The Arabidopsis Receptor-like Kinase CAP1 Promotes Shoot Growth under Ammonium Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Leaf Area and Epidermal Cell Area Measurements
2.3. Gene Expression Analysis
2.4. DAB staining and Image Analysis
3. Results
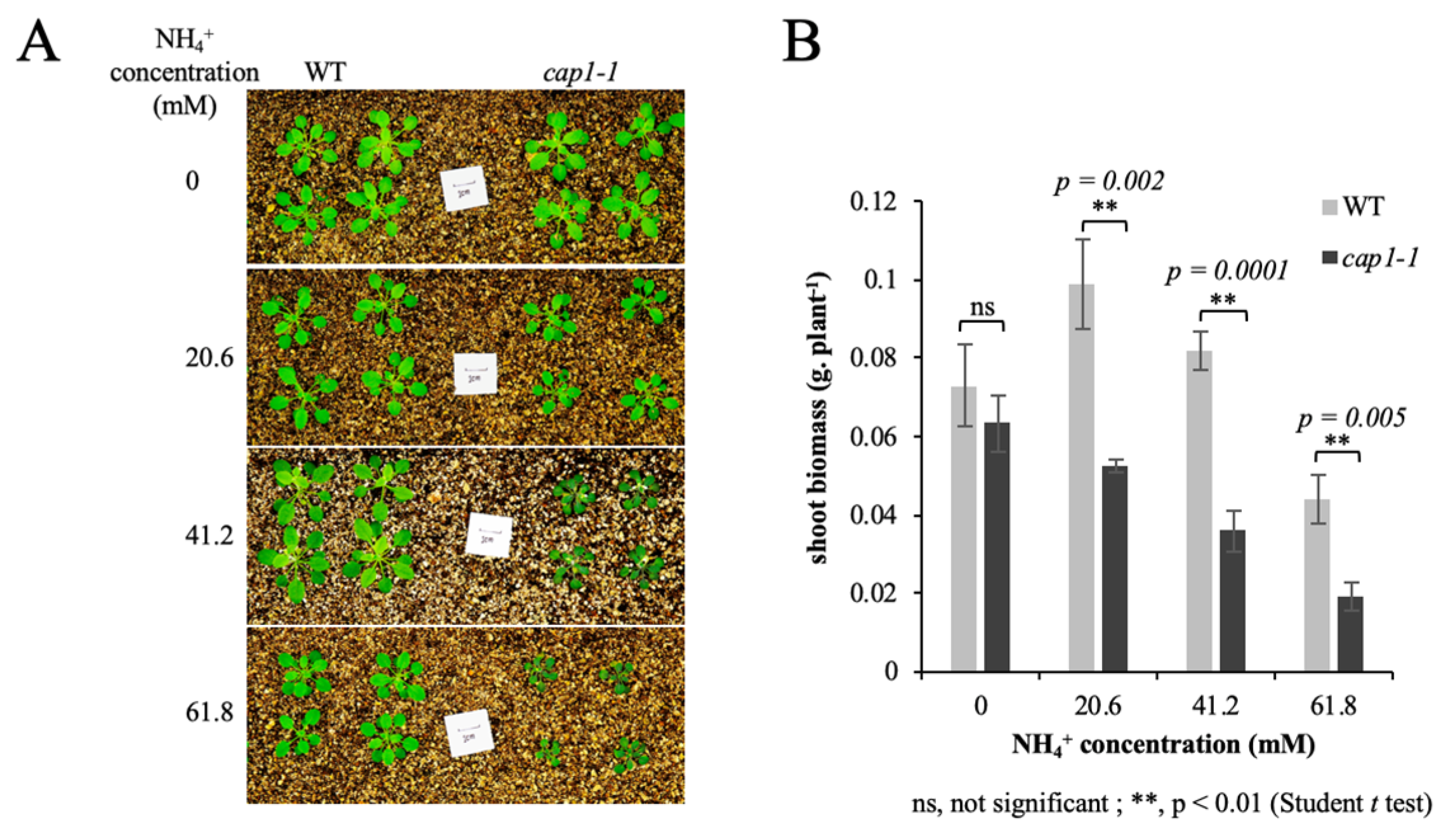
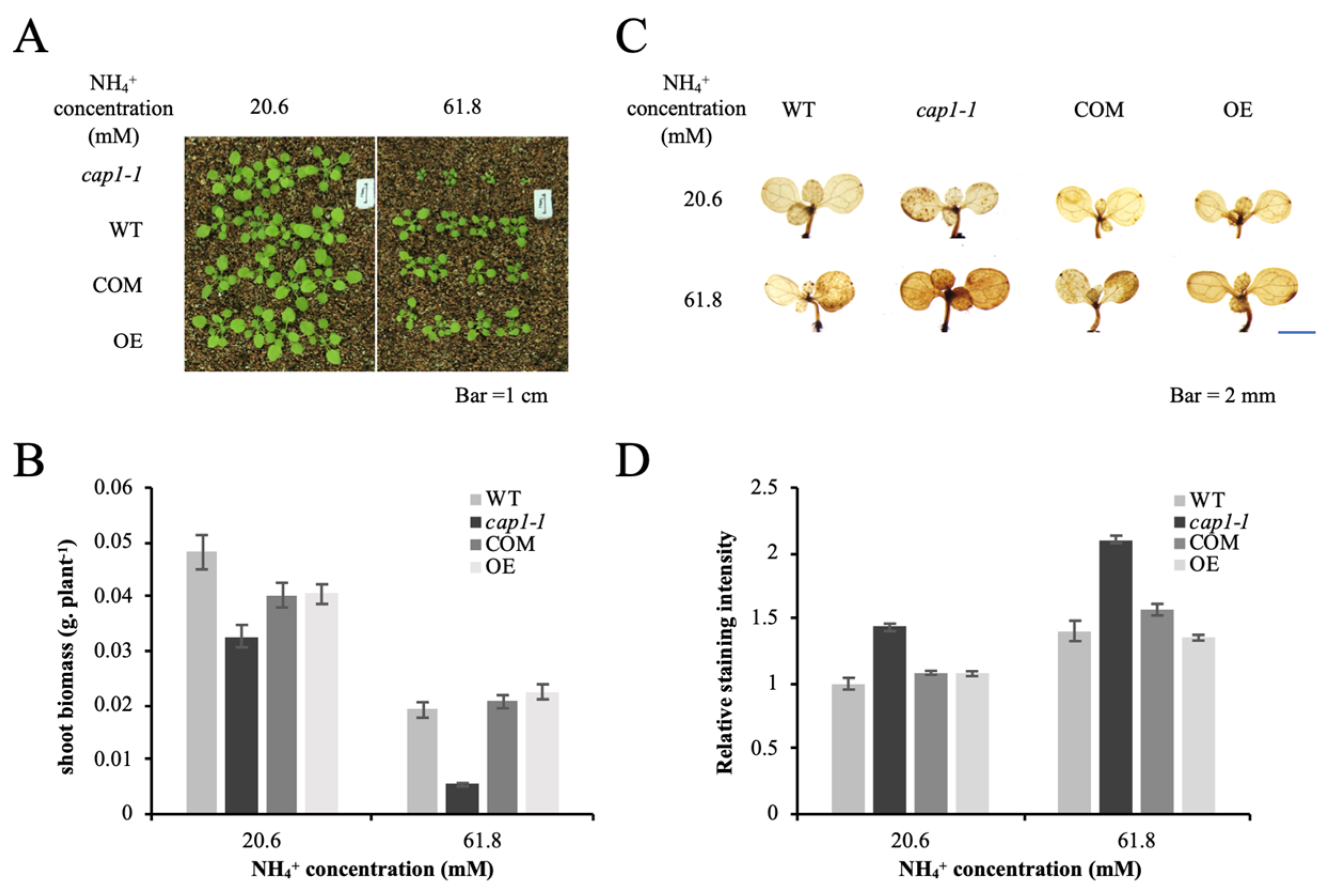
3.1. Deficiency of CAP1 Enhances NH4+-Mediated Inhibition of Shoot Growth in A. thaliana
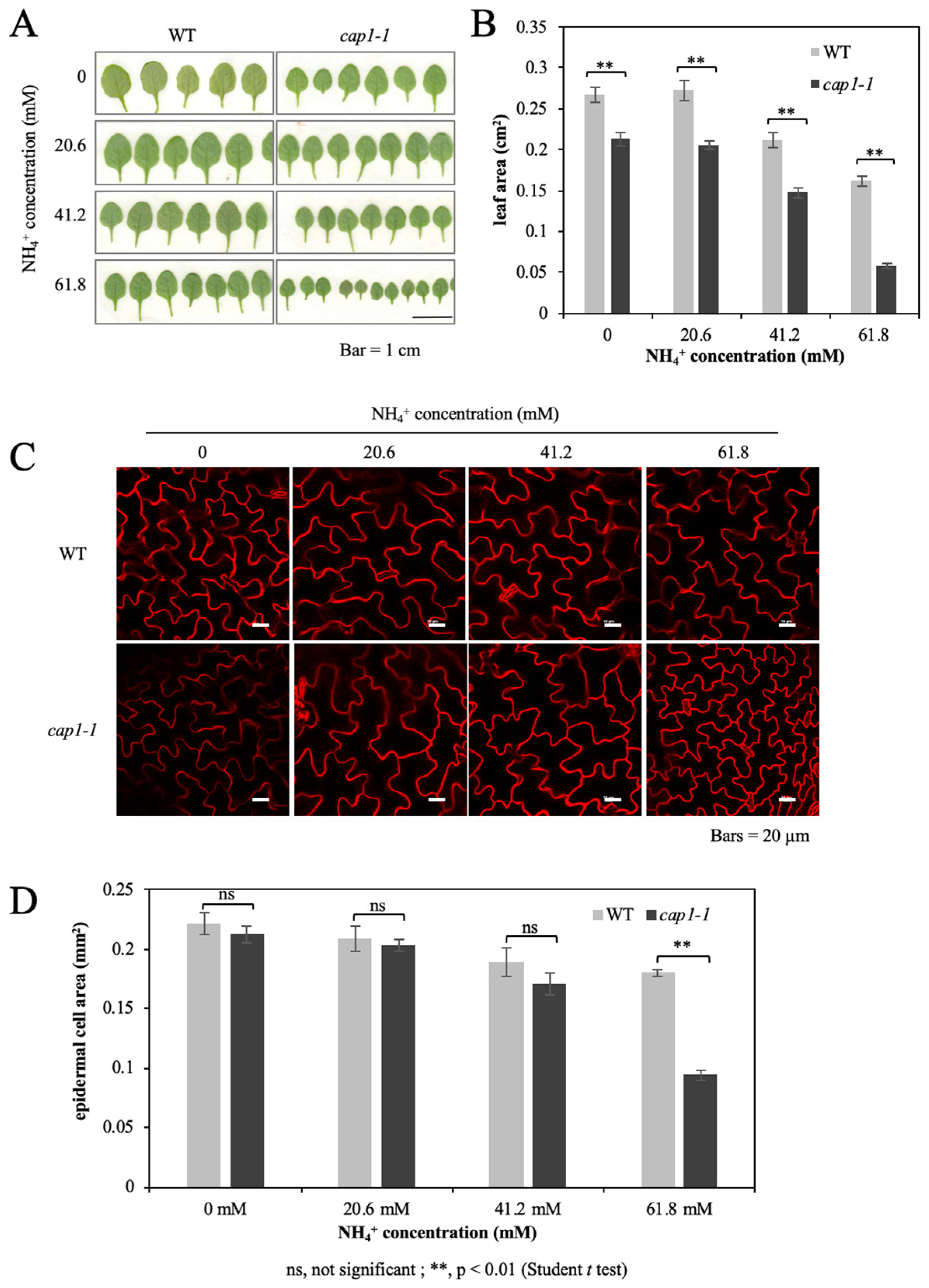
3.2. The cap1-1 Mutant Produces Smaller Leaves under High NH4+
3.3. Deficiency of CAP1 Enhanced the Downregulation of Cell Enlargement-Related Genes under NH4+ Stress
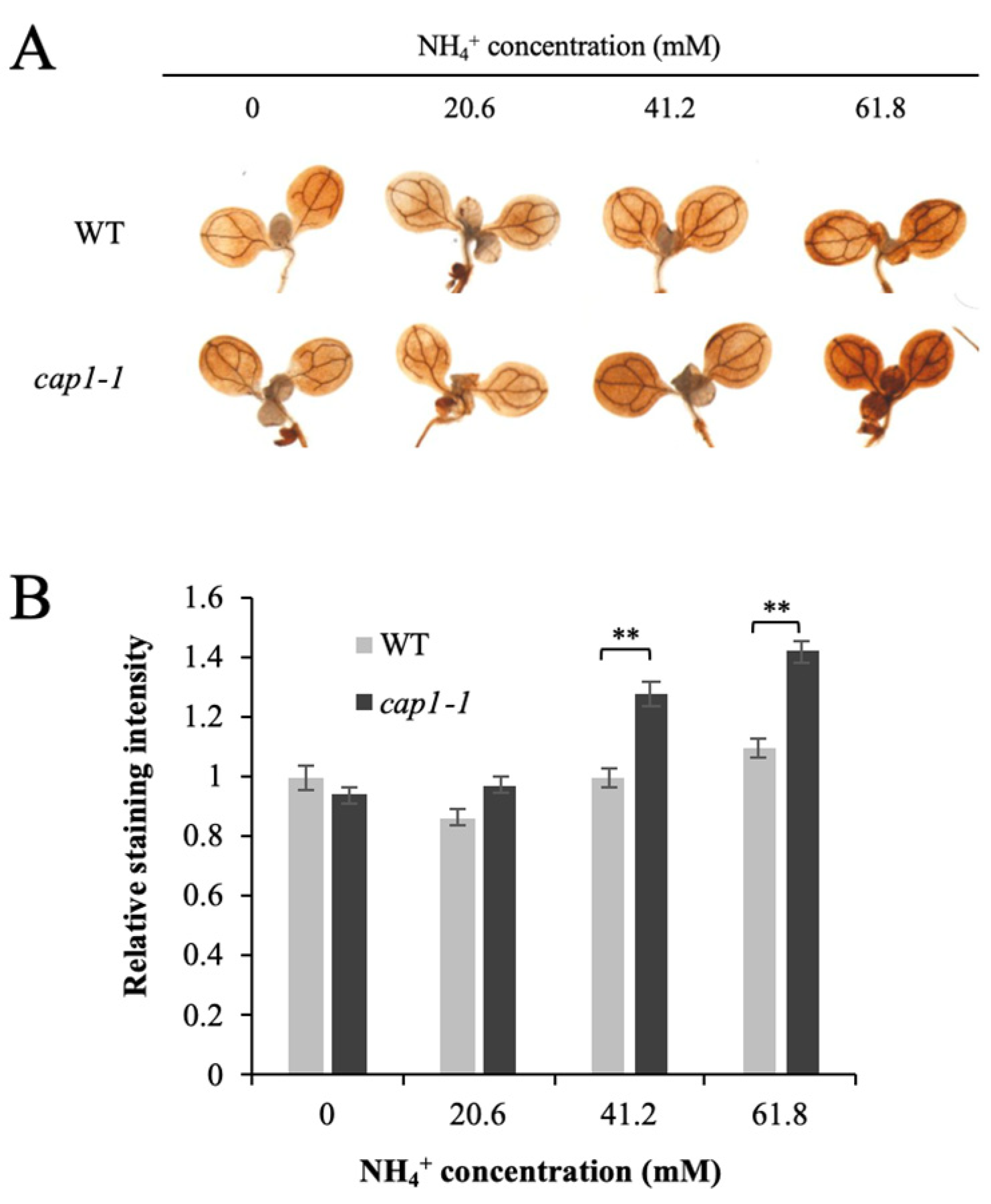
3.4. Deficiency of CAP1 Causes Higher NH4+-Induced ROS Levels in Shoots
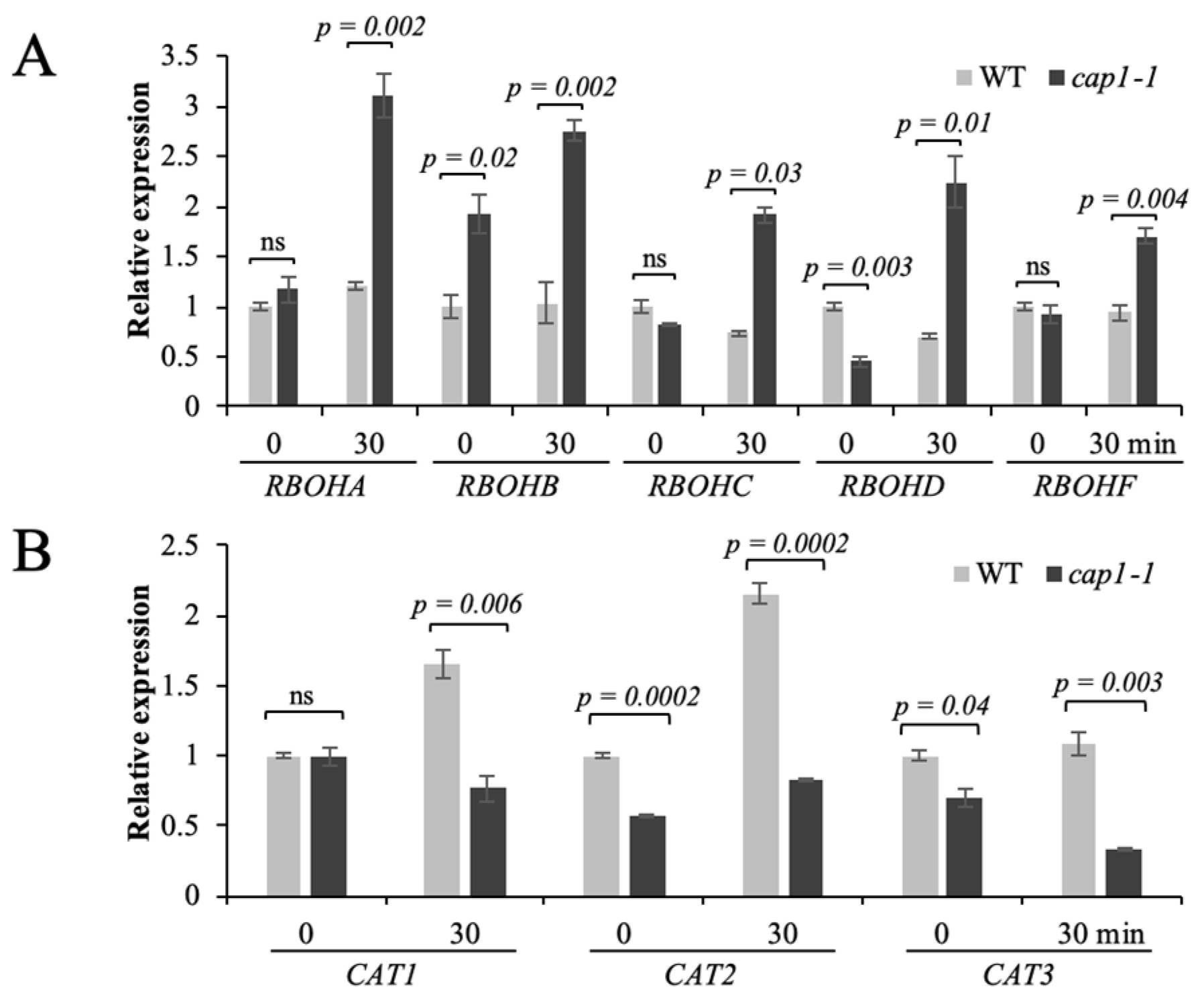
3.5. CAP1 Regulates the Transcription of AtRBOH and CAT Genes in Response to NH4+
3.6. CAP1 Transgenic Lines Suppress the Hypersensitivity of cap1-1 Mutants to NH4+
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Sanchez-Zabala, J.; Gonzalez-Murua, C.; Marino, D. Mild ammonium stress increases chlorophyll content in Arabidopsis thaliana. Plant Signal. Behav. 2015, 10, e991596. [Google Scholar] [CrossRef] [PubMed]
