Mitigating the Mistletoe Menace: Biotechnological and Smart Management Approaches
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Biology of Mistletoe
3. Mistletoe Damages Trees
4. Conventional Control Strategies and Integrated Pest Management Approaches
4.1. Physical Methods
4.2. Chemical Methods
4.3. Silvicultural Practices
5. Mistletoe Control through Biotechnological Interventions and Smart Management
5.1. Mistletoe Biocontrol Agents (MBCAs)
5.1.1. Bacteria and Fungi as MBCAs
5.1.2. Diatoms and Algae as MBCAs
5.1.3. Insects as MBCAs
5.1.4. Hyperparasitic Mistletoes
5.1.5. Higher Animals
5.2. Requirements of an Effective MBCA Selection Program
5.3. Challenges with MBCAs
5.4. Inducing Host Plant Resistance to Mistletoes and/or Herbicides
5.5. Hunting for the Genetic Basis to Hosts’ Inherent Resistance to Mistletoes: Background Studies
5.6. Mistletoe Community Restructuring, Disturbances, and Biological Interactions
5.7. Transcriptomic/Metabolomic Profiling, Transgenic Trees, and Translational Research Pipeline
5.8. Role of Epigenetic Signaling in Mistletoe Parasitism: Moving beyond the Genetic Basis
5.9. Smart Mistletoe Management: How the 21st Century Can Mitigate the Mistletoe Problem
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.B.; Mudgal, G.; Vinayak, A.; Kaur, J.; Chand, K.; Parashar, M.; Verma, A.K.; Goswami, A.; Sharma, S. Molecular Communications between Plants and Microbes. In Plant-Microbial Interactions and Smart Agricultural Biotechnology; Tyagi, S., Kumar, R., Saharan, B., Nadda, A.K., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 147–184. [Google Scholar]
- Yopp, J.H.; Dickison, W.C.; Rothwell, G.W.; Lambers, H.; Schmid, R.; Woodwell, G.M. Plant. Encycl. Br. 2021. Available online: https://www.britannica.com/plant/plant (accessed on 8 October 2022).
- Satterthwaite, D.; McGranahan, G.; Tacoli, C. Urbanization and its implications for food and farming. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2010, 365, 2809–2820. [Google Scholar] [CrossRef]
- Chapagain, T.; Raizada, M.N. Impacts of natural disasters on smallholder farmers: Gaps and recommendations. Agric. Food Secur. 2017, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- FAO. The Impact of Disasters and Crises on Agriculture and Food Security: 2021; Food and Agriculture Organization: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Dubey, P.K.; Singh, G.S.; Abhilash, P.C. Agriculture in a changing climate. In Adaptive Agricultural Practices; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–10. [Google Scholar]
- Oshunsanya, S.O.; Nwosu, N.J.; Li, Y. Abiotic stress in agricultural crops under climatic conditions. In Sustainable Agriculture, Forest and Environmental Management; Springer: Singapore, 2019; pp. 71–100. [Google Scholar]
- Joos, L.; De Tender, C. Soil under stress: The importance of soil life and how it is influenced by (micro)plastic pollution. Comput. Struct. Biotechnol. J. 2022, 20, 1554–1566. [Google Scholar] [CrossRef] [PubMed]
- Klutsch, J.G.; Erbilgin, N. Dwarf mistletoe infection in jack pine alters growth-defense relationships. Tree Physiol. 2018, 38, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Ehleringer, J.; Schulze, E.-D.; Ziegler, H.; Lange, O.; Farquhar, G.; Cowar, I. Xylem-tapping mistletoes: Water or nutrient parasites? Science 1985, 227, 1479–1481. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.D.; Turner, N.; Glatzel, G. Carbon, water and nutrient relations of two mistletoes and their hosts: A hypothesis. Plant Cell Environ. 1984, 7, 293–299. [Google Scholar] [CrossRef]
- The Editors of Encyclopaedia Britannica. Mistletoe. Encyclopedia Britannica. Available online: https://www.britannica.com/plant/mistletoe (accessed on 27 October 2022).
- Halloin, L. Dwarf Mistletoe Biology and Management in Southeast Region; Washington State Department of Natural Resources: Washington, DC, USA, 2003; pp. 1–8. Available online: https://www.dnr.wa.gov/Publications/rp_fh_wadnrdwarfmistletoe.pdf (accessed on 27 October 2022).
- MCFB. Dwarf Mistletoe of Conifers. Available online: https://www.gov.mb.ca/nrnd/forest/pubs/forest_lands/health/dwarf_mistletoe_brochure.pdf (accessed on 22 October 2021).
- Ferguson, B.A. State Forester Forum-Dwarf Mistletoes; Ferguson Forest Pathology Consulting, Inc.: Missoula, MT, USA, 2014; p. 9. Available online: https://www.idl.idaho.gov/wp-content/uploads/sites/2/forestry/forester-forums/id24.pdf (accessed on 8 October 2022).
