Camouflage and Exploratory Avoidance of Newborn Cuttlefish under Warming and Acidification
Abstract
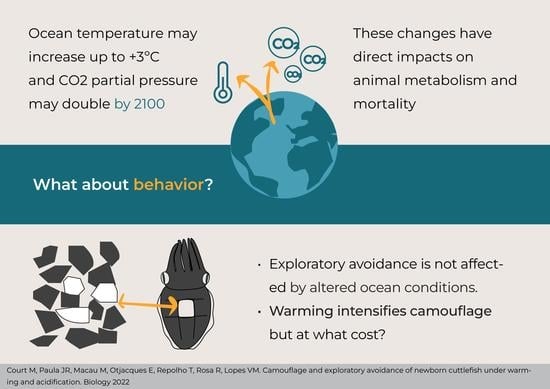
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Egg Collection and Husbandry
2.3. Hatching Success, Development Time and Size
2.4. Exploratory Avoidance Behavior Data Collection and Processing
2.5. Camouflage Data Collection and Processing
2.6. Anesthesia and Humane Killing
2.7. Data Analyses
2.7.1. Survival Analysis
2.7.2. Generalized Linear Models
3. Results
3.1. Development Time, Hatching Success and Size
3.2. Exploration Avoidance
3.3. Camouflage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duarte, C.M. Global change and the future ocean: A grand challenge for marine sciences. Front. Mar. Sci. 2014, 1, 63. [Google Scholar] [CrossRef]
- Knoll, A.H.; Bambach, R.K.; Canfield, D.E.; Grotzinger, J.P. Comparative earth history and late Permian mass extinction. Science 1996, 273, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Bijma, J.; Pörtner, H.O.; Yesson, C.; Rogers, A.D. Climate change and the oceans—What does the future hold? Mar. Poll. Bull. 2013, 74, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, K.; Wickett, M.E. Anthropogenic carbon and ocean pH. Nature 2003, 425, 365. [Google Scholar] [CrossRef]
- Reid, P.C. Ocean warming: Setting the scene. In Explaining Ocean Warming: Causes, Scale, Effects and Consequences; Laffoley, D., Baxter, J.M., Eds.; IUCN: Gland, Switzerland, 2016; pp. 19–45. [Google Scholar]
- Fox-Kemper, B.; Hewitt, H.T.; Xiao, C.; Aðalgeirsdóttir, G.; Drijfhout, S.S.; Edwards, T.L.; Golledge, N.R.; Hemer, M.; Kopp, R.E.; Krinner, G.; et al. Ocean, Cryosphere and Sea Level Change. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Rhein, M.; Rintoul, S.R.; Aoki, S.; Campos, E.; Chambers, D.; Feely, R.A.; Nauels, A.; Xia, Y.; Bex, V.; Wang, F. Observations: Ocean. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Deutsch, C.; Penn, J.L.; Seibel, B. Metabolic trait diversity shapes marine biogeography. Nature 2020, 585, 557–562. [Google Scholar] [CrossRef]
- Clemente, S.; Lorenzo-Morales, J.; Mendoza, J.C.; López, C.; Sangil, C.; Alves, F.; Kaufman, M.; Hernández, J.C. Sea urchin Diadema africanum mass mortality in the subtropical eastern Atlantic: Role of waterborne bacteria in a warming ocean. Mar. Ecol. Prog. Ser. 2014, 506, 1–14. [Google Scholar] [CrossRef]
- Rivetti, I.; Fraschetti, S.; Lionello, P.; Zambianchi, E.; Boero, F. Global warming and mass mortalities of benthic invertebrates in the Mediterranean Sea. PLoS ONE 2014, 9, e115655. [Google Scholar] [CrossRef]
- Genin, A.; Levy, L.; Sharon, G.; Raitsos, D.E.; Diamant, A. Rapid onsets of warming events trigger mass mortality of coral reef fish. Proc. Natl. Acad. Sci. USA 2020, 117, 25378–25385. [Google Scholar] [CrossRef]
- Bindoff, N.L.; Cheung, W.W.L.; Kairo, J.G.; Arístegui, J.; Guinder, V.A.; Hallberg, R.; Williamson, P. Changing Ocean, Marine Ecosystems, and Dependent Communities. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Weyer, N.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2019. [Google Scholar]
- Southward, A.J.; Hawkins, S.J.; Burrows, M.T. Seventy years’ observations of changes in distribution and abundance of zooplankton and intertidal organisms in the western English Channel in relation to rising sea temperature. J. Therm. Biol. 1995, 20, 127–155. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Marotzke, J.; Bala, G.; Cao, L.; Corti, S.; Dunne, J.P.; Engelbrecht, F.; Fischer, E.; Fyfe, J.C.; Jones, C.; et al. Future Global Climate: Scenario-Based Projections and Near-Term Information Supplementary Material. In Climate Change 2021: The Physical Science Basis; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Gaylord, B.; Kroeker, K.J.; Sunday, J.M.; Anderson, K.M.; Barry, J.P.; Brown, N.E.; Connell, S.D.; Dupont, S.; Fabricius, K.E.; Hall-Spencer, J.M.; et al. Ocean acidification through the lens of ecological theory. Ecology 2015, 96, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Wootton, J.T.; Pfister, C.A.; Forester, J.D. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl. Acad. Sci. USA 2008, 105, 18848–18853. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pulido, G.; Gouezo, M.; Tilbrook, B.; Dove, S.; Anthony, K.R. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 2010, 14, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Hennige, S.; Roberts, J.M.; Williamson, P. An Updated Synthesis of the Impacts of Ocean Acidification on Marine Biodiversity; Secretariat of the Convention on Biological Diversity: Montreal, QC, Canada, 2014. [Google Scholar]
- Doubleday, Z.A.; Prowse, T.A.; Arkhipkin, A.; Pierce, G.J.; Semmens, J.; Steer, M.; Leporati, S.C.; Lourenço, S.; Quetglas, A.; Sauer, W.; et al. Global proliferation of cephalopods. Curr. Biol. 2016, 26, R406–R407. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Castro, B.G. On the life cycle of Sepia officinalis (Cephalopoda, Sepioidea) in the Rio de Vigo (NW Spain). Cah. Biol. Mar. 1988, 29, 395–405. [Google Scholar]
- Bettencourt, V.; Guerra, A. Carbon- and oxygen-isotope composition of the cuttlebone of Sepia officinalis: A tool for predicting ecological information? Mar. Biol. 1999, 133, 651–657. [Google Scholar] [CrossRef]
- Guerra, A. Ecology of Sepia officinalis. Vie Milieu 2006, 56, 97–107. [Google Scholar]
- Boyle, P.R.; Boletzky, S. Cephalopod Populations: Definition and Dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 985–1002. [Google Scholar] [CrossRef]
- Pimentel, M.S.; Truebenbach, K.; Faleiro, F.; Boavida-Portugal, J.; Repolho, T.; Rosa, R. Impact of ocean warming on the early ontogeny of cephalopods: A metabolic approach. Mar. Biol. 2012, 159, 2051–2059. [Google Scholar] [CrossRef]
- Rosa, R.; Trubenbach, K.; Repolho, T.; Pimentel, M.; Faleiro, F.; Boavida-Portugal, J.; Baptista, M.; Lopes, V.M.; Dionísio, G.; Leal, M.C.; et al. Lower hypoxia thresholds of cuttlefish early life stages living in a warm acidified ocean. Proc. R. Soc. B 2013, 280, 1768. [Google Scholar] [CrossRef]
- Moura, É.; Pimentel, M.; Santos, C.P.; Sampaio, E.; Pegado, M.R.; Lopes, V.M.; Rosa, R. Cuttlefish Early Development and Behavior Under Future High CO2 Conditions. Front. Physiol. 2019, 10, 975. [Google Scholar] [CrossRef]
- Otjacques, E.; Repolho, T.; Paula, J.R.; Simão, S.; Baptista, M.; Rosa, R. Cuttlefish Buoyancy in Response to Food Availability and Ocean Acidification. Biology 2020, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Dorey, N.; Melzner, F.; Martin, S.; Oberhänsli, F.; Teyssié, J.-L.; Bustamante, P.; Gattuso, J.-P.; Lacoue-Labarthe, T. Ocean acidification and temperature rise: Effects on calcification during early development of the cuttlefish Sepia officinalis. Mar. Biol. 2012, 160, 2007–2022. [Google Scholar] [CrossRef] [Green Version]
- Gurarie, E.; Bracis, C.; Delgado, M.; Meckley, T.D.; Kojola, I.; Wagner, C.M. What is the animal doing? Tools for exploring behavioural structure in animal movements. J. Anim. Ecol. 2016, 85, 69–84. [Google Scholar] [CrossRef]
- Lamprea, M.R.; Cardenas, F.P.; Setem, J.; Morato, S. Thigmotactic responses in an open-field. Braz. J. Med. Biol. Res. 2018, 41, 135–140. [Google Scholar] [CrossRef]
- Kuba, M.J.; Byrne, R.A.; Meisel, D.V.; Mather, J.A. Exploration and Habituation in Intact Free Moving Octopus vulgaris. Int. J. Comp. Psychol. 2006, 19, 426–438. [Google Scholar]