- Glass, A.D.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Okamoto, M.; Rawat, S.; Siddiqi, M.Y.; Unkles, S.E.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A.D.M.; Britto, D.T. Root ammonium transport efficiency as a determinant in forest colonization patterns: An hypothesis. Physiol. Plant. 2003, 117, 164–170. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Li, B.; Li, G.; Kronzucker, H.J.; Baluska, F.; Shi, W. Ammonium stress in Arabidopsis: Signaling, genetic loci, and physiological targets. Trends Plant Sci. 2014, 19, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Review: Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef]
- Bittsánszky, A.; Pilinszky, K.; Gyulai, G.; Komives, T. Overcoming ammonium toxicity. Plant Sci. 2015, 231, 184–190. [Google Scholar] [CrossRef]
- Di, D.W.; Sun, L.; Zhang, X.N.; Li, G.J.; Kronzucker, H.J.; Shi, W.M. Involvement of auxin in the regulation of ammonium tolerance in rice (Oryza sativa L.). Plant Soil 2018, 432, 373–387. [Google Scholar] [CrossRef]
- Raab, T.K.; Terry, N. Nitrogen Source Regulation of Growth and Photosynthesis in Beta vulgaris L. Plant Physiol. 1994, 105, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Gerendás, J.; Zhu, Z.; Bendixen, R.; Ratcliffe, R.G.; Sattelmacher, B. Physiological and biochemical processes related to ammonium toxicity in higher plants. J. Plant Nutr. Soil Sci. 1997, 160, 239–251. [Google Scholar] [CrossRef]