- Mallams, K.M.; Mathiasen, R.L. Mistletoes on Hardwoods in the United States. For. Insect Dis. Leafl. 2010, 147, 1–10. [Google Scholar]
- Aly, R. Conventional and biotechnological approaches for control of parasitic weeds. Vitr. Cell. Dev. Biol. Plant 2007, 43, 304–317. [Google Scholar] [CrossRef]
- Nickrent, D.L. Parasitic angiosperms: How often and how many? Taxon 2020, 69, 5–27. [Google Scholar] [CrossRef]
- Parker, C. Protection of crops against parasitic weeds. Crop Prot. 1991, 10, 6–22. [Google Scholar] [CrossRef]
- Kaiser, B.; Vogg, G.; Fürst, U.B.; Albert, M. Parasitic plants of the genus Cuscuta and their interaction with susceptible and resistant host plants. Front. Plant Sci. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauerborn, J.; Müller-Stöver, D.; Hershenhorn, J. The role of biological control in managing parasitic weeds. Crop Prot. 2007, 26, 246–254. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M. Innovations in parasitic weeds management in legume crops. A review. Agron. Sustain. Dev. 2012, 32, 433–449. [Google Scholar] [CrossRef]
- Albert, M.; Axtell, M.J.; Timko, M.P. Mechanisms of resistance and virulence in parasitic plant–host interactions. Plant Physiol. 2020, 185, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Aparicio, M.; Delavault, P.; Timko, M.P. Management of Infection by Parasitic Weeds: A Review. Plants 2020, 9, 1184. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E. Menace of the mistletoe. Vic. Nat. 1949, 66, 24–32. [Google Scholar]
- Watson, D.M.; Cook, M.E.; Fadini, R.R. Towards best-practice management of mistletoes in horticulture. Botany 2020, 98, 489–498. [Google Scholar] [CrossRef]
- Geils, B.W. Mistletoes of North American Conifers; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2002. [Google Scholar] [CrossRef]
- Pearson, G.; Marsh, R. Timber Growing and Logging Practice in the Southwest and in the Black Hills Region. Available online: https://naldc.nal.usda.gov/download/CAT86200474/PDF (accessed on 22 October 2022).
- Reid, N.; Shamoun, S.F. Contrasting research approaches to managing mistletoes in commercial forests and wooded pastures. Botany 2008, 87, 1–9. [Google Scholar] [CrossRef]
- Watson, D.M. Mistletoe—A Keystone Resource in Forests and Woodlands Worldwide. Annu. Rev. Ecol. Syst. 2001, 32, 219–249. [Google Scholar] [CrossRef] [Green Version]
- Madisa, M.E.; Assefa, Y.; Kelemoge, O.D.; Mathowa, T.; Segwagwe, A. Incidence and level of mistletoe infestation in tree species at Botswana University of Agriculture and Natural Resources’ Sebele Content Farm Campus, Botswana. Int. J. Environ. Agric. Res. 2017, 3, 53–58. [Google Scholar] [CrossRef]
- Stinziano, J. Mistletoe-ing around the Tree Reduces Growth in Forests. Available online: https://botany.one/2017/12/mistletoe-ing-around-tree-reduces-growth-forests/ (accessed on 22 October 2022).
- Rist, L.; Shaanker, R.U.; Ghazoul, J. The Spatial Distribution of Mistletoe in a Southern Indian Tropical Forest at Multiple Scales. Biotropica 2011, 43, 50–57. [Google Scholar] [CrossRef]
- Shamoun, S.; DeWald, L. Management strategies for dwarf mistletoes: Biological, chemical, and genetic approaches. In Mistletoes of North American Conifers; Geils, B.W., Tovar, J.C., Moody, B., Eds.; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2002; Volume 98, pp. 75–82. [Google Scholar]
- Choi, S.-U.; Kim, S.T.; Han, D.G.; Hwang, Y.-H.; Lee, K.Y.; Kim, D.U.; Cho, K.H.; Park, S.Y.; Kim, H.-C.; Kim, S.-B.; et al. Comparative Assessment of Biological Activities of Mistletoes for Cosmetic Applications: Viscum Album Var. Coloratum (Kom.) Ohwi and Loranthus Tanakae Franch. & Sav. J. Cosmet. Sci. 2019, 70, 235–245. [Google Scholar] [PubMed]
- Mudgal, G.; Mudgal, B. Evidence for unusual choice of host and haustoria by Dendrophthoe falcata (Lf) Ettingsh, a leafy mistletoe. Arch. Phytopathol. Plant Prot. 2011, 44, 186–190. [Google Scholar] [CrossRef]
- Mudgal, G.; Mudgal, B.; Gururani, M.A.; Jelli, V. Pseudaulacaspis cockerelli (Cooley) hyperparasitizing Dendrophthoe falcata (Lf) Ettingsh. Arch. Phytopathol. Plant Prot. 2011, 44, 282–286. [Google Scholar] [CrossRef]
- Singh, S.R.; Piloo, N.; Senjam, P.; Hemanta, L. Need of awareness programme to control the loranthus weed–Helixanthera ligustrina. Agri-India Today 2021, 1, 10–13. [Google Scholar]
- Arce-Acosta, I.; Suzán-Azpiri, H.; García-Rubio, O. Biotic factors associated with the spatial distribution of the mistletoe Psittacanthus calyculatus in a tropical deciduous forest of central Mexico. Bot. Sci. 2016, 94, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Fadini, R.F.; Fischer, E.; Castro, S.J.; Araujo, A.C.; Ornelas, J.F.; de Souza, P.R. Bat and bee pollination in Psittacanthus mistletoes, a genus regarded as exclusively hummingbird-pollinated. Ecology 2018, 99, 1239–1241. [Google Scholar] [CrossRef]
- Infante, S.D.; Lara, C.; del Coro Arizmendi, M.; Eguiarte, L.E.; Ornelas, J.F. Reproductive ecology and isolation of Psittacanthus calyculatus and P. auriculatus mistletoes (Loranthaceae). PeerJ 2016, 4, e2491. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Crespo, M.J.; Lara, C.; Ornelas, J.F. Uncorrelated mistletoe infection patterns and mating success with local host specialization in Psittacanthus calyculatus (Loranthaceae). Evol. Ecol. 2016, 30, 1061–1080. [Google Scholar] [CrossRef]
