Carbon Footprint Management by Agricultural Practices
Abstract
:Simple Summary
Abstract
1. Introduction
2. Carbon Footprint Due to Environmental Factors
3. Land-Use Changes and Carbon Footprint
4. Agriculture and Carbon Footprint
5. Role of Soil in Carbon Footprint and Agriculture
5.1. Soil Types
5.2. Soil Health (Feedback Mechanism)
5.3. Carbon Stabilization and Storage
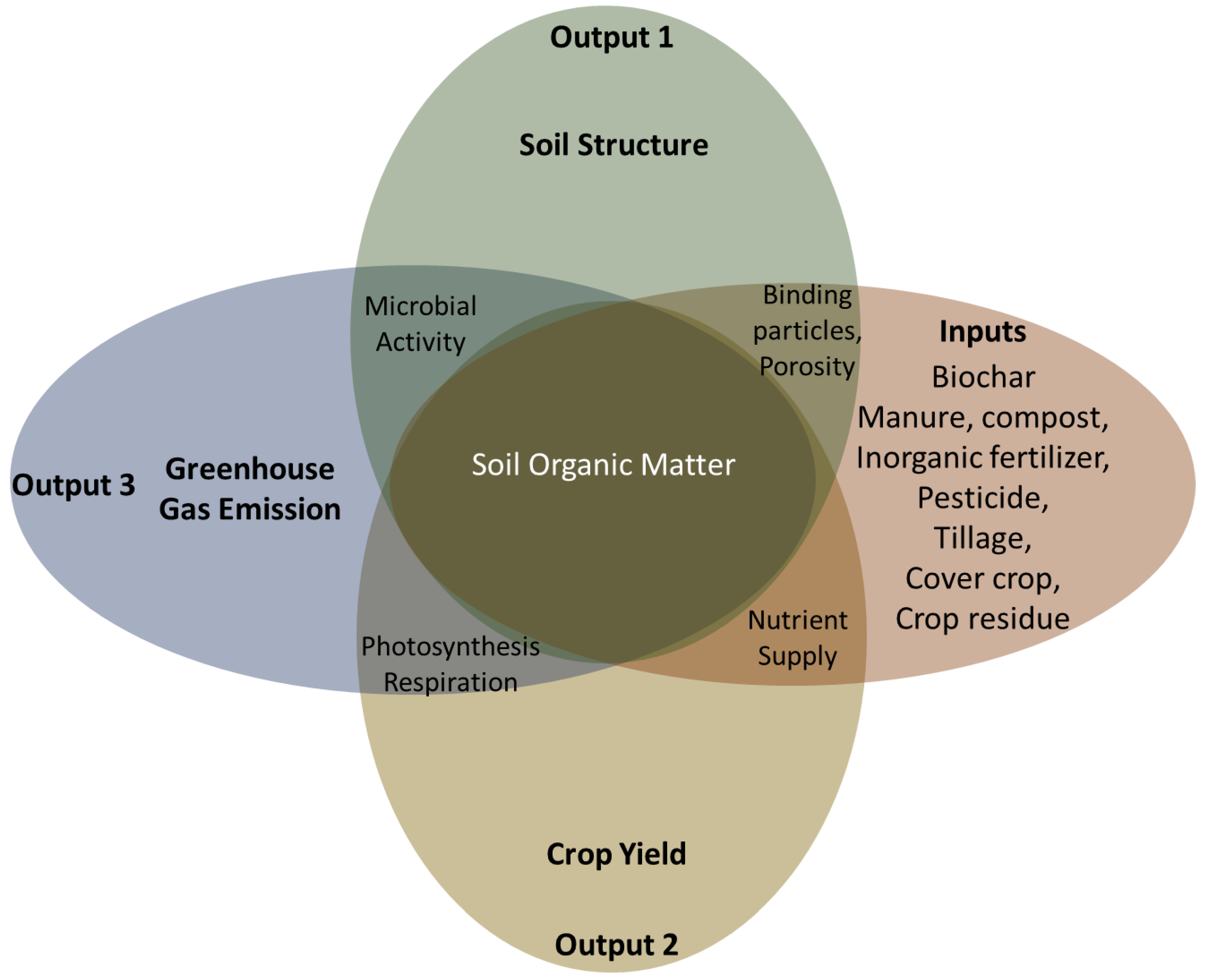
6. How Does the System Work?
6.1. Inputs (Carbon Sequestration)
6.1.1. Fertilizers

6.1.2. Manure Applications
6.1.3. Biochar
6.1.4. Crop Residues
6.1.5. Photosynthesis
6.2. Outputs (Carbon Emissions)
6.2.1. CO2 Emission (Soil Respiration)
6.2.2. CH4 Emission
6.2.3. N2O Emission
6.2.4. Carbon Leaching
6.2.5. Pesticides and Herbicides
6.2.6. Tillage
6.2.7. CO2 (Tractors), Harvesting, and Runoff
7. Carbon Footprint Calculations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prasad, P.V.V.; Thomas, J.M.G.; Narayanan, S. Global Warming Effects. Encycl. Appl. Plant Sci. 2017, 3, 289–299. [Google Scholar] [CrossRef]
- Denise, C. Climate Change Report: Experts React, Live Science. 2013, 150 5th Avenue, 9th Floor, New York. Available online: https://www.livescience.com/40021-ipcc-climate-change-report-reactions.html (accessed on 20 September 2022).
