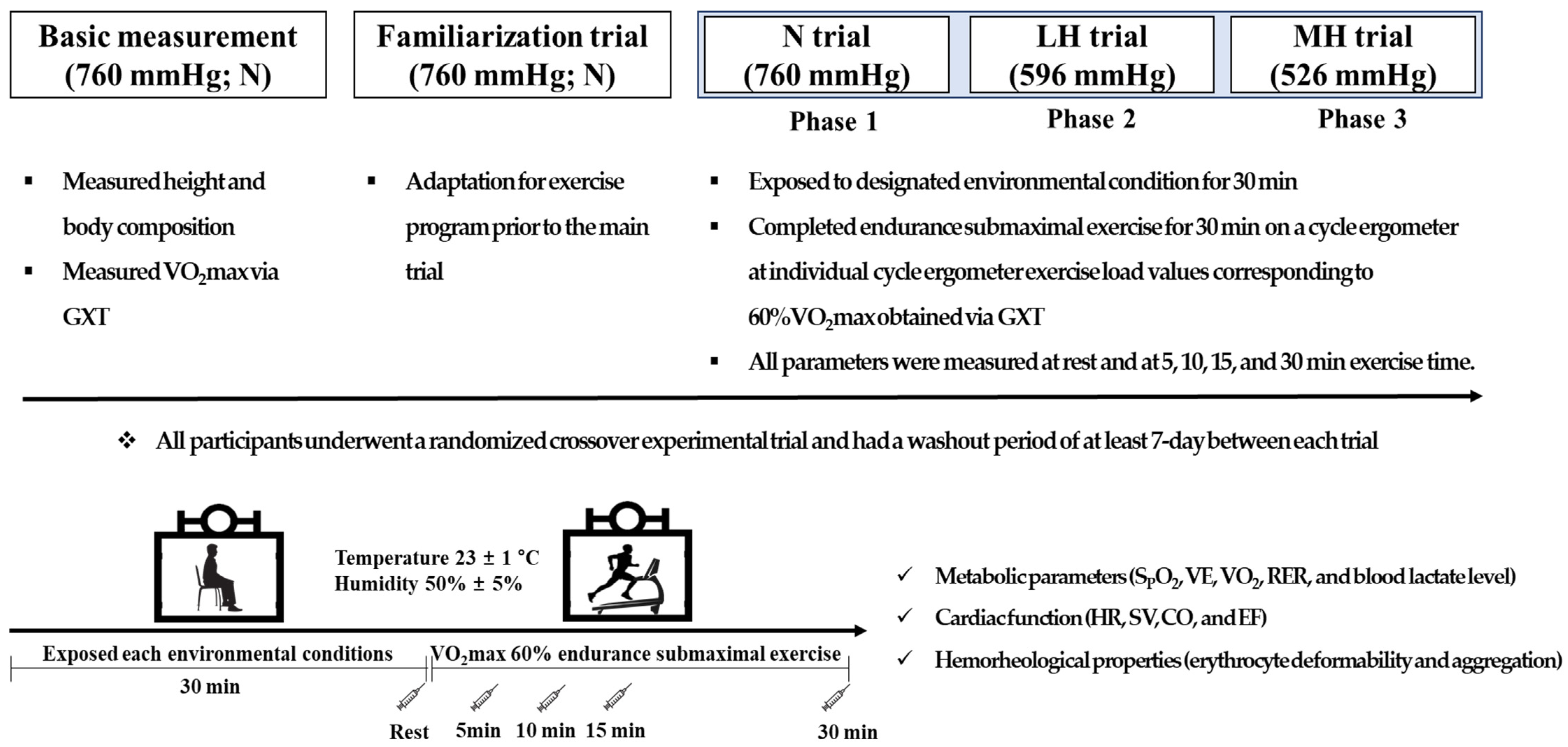
2.2. Study Design
2.3. Measurement
2.4. Statistical Analysis
3. Results
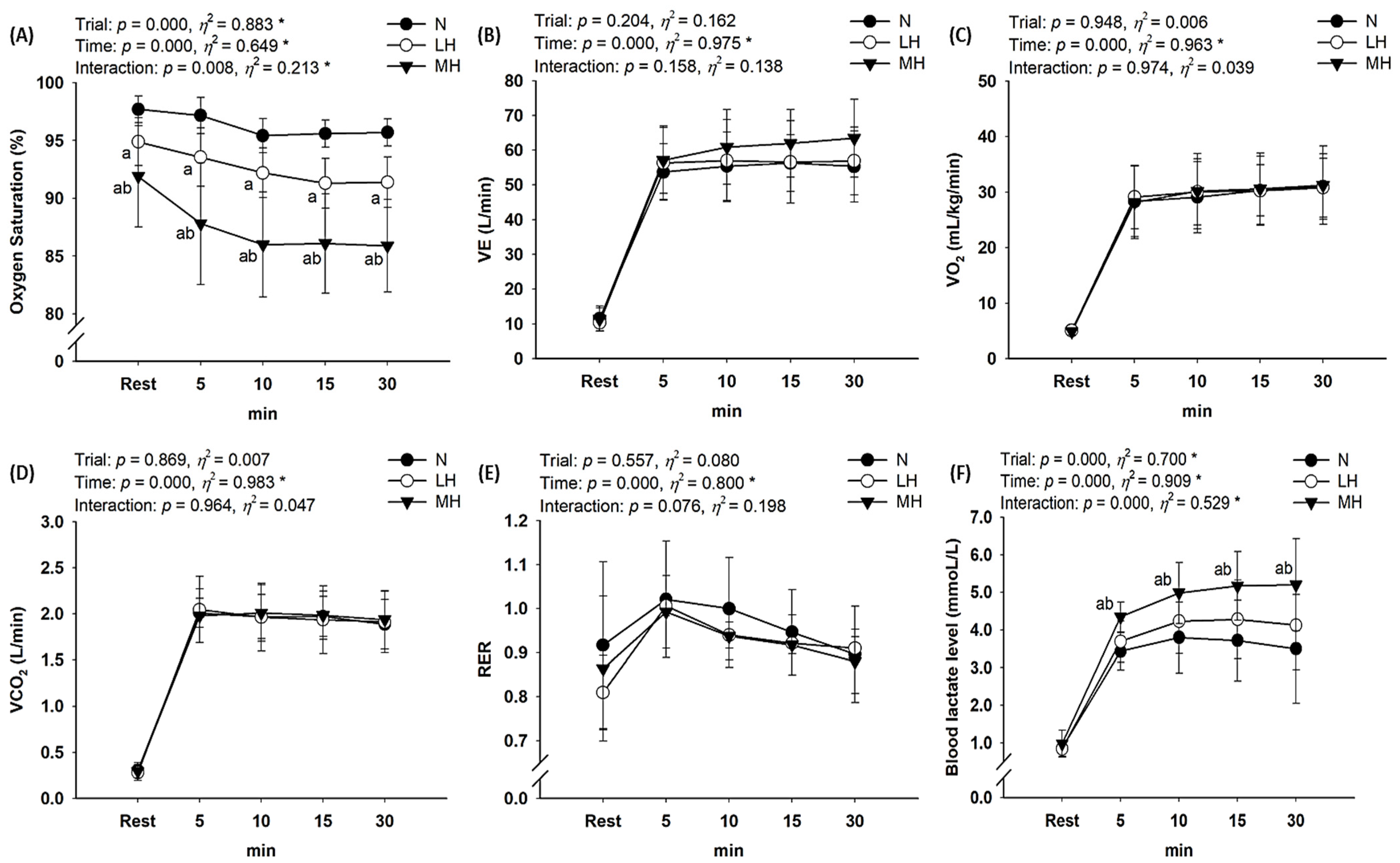
3.1. Metabolic Parameters
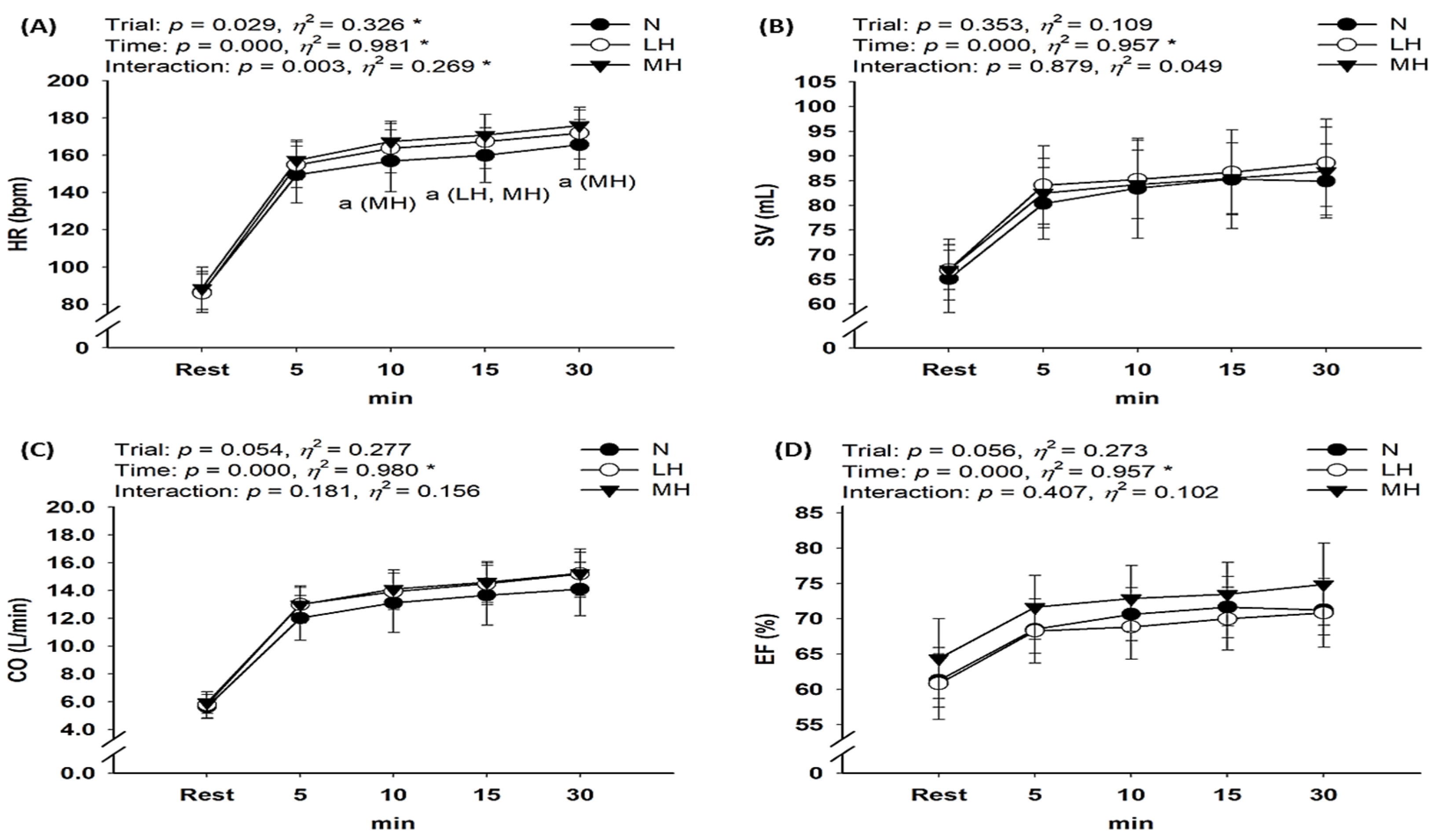
3.2. Cardiac Function
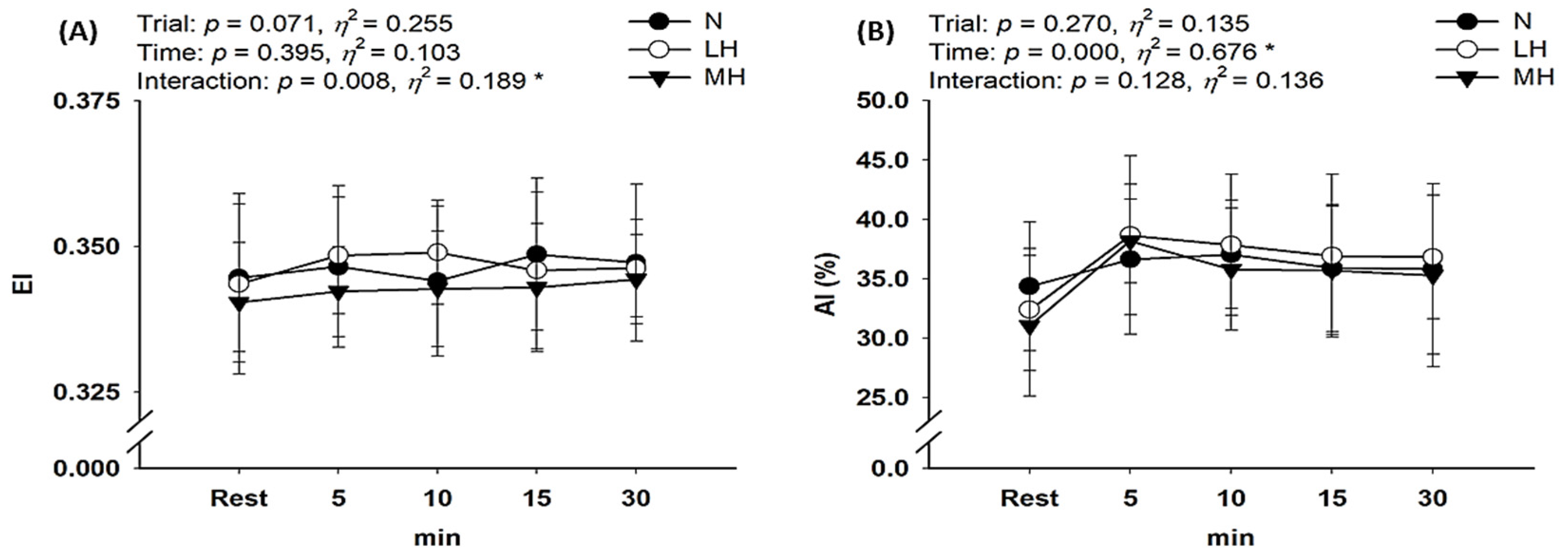
3.3. Hemorheological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasperowski, D. Constructing altitude training standards for the 1968 Mexico Olympics: The impact of ideals of equality and uncertainty. Int. J. Hist. Sport 2009, 26, 1263–1291. [Google Scholar] [CrossRef]
- Feriche, B.; García-Ramos, A.; Morales-Artacho, A.J.; Padial, P. Resistance training using different hypoxic training strategies: A basis for hypertrophy and muscle power development. Sports Med. Open 2017, 3, 12. [Google Scholar] [CrossRef]
- Czuba, M.; Wilk, R.; Karpiński, J.; Chalimoniuk, M.; Zajac, A.; Langfort, J. Intermittent hypoxic training improves anaerobic performance in competitive swimmers when implemented into a direct competition mesocycle. PLoS ONE 2017, 12, e0180380. [Google Scholar] [CrossRef]
- Brocherie, F.; Girard, O.; Faiss, R.; Millet, G.P. Effects of repeated-sprint training in hypoxia on sea-level performance: A meta-analysis. Sports Med. 2017, 47, 1651–1660. [Google Scholar] [CrossRef]
- Sinex, J.A.; Chapman, R.F. Hypoxic training methods for improving endurance exercise performance. J. Sport Health Sci. 2015, 4, 325–332. [Google Scholar] [CrossRef]
- Faiss, R.; Girard, O.; Millet, G.P. Advancing hypoxic training in team sports: From intermittent hypoxic training to repeated sprint training in hypoxia. Br. J. Sports Med. 2013, 47, i45–i50. [Google Scholar] [CrossRef]