- Hanlon, R.T.; Messenger, J.B. Adaptive coloration in young cuttlefish (Sepia officinalis L.): The morphology and development of body patterns and their relation to behaviour. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1988, 320, 1200. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Messenger, J.B. Body Patterning and Colour Change. In Cephalopod Behaviour, 2nd ed.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2018; pp. 45–73. [Google Scholar] [CrossRef]
- Marshall, N.J.; Messenger, J.B. Colour-blind camouflage. Nature 1996, 382, 408–409. [Google Scholar] [CrossRef]
- Mäthger, L.M.; Chiao, C.-C.; Barbosa, A.; Hanlon, R.T. Color matching on natural substrates in cuttlefish, Sepia officinalis. J. Comp. Physiol. A 2008, 194, 577–585. [Google Scholar] [CrossRef]
- Kelman, E.J.; Baddeley, R.J.; Shohet, A.J.; Osorio, D. Perception of visual texture and the expression of disruptive camouflage by the cuttlefish, Sepia officinalis. Proc. R. Soc. B 2007, 274, 1369–1375. [Google Scholar] [CrossRef]
- Zylinski, S.; Osorio, D.; Shohet, A.J. Perception of edges and visual texture in the camouflage of the common cuttlefish, Sepia officinalis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 439–448. [Google Scholar] [CrossRef]
- Troscianko, J.; Skelhorn, J.; Stevens, M. Quantifying camouflage: How to predict detectability from appearance. BMC Evol. Biol. 2017, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Merilaita, S. Defining disruptive coloration and distinguishing its functions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; De Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.W.; Kuba, M.J.; et al. Guidelines for the Care and Welfare of Cephalopods in Research –A consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.; Wallace, D.; Allison, L.J. Program Developed for CO2 System Calculations; Brookhaven National Lab.: New York, NY, USA, 1998. [CrossRef]
- Cooke, G.; Anderson, D.; Bégout, M.L.; Dennison, N.; Osorio, D.; Tonkins, B.; Kristiansen, T.; Fiorito, G.; Galligioni, V.; Ponte, G.; et al. Prospective severity classification of scientific procedures in cephalopods: Report of a COST FA1301 Working Group survey. Lab. Anim. 2019, 53, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.J.; Holcombe, A.; Tresguerres, M. CO2-induced ocean acidification increases anxiety in Rockfish via alteration of GABAA receptor functioning. Proc. R. Soc. B 2014, 281, 1775. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, P.; Andersson, M. ToxTrac: A fast and robust software for tracking organisms. Methods Ecol. Evol. 2018, 9, 460–464. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, M. ToxId: An algorithm to track the identity of multiple animals. Sci. Rep. 2018, 7, 14774. [Google Scholar] [CrossRef]
- Schober, P.; Vetter, T.R. Survival Analysis and Interpretation of Time-of-Event Data: The Tortoise and the Hare. Anesth Analg. 2018, 127, 792–798. [Google Scholar] [CrossRef]
- Gutowska, M.A.; Pörtner, H.O.; Melzner, F. Growth and calcification in the cephalopod Sepia officinalis under elevated seawater pCO2. Mar. Ecol. Prog. Ser. 2008, 373, 303–309. [Google Scholar] [CrossRef]
- Rosa, R.; Trübenbach, K.; Pimentel, M.S.; Boavida-Portugal, J.; Faleiro, F.; Baptista, M.; Dionísio, G.; Calado, R.; Pörtner, H.O.; Repolho, T. Differential impacts of ocean acidification and warming on winter and summer progeny of a coastal squid (Loligo vulgaris). J. Exp. Biol. 2014, 217, 518–525. [Google Scholar] [CrossRef]
- Hu, M.Y.; Tseng, Y.-C.; Stumpp, M.; Gutowska, M.A.; Kiko, R.; Lucassen, M.; Melzner, F. Elevated seawater pCO2 differentially affects branchial acid-base transporters over the course of development in the cephalopod Sepia officinalis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1100–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, R.; Pimentel, M.S.; Boavida-Portugal, J.; Teixeira, T.; Trübenbach, K.; Diniz, M. Ocean warming enhances malformations, premature hatching, metabolic suppression and oxidative stress in the early life stages of a keystone squid. PLoS ONE 2012, 7, e38282. [Google Scholar] [CrossRef] [PubMed]