- Wiedmann, T.; Minx, J. A definition of ‘carbon footprint’. Ecol. Econ. Res. Trends 2008, 1, 1–11. [Google Scholar]
- Gao, T.; Liu, Q.; Wang, J. A comparative study of carbon footprint and assessment standards. Int. J. Low-Carbon Technol. 2013, 9, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Notarnicola, B.; Tassielli, G.; Renzulli, P.A.; Castellani, V.; Sala, S. Environmental impacts of food consumption in Europe. J. Clean. Prod. 2017, 140, 753–765. [Google Scholar] [CrossRef]
- Tandonl, S.; Singh, S. Energy balance in conservation agriculture and conventional farming: A comparison. Conserv. Agric. 2010, 4. [Google Scholar]
- Sejian, V.; Prasadh, R.S.; Lees, A.M.; Lees, J.C.; Al-Hosni, Y.A.; Sullivan, M.L.; Gaughan, J.B. Assessment of the carbon footprint of four commercial dairy production systems in Australia using an integrated farm system model. Carbon Manag. 2018, 9, 57–70. [Google Scholar] [CrossRef]
- Wilson, D.C.; Rodic, L.; Modak, P.; Soos, R.; Carpintero, A.; Velis, K.; Iyer, M.; Simonett, O. Global Waste Management Outlook; United Nations Environment Programme (UNEP): Viena, Austria, 2015; 346p. [Google Scholar]
- Del Prado, A.; Mas, K.; Pardo, G.; Gallejones, P. Modelling the interactions between C and N farm balances and GHG emissions from confinement dairy farms in northern Spain. Sci. Total Environ. 2013, 465, 156–165. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of surface GHG fluxes to long-term manure and inorganic fertilizer application in corn and soybean rotation. Sci. Total Environ. 2018, 626, 817–825. [Google Scholar] [CrossRef]
- Babur, E.; Dindaroglu, T. Seasonal changes of soil organic carbon and microbial biomass carbon in different forest ecosystems. Environ. Factors Affect. Hum. Health 2020, 1, 1–21. [Google Scholar]
- Babur, E.; Dindaroğlu, T.; Riaz, M.; Uslu, O.S. Seasonal Variations in Litter Layers’ Characteristics Control Microbial Respiration and Microbial Carbon Utilization Under Mature Pine, Cedar, and Beech Forest Stands in the Eastern Mediterranean Karstic Ecosystems. Microb. Ecol. 2021, 84, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Oertel, C.; Matschullat, J.; Zurba, K.; Zimmermann, F.; Erasmi, S. Greenhouse gas emissions from soils—A review. Chem. Der Erde-Geochem. 2016, 76, 327–352. [Google Scholar] [CrossRef] [Green Version]
- Fowler, D.; Pilegaard, K.; Sutton, M.; Ambus, P.; Raivonen, M.; Duyzer, J.; Simpson, D.; Fagerli, H.; Fuzzi, S.; Schjørring, J.K. Atmospheric composition change: Ecosystems–atmosphere interactions. Atmos. Environ. 2009, 43, 5193–5267. [Google Scholar] [CrossRef]
- Brümmer, C.; Brüggemann, N.; Butterbach-Bahl, K.; Falk, U.; Szarzynski, J.; Vielhauer, K.; Wassmann, R.; Papen, H. Soil-atmosphere exchange of N2O and NO in near-natural savanna and agricultural land in Burkina Faso (W. Africa). Ecosystems 2008, 11, 582–600. [Google Scholar] [CrossRef]
- Smith, K.; Ball, T.; Conen, F.; Dobbie, K.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef] [Green Version]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Butterbach-Bahl, K. Effects of soil moisture and temperature on NO, NO2, and N2O emissions from European forest soils. J. Geophys. Res. Atmos. 2004, 109, D17. [Google Scholar] [CrossRef]
- Fang, C.; Moncrieff, J. The dependence of soil CO2 efflux on temperature. Soil Biol. Biochem. 2001, 33, 155–165. [Google Scholar] [CrossRef]
- Izaurralde, R.; Lemke, R.L.; Goddard, T.W.; McConkey, B.; Zhang, Z. Nitrous oxide emissions from agricultural toposequences in Alberta and Saskatchewan. Soil Sci. Soc. Am. J. 2004, 68, 1285–1294. [Google Scholar] [CrossRef]
- Kim, Y.S. Soil-atmosphere exchange of CO2, CH4 and N2O in northern temperate forests: Effects of elevated CO2 concentration, N deposition and forest fire. Eurasian J. For. Res. 2013, 16, 1–43. [Google Scholar]
- FAO. FAOSTAT. Food and Agriculture Organization of the United Nations, Rome. 2014. Available online: https://www.fao.org/faostat/en/#home (accessed on 20 September 2022).