- Jin, M.; Guo, M.; Yue, G.; Li, J.; Yang, S.; Zhao, P.; Su, Y. An unusual strategy of stomatal control in the desert shrub Ammopiptanthus mongolicus. Plant Physiol. Biochem. 2018, 125, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Blumwald, E. The Roles of ROS and ABA in Systemic Acquired Acclimation. Plant Cell 2015, 27, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Yang, S.Y.; Hao, D.L.; Jin, M.; Li, Y.; Liu, Z.T.; Huang, Y.N.; Chen, T.X.; Su, Y.H. Internal ammonium excess induces ROS-mediated reactions and causes carbon scarcity in rice. BMC Plant Biol. 2020, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Shi, D.L.; Hu, Z.B.; Xu, F.L.; Chen, Y.H.; Shen, Z.G. Subcellular accumulation and source of O−2 and H2O2 in submerged plant Hydrilla verticillata (L.f.) Royle under NH4+-N stress condition. Aquat. Toxicol. 2019, 207, 1–12. [Google Scholar] [CrossRef]
- Sun, L.; Di, D.W.; Li, G.; Kronzucker, H.J.; Wu, X.; Shi, W. Endogenous ABA alleviates rice ammonium toxicity by reducing ROS and free ammonium via regulation of the SAPK9-bZIP20 pathway. J. Exp. Bot. 2020, 71, 4562–4577. [Google Scholar] [CrossRef]
- Li, G.; Zhang, L.; Wang, M.; Di, D.; Kronzucker, H.J.; Shi, W. The Arabidopsis AMOT1/EIN3 gene plays an important role in the amelioration of ammonium toxicity. J. Exp. Bot. 2019, 70, 1375–1388. [Google Scholar] [CrossRef]
- Smirnoff, N.; Arnaud, D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019, 221, 1197–1214. [Google Scholar] [CrossRef]