- Teodoro, G.S.; van den Berg, E.; Santos, M.d.C.N.; de Freitas Coelho, F. How does a Psittacanthus robustus Mart. population structure relate to a Vochysia thyrsoidea Pohl. host population? Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 797–801. [Google Scholar] [CrossRef]
- Aliero, B.; Samaila, A. The occurrence of Parasitic mistletoe (Tapinanthus spp) on Parkia biglobosa (Jacq.) Benth (African Locust Bean Tree) in Yauri Local Government Area, Kebbi State. Niger. J. Basic Appl. Sci. 2000, 9, 5–10. [Google Scholar]
- Edagbo, D.E.; Ajiboye, T.O.; Borokini, T.I.; Ighere, D.A.; Alowonle, A.A.; Clement, M. The Influence of African Mistletoe (Tapinanthus bangwensis) on the Conservation Status and Productivity of Irvingia gabonensis in Moor Plantation Area of Ibadan, Nigeria. Int. J. Curr. Res. 2012, 4, 484–487. [Google Scholar] [CrossRef]
- Kwon-Ndung, E.; Ismaila, A. Prospects of host resistance in improved and domesticated species of Parkia biglobosa to African mistletoes (Tapinanthus spp.) in Central Nigeria. Electron. J. Environ. Agric. Food Chem. 2009, 8, 382–388. [Google Scholar]
- Ávalos, V.M.C.; Collazo, I.V.; Flores, H.J.M.; Castillo, J.V. Impacto de tierra de diatomeas sobre Arceuthobium globosum Hawksworth & Wiens subsp. grandicaule en Pinus pseudostrobus Lindl. Rev. Mex. Cienc. For. 2010, 1, 39–46. [Google Scholar]
- Gilbert, J.A. The Biology of Dwarf Mistletoes (Arceuthobium spp.) in Manitoba. Master Thesis, University of Manitoba, Winnipeg, MB, Canada, 1984. [Google Scholar]
- Hernández-Benítez, R.; Cano-Santana, Z.; Castellanos-Vargas, I. Incidencia de infestación de Arceuthobium globosum grandicaule (Hawks. y Wiens) en Pinus hartwegii (Lindl.). Cienc. For. En México 2005, 30, 79–86. [Google Scholar]
- Queijeiro-Bolanos, M.E.; Cano-Santana, Z.; Castellanos-Vargas, I. Does disturbance determine the prevalence of dwarf mistletoe (Arceuthobium, Santalales: Viscaceae) in Central Mexico? Rev. Chil. De Hist. Nat. 2013, 86, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Shaw, D.C.; Agne, M.C.J.B. Fire and dwarf mistletoe (Viscaceae: Arceuthobium species) in western North America: Contrasting Arceuthobium tsugense and Arceuthobium americanum. Botany 2017, 95, 231–246. [Google Scholar] [CrossRef]
- Villier, J.A.d.; Reblin, J.S.; Logan, B.A. Needle properties of host white spruce (Picea glauca [Moench] Voss) experiencing eastern dwarf mistletoe (Arceuthobium pusillum Peck) infections of differing severity. Botany 2017, 95, 295–305. [Google Scholar] [CrossRef]
- Anselmo-Moreira, F.; Teixeira-Costa, L.; Ceccantini, G.; Furlan, C.M. Mistletoe effects on the host tree Tapirira guianensis: Insights from primary and secondary metabolites. Chemoecology 2019, 29, 11–24. [Google Scholar] [CrossRef]
- Solís-Gracia, V.; Suzán-Azpiri, H. Análisis de la distribución espacial del muérdago (Phoradendron californicum) en el sur del Desierto Sonorense. Cactáceas Suculentas Mex. 2014, 59, 11–28. [Google Scholar]
- Wiens, D.; Hawksworth, F. New species of Phoradendron (Viscaceae) from Mexico and Guatemala and a synopsis of species in section Pauciflorae. Aliso J. Syst. Evol. Bot. 2002, 21, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Mir, N.; Sultan, S. White-berry mistletoe (Viscum album L.): A hemiparasitic plant: Occurrence and ethnobotanical use in Kashmir. J. Pharmacogn. Phytochem. 2018, 7, 1831–1833. [Google Scholar]
- Alam, M. A critical review on the biology and control of Loranthaceae with a particular reference to Bangladesh. Bano Biggyan Patrika 1984, 13, 7–18. [Google Scholar]
- Barbu, C. Impact of mistletoe attack (Viscum album ssp. abietis) on the radial growth of silver fir. A case study in the North of Eastern Carpathians. Ann. For. Res. 2009, 52, 89–96. [Google Scholar] [CrossRef]
- Bilgili, E.; Coskuner, K.A.; Öztürk, M. Leaf area–sapwood area relationship in Scots pine (Pinus sylvestris L.) under mistletoe (Viscum album ssp. austriacum) infection. Dendrobiology 2020, 84, 1–11. [Google Scholar] [CrossRef]
- Dobbertin, M.; Rigling, A. Pine mistletoe (Viscum album ssp. austriacum) contributes to Scots pine (Pinus sylvestris) mortality in the Rhone valley of Switzerland. For. Pathol. 2006, 36, 309–322. [Google Scholar] [CrossRef]
- Mutlu, S.; Osma, E.; Ilhan, V.; Turkoglu, H.I.; Atici, O. Mistletoe (Viscum album) reduces the growth of the Scots pine by accumulating essential nutrient elements in its structure as a trap. Trees 2016, 30, 815–824. [Google Scholar] [CrossRef]
- Ozturk, M.; Coskuner, K.A.; Serdar, B.; Atar, F.; Bilgili, E. Impact of white mistletoe (Viscum album ssp. abietis) infection severity on morphology, anatomy and photosynthetic pigment content of the needles of cilicican fir (Abies cilicica). Flora 2022, 294, 152135. [Google Scholar] [CrossRef]
- Ozturk, M.; Coskuner, K.A.; Usta, Y.; Serdar, B.; Bilgili, E. The effect of mistletoe (Viscum album) on branch wood and needle anatomy of Scots pine (Pinus sylvestris). IAWA J. 2019, 40, 352–365. [Google Scholar] [CrossRef]
- Glatzel, G.; Geils, B. Mistletoe ecophysiology: Host–parasite interactions. Botany 2008, 87, 10–15. [Google Scholar] [CrossRef]
- Calvin, C.L.; Wilson, C.A. Comparative morphology of epicortical roots in Old and New World Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora-Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 51–64. [Google Scholar] [CrossRef]
- Barlow, B. MIstletoes in Australia. Available online: http://www.anbg.gov.au/mistletoe/haustoria.html (accessed on 12 January 2020).