- Babur, E.; Dindaroğlu, T.; Roy, R.; Seleiman, M.F.; Ozlu, E.; Battaglia, M.L.; Uslu, Ö.S. Relationship between organic matter and microbial biomass in different vegetation types. In Microbial Syntrophy-Mediated Eco-enterprising; Elsevier: Amsterdam, The Netherlands, 2022; pp. 225–245. [Google Scholar]
- Saiz, G.; Byrne, K.A.; BUTTERBACH-BAHL, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Change Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- DeGryze, S.; Six, J.; Paustian, K.; Morris, S.J.; Paul, E.A.; Merckx, R. Soil organic carbon pool changes following land-use conversions. Glob. Change Biol. 2004, 10, 1120–1132. [Google Scholar] [CrossRef] [Green Version]
- Verhoeven, J.T.; Setter, T.L. Agricultural use of wetlands: Opportunities and limitations. Ann. Bot. 2009, 105, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minkkinen, K.; Laine, J. Long-term effect of forest drainage on the peat carbon stores of pine mires in Finland. Can. J. For. Res. 1998, 28, 1267–1275. [Google Scholar] [CrossRef]
- Latham, J.; Cumani, R.; Rosati, I.; Bloise, M. Global Land Cover SHARE (GLC-SHARE) database Beta-Release Version 1.0; FAO-Food and Agriculture Organization of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Lal, R.; Follett, R.F. Soil Carbon Sequestration and the Greenhouse Effect. ASA-CSSA-SSSA: Madison, WI, USA, 2009; Volume 57. [Google Scholar]
- Dorodnikov, M.; Blagodatskaya, E.; Blagodatsky, S.; Marhan, S.; Fangmeier, A.; Kuzyakov, Y. Stimulation of microbial extracellular enzyme activities by elevated CO2 depends on soil aggregate size. Glob. Change Biol. 2009, 15, 1603–1614. [Google Scholar] [CrossRef]
- Ding, W.; Cai, Z.; Tsuruta, H.; Li, X. Key factors affecting spatial variation of methane emissions from freshwater marshes. Chemosphere 2003, 51, 167–173. [Google Scholar] [CrossRef]
- Massé, D.; Talbot, G.; Gilbert, Y. On farm biogas production: A method to reduce GHG emissions and develop more sustainable livestock operations. Anim. Feed Sci. Technol. 2011, 166, 436–445. [Google Scholar] [CrossRef]
- Qi, J.-Y.; Yang, S.-T.; Xue, J.-F.; Liu, C.-X.; Du, T.-Q.; Hao, J.-P.; Cui, F.-Z. Response of carbon footprint of spring maize production to cultivation patterns in the Loess Plateau, China. J. Clean. Prod. 2018, 187, 525–536. [Google Scholar] [CrossRef]
- Lal, R. Reducing carbon footprints of agriculture and food systems. Carbon Footpr. 2022, 1, 3. [Google Scholar] [CrossRef]
- Baldock, J.; Wheeler, I.; McKenzie, N.; McBrateny, A. Soils and climate change: Potential impacts on carbon stocks and greenhouse gas emissions, and future research for Australian agriculture. Crop Pasture Sci. 2012, 63, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Ball, B. Soil structure and greenhouse gas emissions: A synthesis of 20 years of experimentation. Eur. J. Soil Sci. 2013, 64, 357–373. [Google Scholar] [CrossRef]
- Severin, M.; Fuß, R.; Well, R.; Garlipp, F.; Van den Weghe, H. Soil, slurry and application effects on greenhouse gas emissions. Plant Soil Environ. 2016, 61, 344–351. [Google Scholar] [CrossRef] [Green Version]
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar properties influencing greenhouse gas emissions in tropical soils differing in texture and mineralogy. J. Environ. Qual. 2016, 45, 1509–1519. [Google Scholar] [CrossRef] [Green Version]
- Arah, J.; Smith, K.; Crichton, I.; Li, H. Nitrous oxide production and denitrification in Scottish arable soils. J. Soil Sci. 1991, 42, 351–367. [Google Scholar] [CrossRef]
- Ozlu, E. Dynamics of Soil Aggregate Formation in Different Ecosystems; University of Wiconsin-Madison: Madison, WI, USA, 2020. [Google Scholar]
- Dexter, A.R. Soil physical quality: Part I. Theory, effects of soil texture, density, and organic matter, and effects on root growth. Geoderma 2004, 120, 201–214. [Google Scholar] [CrossRef]
- Gu, J.; Nicoullaud, B.; Rochette, P.; Grossel, A.; Hénault, C.; Cellier, P.; Richard, G. A regional experiment suggests that soil texture is a major control of N2O emissions from tile-drained winter wheat fields during the fertilization period. Soil Biol. Biochem. 2013, 60, 134–141. [Google Scholar] [CrossRef]
- Ozlu, E.; Arriaga, F.J. The role of carbon stabilization and minerals on soil aggregation in different ecosystems. Catena 2021, 202, 105303. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Wrage, N.; Metay, A.; CHOTTE, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Change Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Weslien, P.; Kasimir Klemedtsson, Å.; Börjesson, G.; Klemedtsson, L. Strong pH influence on N2O and CH4 fluxes from forested organic soils. Eur. J. Soil Sci. 2009, 60, 311–320. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Hergert, G.W.; Nielsen, R.A. Cattle manure application reduces soil compactibility and increases water retention after 71 years. Soil Sci. Soc. Am. J. 2015, 79, 212–223. [Google Scholar] [CrossRef]