- Sagi, M.; Fluhr, R. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006, 141, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Seo, P.J.; Lee, H.J.; Park, C.M. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. Plant J. 2012, 70, 831–844. [Google Scholar] [CrossRef]
- Xie, Y.J.; Mao, Y.; Xu, S.; Zhou, H.; Duan, X.L.; Cui, W.T.; Zhang, J.; Xu, G.H. Heme-heme oxygenase 1 system is involved in ammonium tolerance by regulating antioxidant defence in Oryza sativa. Plant Cell Environ. 2015, 38, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Li, W.; Zhang, W.J. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquat. Toxicol. 2008, 87, 88–98. [Google Scholar] [CrossRef]
- Nimptsch, J.; Pflugmacher, S. Ammonia triggers the promotion of oxidative stress in the aquatic macrophyte Myriophyllum mattogrossense. Chemosphere 2007, 66, 708–714. [Google Scholar] [CrossRef]
- Husted, S.; Schjoerring, J.K. Apoplastic pH and Ammonium Concentration in Leaves of Brassica napus L. Plant Physiol. 1995, 109, 1453–1460. [Google Scholar] [CrossRef]
- Podgorska, A.; Burian, M.; Gieczewska, K.; Ostaszewska-Bugajska, M.; Zebrowski, J.; Solecka, D.; Szal, B. Altered Cell Wall Plasticity Can Restrict Plant Growth under Ammonium Nutrition. Front. Plant Sci. 2017, 8, 1344. [Google Scholar] [CrossRef]
- Li, B.; Li, Q.; Xiong, L.; Kronzucker, H.J.; Kramer, U.; Shi, W. Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiol. 2012, 160, 2040–2051. [Google Scholar] [CrossRef]