- Nam, S.-S.; Park, H.-Y. Effects of endurance exercise under hypoxia on acid-base and ion balance in healthy males. Phys. Act. Nutr. 2020, 24, 7. [Google Scholar] [CrossRef]
- Sumi, D.; Kojima, C.; Kasai, N.; Goto, K. The effects of endurance exercise in hypoxia on acid-base balance and potassium kinetics: A randomized crossover design in male endurance athletes. Sports Med. Open 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Sumi, D.; Kasai, N.; Ito, H.; Goto, K. The effects of endurance exercise in hypoxia on acid-base balance, potassium kinetics, and exogenous glucose oxidation. Front. Physiol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Seo, J.; Jung, W.-S.; Kim, J.; Park, H.-Y.; Lim, K. Effects of an acute Pilates program under hypoxic conditions on vascular endothelial function in Pilates participants: A randomized crossover trial. Int. J. Environ. Res. Public Health 2020, 17, 2584. [Google Scholar] [CrossRef] [PubMed]
- Girard, O.; Malatesta, D.; Millet, G.P. Walking in hypoxia: An efficient treatment to lessen mechanical constraints and improve health in obese individuals? Front. Physiol. 2017, 8, 73. [Google Scholar] [CrossRef][Green Version]
- Jung, K.; Kim, J.; Park, H.-Y.; Jung, W.-S.; Lim, K. Hypoxic Pilates intervention for obesity: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 7186. [Google Scholar] [CrossRef]
- Serebrovska, T.V.; Serebrovska, Z.O.; Egorov, E. Fitness and therapeutic potential of intermittent hypoxia training: A matter of dose. Fiziol. Zh. 2016, 62, 78–91. [Google Scholar] [CrossRef]