- Wilson, C.A.; Calvin, C.L. An origin of aerial branch parasitism in the mistletoe family, Loranthaceae. Am. J. Bot. 2006, 93, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Stewart, G.R. Effect of Potassium Levels on the Stomatal Behavior of the Hemi-Parasite Striga hermonthica. Plant Physiol. 1990, 94, 1472–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo Gullo, M.A.; Glatzel, G.; Devkota, M.; Raimondo, F.; Trifilò, P.; Richter, H. Mistletoes and mutant albino shoots on woody plants as mineral nutrient traps. Ann. Bot. 2012, 109, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunaichamy, K.S.T.K.; Paliwal, K.; Arp, P.A. Biomass and nutrient dynamics of mistletoe (Dendrophthoe falcata) and neem (Azadirachta indica) seedlings. Curr. Sci. 1999, 76, 840–842. [Google Scholar]
- Zweifel, R.; Bangerter, S.; Rigling, A.; Sterck, F.J. Pine and mistletoes: How to live with a leak in the water flow and storage system? J. Exp. Bot. 2012, 63, 2565–2578. [Google Scholar] [CrossRef] [Green Version]
- Urban, J.; Gebauer, R.; Nadezhdina, N.; Čermák, J. Transpiration and stomatal conductance of mistletoe (Loranthus europaeus) and its host plant, downy oak (Quercus pubescens). Biologia 2012, 67, 917–926. [Google Scholar] [CrossRef] [Green Version]
- Glatzel, G. Mineral nutrition and water relations of hemiparasitic mistletoes: A question of partitioning. Experiments with Loranthus europaeus on Quercus petraea and Quercus robur. Oecologia 1983, 56, 193–201. [Google Scholar] [CrossRef]
- Yang, D.; Goldstein, G.; Wang, M.; Zhang, W.-W.; Wang, A.-Y.; Liu, Y.-Y.; Hao, G.-Y. Microenvironment in the canopy rivals the host tree water status in controlling sap flow of a mistletoe species. Tree Physiol. 2017, 37, 501–510. [Google Scholar] [CrossRef]
- Rigling, A.; Eilmann, B.; Koechli, R.; Dobbertin, M. Mistletoe-induced crown degradation in Scots pine in a xeric environment. Tree Physiol. 2010, 30, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Dobbertin, M.; Hilker, N.; Rebetez, M.; Zimmermann, N.E.; Wohlgemuth, T.; Rigling, A. The upward shift in altitude of pine mistletoe (Viscum album ssp. austriacum) in Switzerland—The result of climate warming? Int. J. Biometeorol. 2005, 50, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doležal, J.; Lehečková, E.; Sohar, K.; Altman, J. Oak decline induced by mistletoe, competition and climate change: A case study from central Europe. Preslia 2016, 88, 323–346. [Google Scholar]
- Matula, R.; Svátek, M.; Pálková, M.; Volařík, D.; Vrška, T. Mistletoe infection in an oak forest is influenced by competition and host size. PLoS ONE 2015, 10, e0127055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aukema, J.E.; Martinez del Rio, C. Mistletoes as parasites and seed-dispersing birds as disease vectors: Current understanding, challenges, and opportunities. In Seed Dispersal and Frugivory: Ecology, Evolution, and Conservation; CABI International: Wallingford, UK, 2002; pp. 99–110. [Google Scholar]
- Aukema, J.E.; Martínez del Rio, C. Where does a fruit-eating bird deposit mistletoe seeds? Seed deposition patterns and an experiment. Ecology 2002, 83, 3489–3496. [Google Scholar] [CrossRef]
- Reid, N. Coevolution of mistletoes and frugivorous birds? Aust. J. Ecol. 1991, 16, 457–469. [Google Scholar] [CrossRef]
- Green, A.K.; Ward, D.; Griffiths, M.E. Directed dispersal of mistletoe (Plicosepalus acaciae) by Yellow-vented Bulbuls (Pycnonotus xanthopygos). J. Ornithol. 2009, 150, 167–173. [Google Scholar] [CrossRef]
- Mathiasen, R.L.; Nickrent, D.L.; Shaw, D.C.; Watson, D.M. Mistletoes: Pathology, systematics, ecology, and management. Plant Dis. 2008, 92, 988–1006. [Google Scholar] [CrossRef] [Green Version]
- Watson, D.M. The Relative Contribution of Specialists and Generalists to Mistletoe Dispersal: Insights from a Neotropical Rain Forest. Biotropica 2013, 45, 195–202. [Google Scholar] [CrossRef]
- Albert, S.; Rhumeur, A.; Rivière, J.L.; Chauvrat, A.; Sauroy-Toucouère, S.; Martos, F.; Strasberg, D. Rediscovery of the mistletoe Bakerella hoyifolia subsp. bojeri (Loranthaceae) on Reunion Island: Population status assessment for its conservation. Bot. Lett. 2017, 164, 229–236. [Google Scholar] [CrossRef]
- Medel, R.; Vergara, E.; Silva, A.; Kalin-Arroyo, M. Effects of vector behavior and host resistance on mistletoe aggregation. Ecology 2004, 85, 120–126. [Google Scholar] [CrossRef]
- Gill, L.S.; Hawksworth, F.G. The Mistletoes: A Literature Review; US Department of Agriculture: Washington, DC, USA, 1961. [Google Scholar]