- Nissen, K.S.; Willats, W.G.T.; Malinovsky, F.G. Understanding CrRLK1L Function: Cell Walls and Growth Control. Trends Plant Sci. 2016, 21, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Wu, H.M. THESEUS 1, FERONIA and relatives: A family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 2011, 14, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Ma, X.; Zhang, G.; Song, S.; Zhou, Y.; Gao, L.; Miao, Y.; Song, C.P. A Receptor-Like Kinase Mediates Ammonium Homeostasis and Is Important for the Polar Growth of Root Hairs in Arabidopsis. Plant Cell 2014, 26, 1497–1511. [Google Scholar] [CrossRef] [PubMed]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.H.; Kronzucker, H.J.; Shi, W.M. Root growth inhibition by NH4+ in Arabidopsis is mediated by the root tip and is linked to NH4+ efflux and GMPase activity. Plant Cell Environ. 2010, 33, 1529–1542. [Google Scholar] [CrossRef]
- Barth, C.; Gouzd, Z.A.; Steele, H.P.; Imperio, R.M. A mutation in GDP-mannose pyrophosphorylase causes conditional hypersensitivity to ammonium, resulting in Arabidopsis root growth inhibition, altered ammonium metabolism, and hormone homeostasis. J. Exp. Bot. 2010, 61, 379–394. [Google Scholar] [CrossRef]
- Qin, C.; Qian, W.; Wang, W.; Wu, Y.; Yu, C.; Jiang, X.; Wang, D.; Wu, P. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 18308–18313. [Google Scholar] [CrossRef]