- Verges, S.; Chacaroun, S.; Godin-Ribuot, D.; Baillieul, S. Hypoxic conditioning as a new therapeutic modality. Front. Pediatr. 2015, 3, 58. [Google Scholar] [CrossRef]
- Chacaroun, S.; Borowik, A.; Vega-Escamilla, Y.G.I.; Doutreleau, S.; Wuyam, B.; Belaidi, E.; Tamisier, R.; Pepin, J.L.; Flore, P.; Verges, S. Hypoxic exercise training to improve exercise capacity in obese individuals. Med. Sci. Sports Exerc. 2020, 52, 1641–1649. [Google Scholar] [CrossRef]
- Bernardi, L.; Passino, C.; Serebrovskaya, Z.; Serebrovskaya, T.; Appenzeller, O. Respiratory and cardiovascular adaptations to progressive hypoxia. Eur. Heart J. 2001, 22, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent hypoxia: Cause of or therapy for systemic hypertension? Exp. Biol. Med. 2008, 233, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic use of exercising in hypoxia: Promises and limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef]
- Leuenberger, U.A.; Gray, K.; Herr, M.D. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J. Appl. Physiol. 1999, 87, 2218–2224. [Google Scholar] [CrossRef] [PubMed]
- Messina, E.; Sun, D.; Koller, A.; Wolin, M.; Kaley, G. Role of endothelium-derived prostaglandins in hypoxia-elicited arteriolar dilation in rat skeletal muscle. Circ. Res. 1992, 71, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-W.; Shin, S.-H.; Lee, C.-H.; Park, H.-Y.; Sunoo, S.; Nam, S.-S. Effects of various acute hypoxic conditions on the hemorheological response during exercise and recovery 1. Clin. Hemorheol. Microcirc. 2016, 63, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Bor-Kucukatay, M.; Colak, R.; Erken, G.; Kilic-Toprak, E.; Kucukatay, V. Altitude training induced alterations in erythrocyte rheological properties: A controlled comparison study in rats. Clin. Hemorheol. Microcirc. 2014, 58, 479–488. [Google Scholar] [CrossRef]