- Oellermann, M.; Pörtner, H.O.; Mark, F.C. Mitochondrial dynamics underlying thermal plasticity of cuttlefish (Sepia officinalis) hearts. J. Exp. Biol. 2012, 215, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Maneja, R.; Piatkowski, U.; Melzner, F. Effects of ocean acidification on statolith calcification and prey capture in early life cuttlefish, Sepia officinalis. J. Shellfish Res. 2011, 30, 1011. [Google Scholar]
- Lee, Y.C.; Darmaillacq, A.S.; Dickel, L.; Chiao, C.C. Effects of embryonic exposure to predators on the postnatal defensive behaviors of cuttlefish. J. Exp. Mar. Biol. Ecol. 2020, 524, 151288. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Jozet-Alves, C.; Mezrai, N.; Bellanger, C.; Darmaillacq, A.-S.; Dickel, L. Maternal and Embryonic Stress Influence Offspring Behavior in the Cuttlefish Sepia officinalis. Front. Physiol. 2017, 8, 981. [Google Scholar] [CrossRef]
- Helfman, G.S. Threat-sensitive predator avoidance in damselfish-trumpetfish interactions. Behav. Ecol. Sociobiol. 1989, 24, 47–58. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, Y.; Fu, C.; Wang, B.; Li, J.; Ren, Y.; Wan, R. Variability of coastal cephalopods in overexploited China Seas under climate change with implications on fisheries management. Fish. Res. 2018, 208, 22–33. [Google Scholar] [CrossRef]
- Allen, J.J.; Mäthger, L.M.; Barbosa, A.; Buresch, K.C.; Sogin, E.; Schwartz, J.; Chubb, C.; Hanlon, R.T. Cuttlefish dynamic camouflage: Responses to substrate choice and integration of multiple visual cues. Proc. R. Soc. B 2010, 277, 1031–1039. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Liu, T.-H.; Su, C.-H.; Chiao, C.-C. Neural Organization of the Optic Lobe Changes Steadily from Late Embryonic Stage to Adulthood in Cuttlefish Sepia pharaonis. Front. Physiol. 2017, 8, 538. [Google Scholar] [CrossRef]
- Stuart-Fox, D.; Moussalli, A. Camouflage, communication and thermoregulation: Lessons from colour changing organisms. Proc. R. Soc. B 2009, 364, 1516. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, G.M.; Gladman, N.W.; Corless, H.F.; Morrell, L.J. Costs of colour change in fish: Food intake and behavioural decisions. J. Exp. Biol. 2013, 216, 2760–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langridge, K.V.; Broom, M.; Osorio, D. Selective signalling by cuttlefish to predators. Curr. Biol. 2007, 17, R1044–R1045. [Google Scholar] [CrossRef]
- Langridge, K.V. Cuttlefish use startle displays, but not against large predators. Anim. Behav. 2009, 77, 847–856. [Google Scholar] [CrossRef]
- Merilaita, S.; Scott-Samuel, N.E.; Cuthill, I.C. How camouflage works. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 1724. [Google Scholar] [CrossRef]
- Chiao, C.-C.; Wickiser, J.K.; Allen, J.J.; Genter, B.; Hanlon, R.T. Hyperspectral imaging of cuttlefish camouflage indicates good color match in the eyes of fish predators. Proc. Natl. Acad. Sci. USA 2011, 108, 9148–9153. [Google Scholar] [CrossRef]
- Buresch, K.C.; Ulmer, K.M.; Akkaynak, D.; Allen, J.J.; Mäthger, L.M.; Nakamura, M.; Hanlon, R.T. Cuttlefish adjust body pattern intensity with respect to substrate intensity to aid camouflage, but do not camouflage in extremely low light. J. Exp. Mar. Biol. Ecol. 2015, 462, 121–126. [Google Scholar] [CrossRef]
- Barbosa, A.; Mäthger, L.M.; Chubb, C.; Florio, C.; Chiao, C.C.; Hanlon, R.T. Disruptive coloration in cuttlefish: A visual perception mechanism that regulates ontogenetic adjustment of skin patterning. J. Exp. Biol. 2007, 210, 1139–1147. [Google Scholar] [CrossRef]