- Ozlu, E. Long-Term Impacts of Annual Cattle Manure and Fertilizer on Soil Quality under Corn-Soybean Rotation in Eastern South Dakota. Master’s Thesis, South Dakota State University, Brookings, SD, USA, 2016. [Google Scholar]
- Ozlu, E.; Kumar, S.; Arriaga, F.J. Responses of Long-Term Cattle Manure on Soil Physical and Hydraulic Properties under a Corn-Soybean Rotation at Two Locations in Eastern South Dakota. Soil Sci. Soc. Am. J. 2019, 83, 1459–1467. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 1996, 47, 151–163. [Google Scholar] [CrossRef]
- Yadav, G.S.; Lal, R.; Meena, R.S.; Datta, M.; Babu, S.; Das, A.; Layek, J.; Saha, P. Energy budgeting for designing sustainable and environmentally clean/safer cropping systems for rainfed rice fallow lands in India. J. Clean. Prod. 2017, 158, 29–37. [Google Scholar] [CrossRef]
- Pishgar-Komleh, S.H.; Akram, A.; Keyhani, A.; Raei, M.; Elshout, P.M.F.; Huijbregts, M.A.J.; van Zelm, R. Variability in the carbon footprint of open-field tomato production in Iran - A case study of Alborz and East-Azerbaijan provinces. J. Clean. Prod. 2017, 142, 1510–1517. [Google Scholar] [CrossRef]
- Günther, J.; Thevs, N.; Gusovius, H.-J.; Sigmund, I.; Brückner, T.; Beckmann, V.; Abdusalik, N. Carbon and phosphorus footprint of the cotton production in Xinjiang, China, in comparison to an alternative fibre (Apocynum) from Central Asia. J. Clean. Prod. 2017, 148, 490–497. [Google Scholar] [CrossRef]
- Bos, J.F.F.P.; Haan, J.d.; Sukkel, W.; Schils, R.L.M. Energy use and greenhouse gas emissions in organic and conventional farming systems in the Netherlands. NJAS Wagen. J. Life Sci. 2014, 68, 61–70. [Google Scholar] [CrossRef]
- Xu, X.; Lan, Y. A comparative study on carbon footprints between plant- and animal-based foods in China. J. Clean. Prod. 2016, 112, 2581–2592. [Google Scholar] [CrossRef]
- Haynes, R.J.; Naidu, R. Influence of lime, fertilizer and manure applications on soil organic matter content and soil physical conditions: A review. Nutr. Cycl. Agroecosystems 1998, 51, 123–137. [Google Scholar] [CrossRef]
- Khalid, A.A.; Tuffour, H.O.; Bonsu, M. Influence of poultry manure and NPK fertilizer on hydraulic properties of a sandy soil in Ghana. Int. J. Sci. Res. Agric. Sci. 2014, 1, 16–22. [Google Scholar] [CrossRef]
- Lawal, H.; Girei, H. Infiltration and organic carbon pools under the long term use of farm yard manure and mineral fertilizer. Int. J. Adv. Agric. Res 2013, 1, 92–101. [Google Scholar]
- Zhao, Y.; Wang, P.; Li, J.; Chen, Y.; Ying, X.; Liu, S. The effects of two organic manures on soil properties and crop yields on a temperate calcareous soil under a wheat–maize cropping system. Eur. J. Agron. 2009, 31, 36–42. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Chapra, S.; Wedepohl, R.; Sims, J.; Daniel, T.C.; Reddy, K. Managing agricultural phosphorus for protection of surface waters: Issues and options. J. Environ. Qual. 1994, 23, 437–451. [Google Scholar] [CrossRef]
- Edwards, D.; Daniel, T. Environmental impacts of on-farm poultry waste disposal—A review. Bioresour. Technol. 1992, 41, 9–33. [Google Scholar] [CrossRef]
- Ozlu, E.; Kumar, S. Response of Soil Organic Carbon, pH, Electrical Conductivity, and Water Stable Aggregates to Long-Term Annual Manure and Inorganic Fertilizer. Soil Sci. Soc. Am. J. 2018, 82, 1243–1251. [Google Scholar] [CrossRef]
- Di, J.; Xu, M.; Zhang, W.; Tong, X.; He, X.; Gao, H.; Liu, H.; Wang, B. Combinations of soil properties, carbon inputs and climate control the saturation deficit dynamics of stable soil carbon over 17-year fertilizaiton. Sci. Rep. 2018, 8, 12653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozlu, E.; Sandhu, S.S.; Kumar, S.; Arriaga, F.J. Soil health indicators impacted by long-term cattle manure and inorganic fertilizer application in a corn-soybean rotation of South Dakota. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartocci, P.; Bidini, G.; Saputo, P.; Fantozzi, F. Biochar pellet carbon footprint. Chem. Eng. 2016, 50, 217–222. [Google Scholar]
- Widowati, U.W.; Soehono, L.; Guritno, B. Effect of biochar on the release and loss of nitrogen from urea fertilization. J. Agric. Food Tech. 2011, 1, 127–132. [Google Scholar]
- Hagemann, N.; Joseph, S.; Schmidt, H.-P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1089. [Google Scholar] [CrossRef]