- Connes, P.; Tripette, J.; Mukisi-Mukaza, M.; Baskurt, O.K.; Toth, K.; Meiselman, H.J.; Hue, O.; Antoine-Jonville, S. Relationships between hemodynamic, hemorheological and metabolic responses during exercise. Biorheology 2009, 46, 133–143. [Google Scholar] [CrossRef]
- Baskurt, O.; Boynard, M.; Cokelet, G.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.; Jung, F.; Meiselman, H. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, M.; Ulker, P.; Meiselman, H.J.; Baskurt, O.K. Influence of tourniquet application on venous blood sampling for serum chemistry, hematological parameters, leukocyte activation and erythrocyte mechanical properties. Clin. Chem. Lab. Med. 2009, 47, 769–776. [Google Scholar] [CrossRef]
- Moon, H.-W.; Sunoo, S.; Park, H.-Y.; Lee, D.-J.; Nam, S.-S. Effects of various acute hypoxic conditions on metabolic parameters and cardiac function during exercise and recovery. Springerplus 2016, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wehrlin, J.P.; Hallén, J. Linear decrease in. VO2max and performance with increasing altitude in endurance athletes. Eur. J. Appl. Physiol. 2006, 96, 404–412. [Google Scholar] [CrossRef]
- Hill, N.; Stacey, M.; Woods, D. Energy at high altitude. J. R. Army Med. Corps 2011, 157, 43–48. [Google Scholar] [CrossRef]
- Mazzeo, R.S. Physiological responses to exercise at altitude. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef]