- Berry, A.M.; Lichter, J.M.; Reid, M.S. New Methods for Mistletoe Control. Available online: http://slosson.ucdavis.edu/newsletters/Berry_199129111.pdf (accessed on 29 December 2019).
- Ko, S.M.; Kwon, Y.K.; Kim, J.H.; Song, I.-J.; Lee, H.-Y.; Choi, D.-W.; Liu, J.R.; Kim, S.W. Transcriptome analysis of mistletoe (Viscum album) haustorium development. Hortic. Environ. Biotechnol. 2014, 55, 352–361. [Google Scholar] [CrossRef]
- Scharpf, R.F.; Smith, R.S.; Vogler, D. Management of Western Dwarf Mistletoe in Ponderosa and Jeffrey Pines in Forest Recreation Areas; Pacific Southwest Research Station, Forest Service; US Department of Agriculture: Washington, DC, USA, 1988; Volume 103, 11p. [Google Scholar]
- Knutson, D. Physiology of mistletoe parasitism and disease responses in the host. In The Biology of Mistletoes; Academic Press: Sydney, Australia, 1983; pp. 295–316. [Google Scholar]
- Nickrent, D.L. Santalales (Mistletoe). In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: New York, NY, USA, 2002. [Google Scholar]
- Geils, B.; Hawksworth, F. Damage, Effects, and Importance of Dwarf Mistletoes; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2002; pp. 57–65. [Google Scholar]
- Hawksworth, F.G. Mistletoes on Introduced Trees of the World; US Department of Agriculture Forest Service: Washington, DC, USA, 1974. [Google Scholar]
- Barney, C.W.; Hawksworth, F.; Geils, B. Hosts of Viscum album. J. For. Pathol. 1998, 28, 187–208. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Wiens, D.; Geils, B.W. Arceuthobium in North America. In General Technical Report; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Washington, DC, USA, 2002; Chapter 4; pp. 29–56. [Google Scholar]
- Ritter, S.M.; Hoffman, C.M.; Ex, S.A.; Stewart, J.E. Impacts of lodgepole pine dwarf mistletoe (Arceuthobium americanum) infestation on stand structure and fuel load in lodgepole pine dominated forests in central Colorado. Botany 2017, 95, 307–321. [Google Scholar] [CrossRef]
- Shaw, D.C.; Watson, D.M.; Mathiasen, R.L. Comparison of dwarf mistletoes (Arceuthobium spp., Viscaceae) in the western United States with mistletoes (Amyema spp., Loranthaceae) in Australia—Ecological analogs and reciprocal models for ecosystem management. Aust. J. Bot. 2004, 52, 481–498. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.E.; Shaw, D.C.; Marosi, T.K. Distribution of Western Hemlock Dwarf Mistletoe (Arceuthobioum tsugense [Rosendahl] GN Jones Subsp. tsugense) in Mature and Old-growth Douglas-fir (Pseudotsuga menziesii [Mirb.] Franco) Forests. Northwest Sci. 2006, 80, 207. [Google Scholar]
- Yi, J.-S.; Kim, C.-W.; Lee, J.-K.; Meng, F.-J. Identification of Populations of Korean Mistletoe (Viscum Album L. Var. Coloratum) from Gangwon-Do in Korea by AFLP. Biotechnol. Biotechnol. Equip. 2013, 27, 4091–4097. [Google Scholar] [CrossRef]
- Kim, C.-S.; Kim, S.-Y.; Sun, B.-Y.; Yi, J.S. A review of the taxonomic and ecological characteristics of Korean mistletoe types (Viscum, Korthalsella, Loranthus and Taxillus). Korean J. Plant Taxon. 2013, 43, 81–89. [Google Scholar] [CrossRef]
- Sivaramakrishna, P.; Yugandhar, P.; Ekka, G.A. A new species Dendrophthoe laljii (Loranthaceae) infesting Artocarpus heterophyllus Lam. (Moraceae) in Andaman and Nicobar Islands, India. J. Asia-Pac. Biodivers. 2021, 14, 452–459. [Google Scholar] [CrossRef]
- Singh, L.J.; Ranjan, V.; Rasingam, L.; Swamy, J. A new species of genus Dendrophthoe Mart. (Lorantheae-Loranthaceae) from the Peninsular India. J. Asia-Pac. Biodivers. 2020, 13, 487–493. [Google Scholar] [CrossRef]
- Devkota, M.P. Biology of mistletoes and their status in Nepal Himalaya. Himal. J. Sci. 2005, 3, 84–88. [Google Scholar] [CrossRef]
- Bilgili, E.; Coskuner, K.A.; Baysal, I.; Ozturk, M.; Usta, Y.; Eroglu, M.; Norton, D. The distribution of pine mistletoe (Viscum album ssp. austriacum) in Scots pine (Pinus sylvestris) forests: From stand to tree level. Scand. J. For. Res. 2020, 35, 20–28. [Google Scholar] [CrossRef]
- Aukema, J.E. Vectors, viscin, and Viscaceae: Mistletoes as parasites, mutualists, and resources. Front. Ecol. Environ. 2003, 1, 212–219. [Google Scholar] [CrossRef]
- Kuijt, J. Dwarf mistletoes. Bot. Rev. 1955, 21, 569–619. [Google Scholar] [CrossRef]