- Li, G.; Dong, G.; Li, B.; Li, Q.; Kronzucker, H.J.; Shi, W. Isolation and characterization of a novel ammonium overly sensitive mutant, amos2, in Arabidopsis thaliana. Planta 2012, 235, 239–252. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J. Exp. Bot. 2000, 51, 227–237. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, N.W.; Gao, K.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Ammonium Inhibits Primary Root Growth by Reducing the Length of Meristem and Elongation Zone and Decreasing Elemental Expansion Rate in the Root Apex in Arabidopsis thaliana. PLoS ONE 2013, 8, e61031. [Google Scholar] [CrossRef]
- Duan, Q.; Kita, D.; Li, C.; Cheung, A.Y.; Wu, H.M. FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 2010, 107, 17821–17826. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, L.; Ye, H.; Yu, X.; Algreen, A.; Yin, Y. Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2009, 106, 7648–7653. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Restrepo, J.M.; Huck, N.; Kessler, S.; Gagliardini, V.; Gheyselinck, J.; Yang, W.C.; Grossniklaus, U. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 2007, 317, 656–660. [Google Scholar] [CrossRef] [PubMed]
- Hématy, K.; Sado, P.E.; Van Tuinen, A.; Rochange, S.; Desnos, T.; Balzergue, S.; Pelletier, S.; Renou, J.P.; Hofte, H. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 2007, 17, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, Y.Y.; Gao, X.; Du, G.; Ma, X.X.; Liu, M.; Guo, J.S.; Chen, Y.P. Ammonium-induced oxidative stress on plant growth and antioxidative response of duckweed (Lemna minor L.). Ecol. Eng. 2013, 58, 355–362. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Li, W.; Lu, J. Effects of ammonium on the antioxidative response in Hydrilla verticillata (L.f.) Royle plants. Ecotoxicol. Environ. Saf. 2010, 73, 189–195. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014, 5, 771. [Google Scholar] [CrossRef]
- Podgórska, A.; Ostaszewska, M.; Gardeström, P.; Rasmusson, A.G.; Szal, B. In comparison with nitrate nutrition, ammonium nutrition increases growth of the frostbite1 Arabidopsis mutant. Plant Cell Environ. 2015, 38, 224–237. [Google Scholar] [CrossRef]


| Gene | Primer Sequence |
|---|---|
| EXP1 (At1g69530) | F: GAAGAGTGCCGTGCGTGAG R: TAGGTTGAAGTAAGAGTGTCCGTTT |
| EXP17 (At4g01630) | F: GCCTCTGGTACAATGGGTGG R: TGAGCAAAGTTCGGTGGACA |
| PG1 (At3g26610) | F: CCAATGAAACCAACGGCACT R: TGACTTTGACTCCATCCGAATC |
| Putative-PG (At2g43890) | F: ACAGGGTTCTGGAGTGAAGATTAGTC R: TCGCTTGGCACGGATTACT |
| FER (At3g51550) | F: TGCCGTCACTTCTCGTTTGC R: CTTGCTCGGACATTGGGTTG |
| THE1 (At5g54380) | F: TCAAGAAGGCGGTAATGGACA R: CAACCCCAAGCAACGAACTC |
| RBOHA (At5g07390) | F: AGGGGTCGTTTGACTGGTTC R: CTCGTAAACGCTGGTGCAGT |
| RBOHB (At1g09090) | F: CGAGGTGATGGGCTACTGTG R: TGTTCCTACAAACGGGCAAG |
| RBOHC (At5g51060) | F: GAGAACAGTCAGAGAACACGTA R: TTCGTTTCAGCAAAGTAAGCTC |
| RBOHD (At5g47910) | F: GCTCCGTGCTTTCAGATCAA R: TTTGAATCCTTGTGGCTTCG |