- Calbet, J.; Boushel, R.; Rådegran, G.; Søndergaard, H.; Wagner, P.D.; Saltin, B. Determinants of maximal oxygen uptake in severe acute hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R291–R303. [Google Scholar] [CrossRef]
- Mazzeo, R.S.; Brooks, G.A.; Butterfield, G.E.; Podolin, D.A.; Wolfel, E.E.; Reeves, J.T. Acclimatization to high altitude increase muscle sympathetic activity both at rest and during exercise. Am. J. Physiol. 1995, 269, R201–R207. [Google Scholar] [CrossRef]
- Brooks, G.A. The science and translation of lactate shuttle theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Wolfel, E.E.; Butterfield, G.E.; Cymerman, A.; Roberts, A.C.; Mazzeo, R.S.; Reeves, J.T. Poor relationship between arterial [lactate] and leg net release during exercise at 4,300 m altitude. Am. J. Physiol. 1998, 275, R1192–R1201. [Google Scholar] [CrossRef] [PubMed]
- Ofner, M.; Wonisch, M.; Frei, M.; Tschakert, G.; Domej, W.; Kröpfl, J.M.; Hofmann, P. Influence of acute normobaric hypoxia on physiological variables and lactate turn point determination in trained men. J. Sports Sci. Med. 2014, 13, 774–781. [Google Scholar] [PubMed]
- Lühker, O.; Berger, M.M.; Pohlmann, A.; Hotz, L.; Gruhlke, T.; Hochreiter, M. Changes in acid-base and ion balance during exercise in normoxia and normobaric hypoxia. Eur. J. Appl. Physiol. 2017, 117, 2251–2261. [Google Scholar] [CrossRef]
- Connes, P.; Caillaud, C.; Py, G.; Mercier, J.; Hue, O.; Brun, J.-F. Maximal exercise and lactate do not change red blood cell aggregation in well trained athletes. Clin. Hemorheol. Microcirc. 2007, 36, 319–326. [Google Scholar]