- Skjemstad, J.; Spouncer, L.; Cowie, B.; Swift, R. Calibration of the Rothamsted organic carbon turnover model (RothC ver. 26.3), using measurable soil organic carbon pools. Soil Res. 2004, 42, 79–88. [Google Scholar] [CrossRef]
- Lejay, L.; Wirth, J.; Pervent, M.; Cross, J.M.-F.; Tillard, P.; Gojon, A. Oxidative pentose phosphate pathway-dependent sugar sensing as a mechanism for regulation of root ion transporters by photosynthesis. Plant Physiol. 2008, 146, 2036–2053. [Google Scholar] [CrossRef] [Green Version]
- Jansson, C.; Wullschleger, S.D.; Kalluri, U.C.; Tuskan, G.A. Phytosequestration: Carbon biosequestration by plants and the prospects of genetic engineering. Bioscience 2010, 60, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Gifford, R. The global carbon cycle: A viewpoint on the missing sink. Funct. Plant Biol. 1994, 21, 1–15. [Google Scholar] [CrossRef]
- Raven, J.A.; Karley, A.J. Carbon sequestration: Photosynthesis and subsequent processes. Curr. Biol. 2006, 16, R165–R167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Gamage, D.; Hirotsu, N.; Martin, A.; Seneweera, S. Effects of elevated carbon dioxide on photosynthesis and carbon partitioning: A perspective on root sugar sensing and hormonal crosstalk. Front. Physiol. 2017, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- East, R. Soil science comes to life. Nature 2013, 501, S18. [Google Scholar] [CrossRef] [PubMed]
- Tezara, W.; Mitchell, V.; Driscoll, S.; Lawlor, D. Water stress inhibits plant photosynthesis by decreasing coupling factor and ATP. Nature 1999, 401, 914. [Google Scholar] [CrossRef]
- Jobin, L.; Jose, C.; Pages, C.; Raffin, G.; Saupin, X.; Jame, P.; Jaffrezic-Renault, N.; Namour, P. Methanogenesis control in bioelectrochemical systems: A carbon footprint reduction assessment. J. Environ. Chem. Eng. 2018, 6, 803–810. [Google Scholar] [CrossRef]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779. [Google Scholar] [CrossRef]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2 and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Tillage Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Biala, J.; Grace, P.R.; Scheer, C.; Rowlings, D.W. Greenhouse gas emissions from sub-tropical agricultural soils after addition of organic by-products. SpringerPlus 2014, 3, 491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohila, A.; Aurela, M.; Regina, K.; Laurila, T. Soil and total ecosystem respiration in agricultural fields: Effect of soil and crop type. Plant Soil 2003, 251, 303–317. [Google Scholar] [CrossRef]
- Köster, J.R.; Cárdenas, L.; Senbayram, M.; Bol, R.; Well, R.; Butler, M.; Mühling, K.H.; Dittert, K. Rapid shift from denitrification to nitrification in soil after biogas residue application as indicated by nitrous oxide isotopomers. Soil Biol. Biochem. 2011, 43, 1671–1677. [Google Scholar] [CrossRef]
- Spokas, K.A. Impact of biochar field aging on laboratory greenhouse gas production potentials. Gcb Bioenergy 2013, 5, 165–176. [Google Scholar] [CrossRef]
- Mørkved, P.T.; Dörsch, P.; Bakken, L.R. The N2O product ratio of nitrification and its dependence on long-term changes in soil pH. Soil Biol. Biochem. 2007, 39, 2048–2057. [Google Scholar] [CrossRef]
- Yu, L.; Tang, J.; Zhang, R.; Wu, Q.; Gong, M. Effects of biochar application on soil methane emission at different soil moisture levels. Biol. Fertil. Soils 2013, 49, 119–128. [Google Scholar] [CrossRef]
- Maestrini, B.; Nannipieri, P.; Abiven, S. A meta-analysis on pyrogenic organic matter induced priming effect. Gcb Bioenergy 2015, 7, 577–590. [Google Scholar] [CrossRef]
- Maestrini, B.; Herrmann, A.M.; Nannipieri, P.; Schmidt, M.W.; Abiven, S. Ryegrass-derived pyrogenic organic matter changes organic carbon and nitrogen mineralization in a temperate forest soil. Soil Biol. Biochem. 2014, 69, 291–301. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L. Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci. Rep. 2014, 4, 3687. [Google Scholar] [CrossRef] [Green Version]
- Briones, A. The secrets of El Dorado viewed through a microbial perspective. Front. Microbiol. 2012, 3, 239. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, J.A.; Laird, D.A.; Busscher, W.J. Environmental benefits of biochar. J. Environ. Qual. 2012, 41, 967–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spokas, K.A.; Baker, J.M.; Reicosky, D.C. Ethylene: Potential key for biochar amendment impacts. Plant Soil 2010, 333, 443–452. [Google Scholar] [CrossRef]
- Khalil, M.; Baggs, E. CH4 oxidation and N2O emissions at varied soil water-filled pore spaces and headspace CH4 concentrations. Soil Biol. Biochem. 2005, 37, 1785–1794. [Google Scholar] [CrossRef]