- Pennings, S.C.; Callaway, R.M. Parasitic plants: Parallels and contrasts with herbivores. Oecologia 2002, 131, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Press, M.C.; Phoenix, G.K. Impacts of parasitic plants on natural communities. New Phytol. 2005, 166, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Okubamichael, D.Y.; Griffiths, M.E.; Ward, D. Host specificity, nutrient and water dynamics of the mistletoe Viscum rotundifolium and its potential host species in the Kalahari of South Africa. J. Arid Environ. 2011, 75, 898–902. [Google Scholar] [CrossRef]
- Yan, C.-F.; Gessler, A.; Rigling, A.; Dobbertin, M.; Han, X.-G.; Li, M.-H. Effects of mistletoe removal on growth, N and C reserves, and carbon and oxygen isotope composition in Scots pine hosts. Tree Physiol. 2016, 36, 562–575. [Google Scholar] [CrossRef] [Green Version]
- Schulze, E.-D.; Kelliher, F.M.; Körner, C.; Lloyd, J.; Leuning, R. Relationships among maximum stomatal conductance, ecosystem surface conductance, carbon assimilation rate, and plant nitrogen nutrition: A global ecology scaling exercise. Annu. Rev. Ecol. Syst. 1994, 25, 629–662. [Google Scholar] [CrossRef]
- Ehleringer, J.R.; Cook, C.S.; Tieszen, L.L. Comparative water use and nitrogen relationships in a mistletoe and its host. Oecologia 1986, 68, 279–284. [Google Scholar] [CrossRef]
- Sangüesa-Barreda, G.; Linares, J.C.; Camarero, J.J. Mistletoe effects on Scots pine decline following drought events: Insights from within-tree spatial patterns, growth and carbohydrates. Tree Physiol. 2012, 32, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Scalon, M.C.; dos Reis, S.A.; Rossatto, D.R. Shifting from acquisitive to conservative: The effects of Phoradendron affine (Santalaceae) infection in leaf morpho-physiological traits of a Neotropical tree species. Aust. J. Bot. 2017, 65, 31–37. [Google Scholar] [CrossRef]
- Teixeira-Costa, L.; Ceccantini, G. Embolism increase and anatomical modifications caused by a parasitic plant: Phoradendron crassifolium (Santalaceae) on Tapirira guianensis (Anacardiaceae). IAWA J. 2015, 36, 138–151. [Google Scholar] [CrossRef]
- Roth, L.F. Dwarf mistletoe-induced mortality in northwest ponderosa pine growing stock. West. J. Appl. For. 2001, 16, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Raftoyannis, Y.; Radoglou, K.; Bredemeier, M. Effects of mistletoe infestation on the decline and mortality of Abies cephalonica in Greece. Ann. For. Res. 2015, 58, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Fisher, J.T. Water relations of mistletoes and their hosts. In The Biology of Mistletoes; Calder, M., Bernhardt, P., Eds.; Academic: Sydney, Australia, 1983; pp. 161–184. [Google Scholar]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Waringford, UK, 1993; Volume 48. [Google Scholar]
- Szmidla, H.; Tkaczyk, M.; Plewa, R.; Tarwacki, G.; Sierota, Z. Impact of Common Mistletoe (Viscum album L.) on Scots Pine Forests—A Call for Action. Forests 2019, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.M.; Kartoolinejad, D.; Mirnia, S.K.; Tabibzadeh, Z.; Akbarinia, M.; Shayanmehr, F. The effects of Viscum album L. on foliar weight and nutrients content of host trees in Caspian forests (Iran). Pol. J. Ecol. 2007, 55, 579. [Google Scholar]
- Catal, Y.; Carus, S. Effect of pine mistletoe on radial growth of crimean pine (Pinus nigra) in Turkey. J. Environ. Biol. 2011, 32, 263–270. [Google Scholar]
- Queijeiro-Bolaños, M.E.; Malda-Barrera, G.X.; Carrillo-Angeles, I.G.; Suzán-Azpiri, H. Contrasting gas exchange effects on the interactions of two mistletoe species and their host Acacia schaffneri. J. Arid Environ. 2020, 173, 104041. [Google Scholar] [CrossRef]
- Bilgili, E.; Ozturk, M.; Coskuner, K.; Baysal, I.; Serdar, B.; Yavuz, H.; Eroglu, M.; Usta, Y. Quantifying the effect of pine mistletoe on the growth of Scots pine. For. Pathol. 2018, 48, e12435. [Google Scholar] [CrossRef]
- Kollas, C.; Gutsch, M.; Hommel, R.; Lasch-Born, P.; Suckow, F. Mistletoe-induced growth reductions at the forest stand scale. Tree Physiol. 2017, 38, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Pilichowski, S.; Filip, R.; Koscielska, A.; Zaroffe, G.; Zyzniewska, A.; Iszkulo, G. Influence of Viscum album ssp austriacum (Wiesb.) Vollm. on tree radial growth of Pinus sylvestris L. Sylwan 2018, 162, 452–459. [Google Scholar]
- Hawksworth, F.G.; Wiens, D. Dwarf Mistletoes: Biology, Pathology, and Systematics; Diane Publishing: Washington, DC, USA; Department of Agriculture, Forest Service: Washington, DC, USA, 1996; p. 410. [Google Scholar]