- Melzner, F.; Gutowska, M.A.; Langenbuch, M.; Dupont, S.; Lucassen, M.; Thorndyke, M.C.; Bleich, M.; Pörtner, H.O. Physiological basis for high CO2 tolerance in marine ectothermic animals: Pre-adaptation through lifestyle and ontogeny? Biogeosciences 2009, 6, 2313–2331. [Google Scholar] [CrossRef] [Green Version]



| Model | Response | Predictor | χ2 | d.f. | p-Value |
|---|---|---|---|---|---|
| LM, identity link | Mantle length | Treatment | 12.08 | 3 | 0.0071 |
| Cox model | Hatching over time | Treatment | 104 | 3 | <2 × 10−16 |
| Model | Response | n | Predictor | χ2 | d.f. | p-Value |
|---|---|---|---|---|---|---|
| GLM, beta, log link | Proximity to the object | 140 | Treatment | 3.18 | 3 | 0.3649 |
| LM, identity link | Average acceleration | 142 | Treatment | 1.35 | 3 | 0.7162 |
| Visibility rate | 24.16 | 1 | 8.8 × 10−7 | |||
| Visibility rate: Treatment | 7.89 | 3 | 0.0484 | |||
| GLM, binomial, logit link | Ink ejection | 158 | Visibility rate | 0.31828 | 1 | 0.5726 |
| Treatment | 0.80615 | 3 | 0.8480 | |||
| Visibility rate: Treatment | 0.17741 | 3 | 0.9812 |
| Model | Response | n | Predictor | χ2 | d.f. | p-Value |
|---|---|---|---|---|---|---|
| GLM, binomial, logit link | Latency to camouflage (gravel) | 134 | First substrate | 4.29 | 1 | 0.0383 |
| Replicate | 2.44 | 3 | 0.4867 | |||
| Treatment | 10.34 | 3 | 0.0159 | |||
| First substrate:Treatment | 19.46 | 3 | 0.0002 | |||
| Replicate:Treatment | 17.40 | 9 | 0.0428 | |||
| GLM, binomial, logit link | Latency to camouflage (sand) | 134 | First substrate | 6.69 | 1 | 0.0097 |
| Treatment | 1.01 | 3 | 0.7984 | |||
| First substrate:Treatment | 1.27 | 3 | 0.7352 | |||
| LM, identity link | Pixel value difference in body planes | 134 | First substrate | 8.74 | 1 | 0.0032 |
| Treatment | 17.71 | 3 | 0.0005 | |||
| First substrate:Treatment | 3.47 | 3 | 0.3243 | |||
| LM, identity link | Pixel integrated density light region-white gravel | 134 | Treatment | 2.13 | 3 | 0.5453 |
| LM, identity link GLM, binomial, logit link | Pixel integrated density dark region-black gravel | 134 | Treatment | 4.92 | 3 | 0.1776 |
| Replicate | 7.96 | 3 | 0.0476 | |||
| Treatment | 2.36 | 3 | 0.5018 | |||
| GLM, binomial, logit link | Acclimation (sand) | 126 | Treatment | 2.59 | 3 | 0.4587 |
| GLM, binomial, logit link | Acclimation (gravel) | 126 | First substrate | 12.26 | 1 | 0.0004 |
| GLM, binomial, logit link | Burial in sand | 134 | Treatment | 1.03 | 3 | 0.7948 |
| First substrate:Treatment | 5.81 | 3 | 0.1215 | |||
| GLM, binomial, logit link | Acclimation | 252 | Substrate | 0.46 | 1 | 0.4998 |
| GLM, binomial, logit link | Latency to camouflage | 268 | Substrate | 3.95 | 1 | 0.0469 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Court, M.; Paula, J.R.; Macau, M.; Otjacques, E.; Repolho, T.; Rosa, R.; Lopes, V.M. Camouflage and Exploratory Avoidance of Newborn Cuttlefish under Warming and Acidification. Biology 2022, 11, 1394. https://doi.org/10.3390/biology11101394
Court M, Paula JR, Macau M, Otjacques E, Repolho T, Rosa R, Lopes VM. Camouflage and Exploratory Avoidance of Newborn Cuttlefish under Warming and Acidification. Biology. 2022; 11(10):1394. https://doi.org/10.3390/biology11101394
Chicago/Turabian StyleCourt, Mélanie, José Ricardo Paula, Marta Macau, Eve Otjacques, Tiago Repolho, Rui Rosa, and Vanessa Madeira Lopes. 2022. "Camouflage and Exploratory Avoidance of Newborn Cuttlefish under Warming and Acidification" Biology 11, no. 10: 1394. https://doi.org/10.3390/biology11101394
APA StyleCourt, M., Paula, J. R., Macau, M., Otjacques, E., Repolho, T., Rosa, R., & Lopes, V. M. (2022). Camouflage and Exploratory Avoidance of Newborn Cuttlefish under Warming and Acidification. Biology, 11(10), 1394. https://doi.org/10.3390/biology11101394