- Ball, B.; Dobbie, K.; Parker, J.; Smith, K. The influence of gas transport and porosity on methane oxidation in soils. J. Geophys. Res. Atmos. 1997, 102, 23301–23308. [Google Scholar] [CrossRef]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils 1. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Conrad, R. Quantification of methanogenic pathways using stable carbon isotopic signatures: A review and a proposal. Org. Geochem. 2005, 36, 739–752. [Google Scholar] [CrossRef]
- Dalal, R.; Allen, D.; Livesley, S.; Richards, G. Magnitude and biophysical regulators of methane emission and consumption in the Australian agricultural, forest, and submerged landscapes: A review. Plant Soil 2008, 309, 43–76. [Google Scholar] [CrossRef]
- Schmer, M.; Liebig, M.; Hendrickson, J.; Tanaka, D.; Phillips, R. Growing season greenhouse gas flux from switchgrass in the northern great plains. Biomass Bioenergy 2012, 45, 315–319. [Google Scholar] [CrossRef]
- Davidson, E.A.; Swank, W.T.; Perry, T.O. Distinguishing between nitrification and denitrification as sources of gaseous nitrogen production in soil. Appl. Environ. Microbiol. 1986, 52, 1280–1286. [Google Scholar] [CrossRef] [Green Version]
- Dalal, R.C.; Wang, W.; Robertson, G.P.; Parton, W.J. Nitrous oxide emission from Australian agricultural lands and mitigation options: A review. Soil Res. 2003, 41, 165–195. [Google Scholar] [CrossRef]
- Stevens, R.; Laughlin, R.; Burns, L.; Arah, J.; Hood, R. Measuring the contributions of nitrification and denitrification to the flux of nitrous oxide from soil. Soil Biol. Biochem. 1997, 29, 139–151. [Google Scholar] [CrossRef]
- Reay, D.S.; Smith, K.A.; Edwards, A.C. Nitrous oxide emission from agricultural drainage waters. Glob. Change Biol. 2003, 9, 195–203. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Hu, F.; Shi, W. Soil nitrous oxide emissions following crop residue addition: A meta-analysis. Glob. Change Biol. 2013, 19, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- De Visscher, A.; Boeckx, P.; Van Cleemput, O. Interaction between nitrous oxide formation and methane oxidation in soils: Influence of cation exchange phenomena. J. Environ. Qual. 1998, 27, 679–687. [Google Scholar] [CrossRef]
- Cayuela, M.; Velthof, G.; Mondini, C.; Sinicco, T.; Van Groenigen, J. Nitrous oxide and carbon dioxide emissions during initial decomposition of animal by-products applied as fertilisers to soils. Geoderma 2010, 157, 235–242. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Sanz-Cobena, A.; Garnier, J.; Vallejo, A. The potential of organic fertilizers and water management to reduce N2O emissions in Mediterranean climate cropping systems. A review. Agric. Ecosyst. Environ. 2013, 164, 32–52. [Google Scholar] [CrossRef] [Green Version]
- Davidson, E.A.; Verchot, L.V.; Cattanio, J.H.; Ackerman, I.L.; Carvalho, J. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Bhatia, A.; Pathak, H.; Jain, N.; Singh, P.; Singh, A. Global warming potential of manure amended soils under rice–wheat system in the Indo-Gangetic plains. Atmos. Environ. 2005, 39, 6976–6984. [Google Scholar] [CrossRef]
- Nyord, T.; Søgaard, H.; Hansen, M.; Jensen, L.S. Injection methods to reduce ammonia emission from volatile liquid fertilisers applied to growing crops. Biosyst. Eng. 2008, 100, 235–244. [Google Scholar] [CrossRef]
- Flessa, H.; Beese, F. Laboratory estimates of trace gas emissions following surface application and injection of cattle slurry. J. Environ. Qual. 2000, 29, 262–268. [Google Scholar] [CrossRef]
- Sun, F.; Harrison, J.; Ndegwa, P.; Johnson, K. Effect of manure treatment on ammonia and greenhouse gases emissions following surface application. Water Air Soil Pollut. 2014, 225, 1923. [Google Scholar] [CrossRef]
- Robertson, G.P.; Tiedje, J.M. Deforestation alters denitrification in a lowland tropical rain forest. Nature 1988, 336, 756. [Google Scholar] [CrossRef]
- Tenuta, E.G.; Beauchamp, M. Nitrous oxide production from granular nitrogen fertilizers applied to a silt loam soil. Can. J. Soil Sci. 2003, 83, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Gilliam, F.S.; Yu, G.; Chen, H.; Mo, J. Long-term nitrogen addition decreases carbon leaching in a nitrogen-rich forest ecosystem. 2013. Biogeosciences 2013, 10, 3931–3941. [Google Scholar] [CrossRef] [Green Version]
- Heimann, M.; Reichstein, M. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 2008, 451, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindler, R.; Siemens, J.; Kaiser, K.; Walmsley, D.C.; Bernhofer, C.; Buchmann, N.; Cellier, P.; Eugster, W.; Gleixner, G.; Grũnwald, T. Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob. Change Biol. 2011, 17, 1167–1185. [Google Scholar] [CrossRef] [Green Version]