- Varga, I.; Taller, J.; Baltazár, T.; Hyvönen, J.; Poczai, P. Leaf-spot disease on European mistletoe (Viscum album) caused by Phaeobotryosphaeria visci: A potential candidate for biological control. Biotechnol. Lett. 2012, 34, 1059–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro-González, A.; Hódar, J.A.; Zamora, R. Mistletoe Versus Host Pine: Does Increased Parasite Load Alter the Host Chemical Profile? J. Chem. Ecol. 2019, 45, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-González, A.; Gargallo-Garriga, A.; Hódar, J.A.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J.; Zamora, R. Implications of mistletoe parasitism for the host metabolome: A new plant identity in the forest canopy. Plant. Cell Environ. 2021, 44, 3655–3666. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Guimarães, A.F.; Teodoro, G.S.; Bastos, S.S.; de Castro, E.M.; van den Berg, E. The enemy within: The effects of mistletoe parasitism on infected and uninfected host branches. Plant Ecol. 2021, 222, 639–645. [Google Scholar] [CrossRef]
- Gehring, C.A.; Whitham, T.G. Reduced mycorrhizae on Juniperus monosperma with mistletoe: The influence of environmental stress and tree gender on a plant parasite and a plant-fungal mutualism. Oecologia 1992, 89, 298–303. [Google Scholar] [CrossRef]
- Cullings, K.; Raleigh, C.; Vogler, D.R. Effects of severe dwarf mistletoe infection on the ectomycorrhizal community of a Pinus contorta stand in Yellowstone Park. Botany 2005, 83, 1174–1180. [Google Scholar] [CrossRef]
- Stermitz, F.R.; Belofsky, G.N.; Ng, D.; Singer, M.C. Quinolizidine alkaloids obtained by Pedicularis semibarbata (Scrophulariaceae) from Lupinus fulcratus (Leguminosae) fail to influence the specialist herbivore Euphydryas editha (Lepidoptera). J. Chem. Ecol. 1989, 15, 2521–2530. [Google Scholar] [CrossRef]
- Marko, M.D.; Stermitz, F.R. Transfer of alkaloids from Delphinium to Castilleja via root parasitism. Norditerpenoid alkaloid analysis by electrospray mass spectrometry. Biochem. Syst. Ecol. 1997, 25, 279–285. [Google Scholar] [CrossRef]
- Schneider, M.J.; Stermitz, F.R. Uptake of host plant alkaloids by root parasitic Pedicularis species. Phytochemistry 1990, 29, 1811–1814. [Google Scholar] [CrossRef]
- Ehleringer, J.; Ullmann, I.; Lange, O.; Farquhar, G.; Cowan, I.; Schulze, E.-D.; Ziegler, H. Mistletoes: A hypothesis concerning morphological and chemical avoidance of herbivory. Oecologia 1986, 70, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Barlow, B.A.; Wiens, D. Host-Parasite Resemblance in Australian Mistletoes: The Case for Cryptic Mimicry. Evolution 1977, 31, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Mescher, M.C.; De Moraes, C.M. Implications of bioactive solute transfer from hosts to parasitic plants. Curr. Opin. Plant Biol. 2013, 16, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Blick, R.A.J.; Burns, K.C.; Moles, A.T. Predicting network topology of mistletoe–host interactions: Do mistletoes really mimic their hosts? Oikos 2012, 121, 761–771. [Google Scholar] [CrossRef]
- Beatty, J.S.; Mathiasen, R.L. Dwarf Mistletoes of Ponderosa Pine; US Department of Agriculture, Forest Service: Washington, DC, USA, 2003. [Google Scholar]
- Knutson, D. Biological and chemical control of dwarf mistletoe. In Proceedings of the Symposium on Dwarf Mistletoe Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; Gen. Tech. Rep. PSW-31. Scharpf, R.F., Parmeter, J.R., Jr., Eds.; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1978; pp. 151–155. [Google Scholar]
- Milenkovic, M.; Karadzic, D.; Janjic, V.; Mihajlovic, L. Possibilities of mistletoe control. In Proceedings of the Peti Kongres o Korovima, Banja Koviljaca, Serbia, 18–21 June 1996. [Google Scholar]
- Watson, D. Reconnaissance and Recommendations for Mistletoe Management in Macadamia Orchards; Final Report; Hort Innovation: North Sydney, Australia, 2019; pp. 1–30. [Google Scholar]
- Walldén, B. Misteln vid dess nordgräns: Die Mistel an Ihrer Nordgrenze. Svensk Botanisk Tidskrift 1961, 55, 123. [Google Scholar]
- Weber, H. Untersuchungen zur Entwicklungsweise der Laubholzmistel Viscum album L.(Viscaceae) und über Zuwachsraten während ihrer ersten Stadien. Beiträge Zur Biol. Der Pflanz. 1993, 67, 319–331. [Google Scholar]
- Hoffman, J. Management Guide for Dwarf Mistletoe. Arceuthobium spp. Forest Health Protection and State Forestry Organizations. Available online: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5187427.pdf (accessed on 22 October 2022).