| RBOHF (At1g64060) | F: GCCGACGAAACAACAAAGAA R: CACCAATGCCAAGACCAACT |
| CAT1 (At1g20630) | F: CTGGGATTCAGACAGGCAAGA R: AACCAAACCGTAAGAGGAGCAT |
| CAT2 (At4g35090) | F: CCCGTGTCTTCTCCTATGCC R: TAACCTCCTCGTCCCTGTGC |
| CAT3 (At1g20620) | F: AATCACAGCCACGCCACTAA R: TCAGAACCAAGCGACCAACC |
| UBC9 (At4g27960) | F: CACAATTTCCAAGGTGCTGCTATCG R: GTACATGTGAGCTATCTCAGGGACCAAA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, Q.; Dong, N.; Yang, H.; Feng, F.; Xu, Y.; Wang, C.; Yang, Y.; Ma, X.; Bai, L. The Arabidopsis Receptor-like Kinase CAP1 Promotes Shoot Growth under Ammonium Stress. Biology 2022, 11, 1452. https://doi.org/10.3390/biology11101452
You Q, Dong N, Yang H, Feng F, Xu Y, Wang C, Yang Y, Ma X, Bai L. The Arabidopsis Receptor-like Kinase CAP1 Promotes Shoot Growth under Ammonium Stress. Biology. 2022; 11(10):1452. https://doi.org/10.3390/biology11101452
Chicago/Turabian StyleYou, Qingye, Nannan Dong, Hong Yang, Fang Feng, Yifei Xu, Chong Wang, Yilan Yang, Xiaonan Ma, and Ling Bai. 2022. "The Arabidopsis Receptor-like Kinase CAP1 Promotes Shoot Growth under Ammonium Stress" Biology 11, no. 10: 1452. https://doi.org/10.3390/biology11101452
APA StyleYou, Q., Dong, N., Yang, H., Feng, F., Xu, Y., Wang, C., Yang, Y., Ma, X., & Bai, L. (2022). The Arabidopsis Receptor-like Kinase CAP1 Promotes Shoot Growth under Ammonium Stress. Biology, 11(10), 1452. https://doi.org/10.3390/biology11101452