- Uselman, S.M.; Qualls, R.G.; Lilienfein, J. Contribution of root vs. leaf litter to dissolved organic carbon leaching through soil. Soil Sci. Soc. Am. J. 2007, 71, 1555–1563. [Google Scholar] [CrossRef]
- McDowell, W.H.; Currie, W.S.; Aber, J.D.; Yang, Y. Effects of chronic nitrogen amendments on production of dissolved organic carbon and nitrogen in forest soils. In Biogeochemical Investigations at Watershed, Landscape, and Regional Scales; Springer: Berlin/Heidelberg, Germany, 1998; pp. 175–182. [Google Scholar]
- Fröberg, M.; Berggren Kleja, D.; Hagedorn, F. The contribution of fresh litter to dissolved organic carbon leached from a coniferous forest floor. Eur. J. Soil Sci. 2007, 58, 108–114. [Google Scholar] [CrossRef]
- Evans, C.D.; Goodale, C.L.; Caporn, S.J.; Dise, N.B.; Emmett, B.A.; Fernandez, I.J.; Field, C.D.; Findlay, S.E.; Lovett, G.M.; Meesenburg, H. Does elevated nitrogen deposition or ecosystem recovery from acidification drive increased dissolved organic carbon loss from upland soil? A review of evidence from field nitrogen addition experiments. Biogeochemistry 2008, 91, 13–35. [Google Scholar] [CrossRef]
- Gödde, M.; David, M.B.; Christ, M.J.; Kaupenjohann, M.; Vance, G.F. Carbon mobilization from the forest floor under red spruce in the northeastern USA. Soil Biol. Biochem. 1996, 28, 1181–1189. [Google Scholar] [CrossRef]
- Aitkenhead, J.; McDowell, W.H. Soil C: N ratio as a predictor of annual riverine DOC flux at local and global scales. Glob. Biogeochem. Cycles 2000, 14, 127–138. [Google Scholar] [CrossRef]
- Royer, I.; Angers, D.A.; Chantigny, M.H.; Simard, R.R.; Cluis, D. Dissolved organic carbon in runoff and tile-drain water under corn and forage fertilized with hog manure. J. Environ. Qual. 2007, 36, 855–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Major, J.; Lehmann, J.; Rondon, M.; Goodale, C. Fate of soil-applied black carbon: Downward migration, leaching and soil respiration. Glob. Change Biol. 2010, 16, 1366–1379. [Google Scholar] [CrossRef]
- Walmsley, D.C.; Siemens, J.; Kindler, R.; Kirwan, L.; Kaiser, K.; Saunders, M.; Kaupenjohann, M.; Osborne, B.A. Dissolved carbon leaching from an Irish cropland soil is increased by reduced tillage and cover cropping. Agric. Ecosyst. Environ. 2011, 142, 393–402. [Google Scholar] [CrossRef]
- Ruark, M.D.; Brouder, S.M.; Turco, R.F. Dissolved organic carbon losses from tile drained agroecosystems. J. Environ. Qual. 2009, 38, 1205–1215. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, W.; Gundersen, P.; Mo, J.; Zhou, G.; Yoh, M. Large loss of dissolved organic nitrogen from nitrogen-saturated forests in subtropical China. Ecosystems 2009, 12, 33–45. [Google Scholar] [CrossRef]
- Long, G.-Q.; Jiang, Y.-J.; Sun, B. Seasonal and inter-annual variation of leaching of dissolved organic carbon and nitrogen under long-term manure application in an acidic clay soil in subtropical China. Soil Tillage Res. 2015, 146, 270–278. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, W.; Zhu, W.; Gundersen, P.; Fang, Y.; Li, D.; Wang, H. Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob. Change Biol. 2008, 14, 403–412. [Google Scholar] [CrossRef]
- Bragazza, L.; Freeman, C.; Jones, T.; Rydin, H.; Limpens, J.; Fenner, N.; Ellis, T.; Gerdol, R.; Hájek, M.; Hájek, T. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc. Natl. Acad. Sci. 2006, 103, 19386–19389. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Schoenau, J. Fluxes of water-soluble nitrogen and phosphorus in the forest floor and surface mineral soil of a boreal aspen stand. Geoderma 1998, 81, 251–264. [Google Scholar] [CrossRef]
- Sjöberg, G.; Bergkvist, B.; Berggren, D.; Nilsson, S. Long-term N addition effects on the C mineralization and DOC production in mor humus under spruce. Soil Biol. Biochem. 2003, 35, 1305–1315. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Fujii, K.; Hartono, A.; Funakawa, S.; Uemura, M.; Kosaki, T. Fluxes of dissolved organic carbon in three tropical secondary forests developed on serpentine and mudstone. Geoderma 2011, 163, 119–126. [Google Scholar] [CrossRef]
- Don, A.; Schulze, E.-D. Controls on fluxes and export of dissolved organic carbon in grasslands with contrasting soil types. Biogeochemistry 2008, 91, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Berry, P.; Kindred, D.; Paveley, N. Quantifying the effects of fungicides and disease resistance on greenhouse gas emissions associated with wheat production. Plant Pathol. 2008, 57, 1000–1008. [Google Scholar] [CrossRef]