- Muravyov, A.; Draygin, S.; Eremin, N.; Muravyov, A. The microrheological behavior of young and old red blood cells in athletes. Clin. Hemorheol. Microcirc. 2002, 26, 183–188. [Google Scholar]
- Yalcin, O.; Erman, A.; Muratli, S.; Bor-Kucukatay, M.; Baskurt, O.K. Time course of hemorheological alterations after heavy anaerobic exercise in untrained human subjects. J. Appl. Physiol. 2003, 94, 997–1002. [Google Scholar] [CrossRef]
- Grau, M.; Lauten, A.; Hoeppener, S.; Goebel, B.; Brenig, J.; Jung, C.; Bloch, W.; Suhr, F. Regulation of red blood cell deformability is independent of red blood cell-nitric oxide synthase under hypoxia. Clin. Hemorheol. Microcirc. 2016, 63, 199–215. [Google Scholar] [CrossRef]
- Lin, C.-L.; Wang, J.-S.; Fu, T.-C.; Hsu, C.-C.; Huang, Y.-C. Hypoxic exercise training elevates erythrocyte aggregation. Appl. Sci. 2021, 11, 6038. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-Y.; Kim, J.-W.; Nam, S.-S. Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men. Biology 2022, 11, 144. https://doi.org/10.3390/biology11010144
Park H-Y, Kim J-W, Nam S-S. Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men. Biology. 2022; 11(1):144. https://doi.org/10.3390/biology11010144
Chicago/Turabian StylePark, Hun-Young, Jeong-Weon Kim, and Sang-Seok Nam. 2022. "Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men" Biology 11, no. 1: 144. https://doi.org/10.3390/biology11010144
APA StylePark, H.-Y., Kim, J.-W., & Nam, S.-S. (2022). Metabolic, Cardiac, and Hemorheological Responses to Submaximal Exercise under Light and Moderate Hypobaric Hypoxia in Healthy Men. Biology, 11(1), 144. https://doi.org/10.3390/biology11010144