- Lal, R. Carbon emission from farm operations. Environ. Int. 2004, 30, 981–990. [Google Scholar] [CrossRef] [PubMed]
- West, T.O.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Hamel, C.; Cutforth, H.; Wang, H. Strategies for reducing the carbon footprint of field crops for semiarid areas. A review. Agron. Sustain. Dev. 2011, 31, 643–656. [Google Scholar] [CrossRef]
- Gan, Y.; Liang, C.; Huang, G.; Malhi, S.S.; Brandt, S.A.; Katepa-Mupondwa, F. Carbon footprint of canola and mustard is a function of the rate of N fertilizer. Int. J. Life Cycle Assess. 2012, 17, 58–68. [Google Scholar] [CrossRef]
- Beckie, H.J. Beneficial management practices to combat herbicide-resistant grass weeds in the northern Great Plains. Weed Technol. 2007, 21, 290–299. [Google Scholar] [CrossRef]
- Derksen, D.A.; Anderson, R.L.; Blackshaw, R.E.; Maxwell, B. Weed dynamics and management strategies for cropping systems in the northern Great Plains. Agron. J. 2002, 94, 174–185. [Google Scholar] [CrossRef]
- Tu, C. Effects of fungicides on microbial activities in sandy soil. Int. J. Environ. Health Res. 1994, 4, 133–140. [Google Scholar] [CrossRef]
- Kinney, C.A.; Mosier, A.R.; Ferrer, I.; Furlong, E.T.; Mandernack, K.W. Effects of the fungicides mancozeb and chlorothalonil on fluxes of CO2, N2O, and CH4 in a fertilized Colorado grassland soil. J. Geophys. Res. Atmos. 2004, 109. [Google Scholar] [CrossRef]
- Hynes, R.K.; Leung, G.C.; Hirkala, D.L.; Nelson, L.M. Isolation, selection, and characterization of beneficial rhizobacteria from pea, lentil, and chickpea grown in western Canada. Can. J. Microbiol. 2008, 54, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.J.; Clements, D.R.; Derksen, D.A. Weed succession under conservation tillage: A hierarchical framework for research and management. Weed Technol. 1993, 7, 286–297. [Google Scholar] [CrossRef]
- Bilen, S.; Jacinthe, P.-A.; Shrestha, R.; Jagadamma, S.; Nakajima, T.; Kendall, J.; Doohan, T.; Lal, R.; Dick, W. Greenhouse gas fluxes in a no-tillage chronosequence in Central Ohio. Soil Tillage Res. 2022, 218, 105313. [Google Scholar] [CrossRef]
- Altikat, S.; Celik, A.; Bilen, S. Effects of various tillage systems on soil CO2-C fluxes and on bacteria and fungi populations in Zea mays. Afr. J. Agric. Res. 2012, 7, 2926–2934. [Google Scholar] [CrossRef]
- Bilen, S.; Çelik, A.; Altıkat, S. Effects of strip and full-width tillage on soil carbon IV oxide-carbon (CO2-C) fluxes and on bacterial and fungal populations in sunflower. Afr. J. Biotechnol. 2010, 9, 6312–6319. [Google Scholar]
- La Scala Jr, N.; Bolonhezi, D.; Pereira, G. Short-term soil CO2 emission after conventional and reduced tillage of a no-till sugar cane area in southern Brazil. Soil Tillage Res. 2006, 91, 244–248. [Google Scholar] [CrossRef]
- Baggs, E.; Stevenson, M.; Pihlatie, M.; Regar, A.; Cook, H.; Cadisch, G. Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage. Plant Soil 2003, 254, 361–370. [Google Scholar] [CrossRef]
- Srivastava, P.; Kumar, A.; Behera, S.K.; Sharma, Y.K.; Singh, N. Soil carbon sequestration: An innovative strategy for reducing atmospheric carbon dioxide concentration. Biodivers. Conserv. 2012, 21, 1343–1358. [Google Scholar] [CrossRef]
- Sørensen, C.G.; Halberg, N.; Oudshoorn, F.W.; Petersen, B.M.; Dalgaard, R. Energy inputs and GHG emissions of tillage systems. Biosyst. Eng. 2014, 120, 2–14. [Google Scholar] [CrossRef]
- Lockeretz, W. Agriculture and Energy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Singh, K.P.; Prakash, V.; Srinivas, K.; Srivastva, A.K. Effect of tillage management on energy-use efficiency and economics of soybean (Glycine max) based cropping systems under the rainfed conditions in North-West Himalayan Region. Soil Tillage Res. 2008, 100, 78–82. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozlu, E.; Arriaga, F.J.; Bilen, S.; Gozukara, G.; Babur, E. Carbon Footprint Management by Agricultural Practices. Biology 2022, 11, 1453. https://doi.org/10.3390/biology11101453
Ozlu E, Arriaga FJ, Bilen S, Gozukara G, Babur E. Carbon Footprint Management by Agricultural Practices. Biology. 2022; 11(10):1453. https://doi.org/10.3390/biology11101453
Chicago/Turabian StyleOzlu, Ekrem, Francisco Javier Arriaga, Serdar Bilen, Gafur Gozukara, and Emre Babur. 2022. "Carbon Footprint Management by Agricultural Practices" Biology 11, no. 10: 1453. https://doi.org/10.3390/biology11101453
APA StyleOzlu, E., Arriaga, F. J., Bilen, S., Gozukara, G., & Babur, E. (2022). Carbon Footprint Management by Agricultural Practices. Biology, 11(10), 1453. https://doi.org/10.3390/biology11101453