- Zielke, K.; Bancroft, B. Introduction to Silvicultural Systems (Web-Based Workbook). Available online: https://www.for.gov.bc.ca/hfd/pubs/ssintroworkbook/append2.htm (accessed on 20 July 2020).
- Parmeter, J.; Scharpf, R.F. Spread of Dwarf Mistletoe from Discrete Seed Sources into Young Stands of Ponderosa and Jeffrey Pines; US Forest Service: Washington, DC, USA; US Department of Agriculture, Pacific Southwest Forest and Range: Vallejo, CA, USA, 1972; Volume 269. [Google Scholar]
- Scharpf, R.F.; Parmeter, J. Seed Production and Dispersal by Dwarf Mistletoe in Overstory Jeffrey Pines in California; US Forest Service: Washington, DC, USA; US Department of Agriculture, Pacific Southwest Forest and Range: Berkeley CA, USA, 1971; Volume 247. [Google Scholar]
- Scharpf, R.F.; Parmeter, J.R., Jr. Spread of Dwarf Mistletoe into Jeffrey Pine Plantation-Trees Infected after 22 Years; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1976; 6p. [Google Scholar]
- Reid, N.; Yan, Z. Mistletoes and Other Phanerogams Parasitic on Eucalypts; CSIRO: Canberra, Australia, 2000; pp. 353–384. [Google Scholar]
- Kelly, P.; Reid, N.; Davies, I. Effects of experimental burning, defoliation, and pruning on survival and vegetative resprouting in mistletoes (Amyema miquelii and Amyema pendula). Int. J. Plant Sci. 1997, 158, 856–861. [Google Scholar] [CrossRef]
- Conklin, D.A.; Geils, B.W. Survival and sanitation of dwarf mistletoe-infected ponderosa pine following prescribed underburning. West. J. Appl. For. 2008, 23, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Koonce, A.L.; Roth, L.F. The effects of prescribed burning on dwarf mistletoe in ponderosa pine. In Proceedings of the Sixth Conference on Fire and Forest Meteorology, Seattle, WA, USA, 22–24 April 1980; pp. 22–24. [Google Scholar]
- Ritter, S.; Hoffman, C.; Stewart, J.; Zimmerman, T. The influence of prescribed crown fire on lodgepole pine dwarf mistletoe (Arceuthobium americanum) populations 33 years post-fire. For. Pathol. 2018, 48, e12419. [Google Scholar] [CrossRef]
- Alexander, M.E.; Hawksworth, F.G. Fire and dwarf mistletoes in North American coniferous forests. J. For. 1976, 74, 446–449. [Google Scholar] [CrossRef]
- Kipfmueller, K.F.; Baker, W.L. Fires and dwarf mistletoe in a Rocky Mountain lodgepole pine ecosystem. For. Ecol. Manag. 1998, 108, 77–84. [Google Scholar] [CrossRef]
- Manion, P. Tree Diseases Concepts, 2nd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1991. [Google Scholar]
- Knight, D.H. Parasites, lightning, and the vegetation mosaic in wilderness landscapes. In Landscape Heterogeneity and Disturbance; Springer: Berlin/Heidelberg, Germany, 1987; pp. 59–83. [Google Scholar]
- Conklin, D.A.; Armstrong, W.A. Effects of Three Prescribed Fires on Dwarf Mistletoe Infection in Southwestern Ponderosa Pine; US Department of Agriculture, Forest Service, Southwestern Region: Berkeley CA, USA, 2001. [Google Scholar]
- Haikerwal, A.; Reisen, F.; Sim, M.R.; Abramson, M.J.; Meyer, C.P.; Johnston, F.H.; Dennekamp, M. Impact of smoke from prescribed burning: Is it a public health concern? J. Air Waste Manag. Assoc. 2015, 65, 592–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afrin, S.; Garcia-Menendez, F. Potential impacts of prescribed fire smoke on public health and socially vulnerable populations in a Southeastern, U.S. state. Sci. Total Environ. 2021, 794, 148712. [Google Scholar] [CrossRef] [PubMed]
- Cirocco, R.M.; Facelli, J.M.; Watling, J.R. High water availability increases the negative impact of a native hemiparasite on its non-native host. J. Exp. Bot. 2015, 67, 1567–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griebel, A.; Metzen, D.; Pendall, E.; Nolan, R.H.; Clarke, H.; Renchon, A.A.; Boer, M.M. Recovery from Severe Mistletoe Infection After Heat- and Drought-Induced Mistletoe Death. Ecosystems 2022, 25, 1–16. [Google Scholar] [CrossRef]
- Griebel, A.; Peters, J.M.R.; Metzen, D.; Maier, C.; Barton, C.V.M.; Speckman, H.N.; Boer, M.M.; Nolan, R.H.; Choat, B.; Pendall, E. Tapping into the physiological responses to mistletoe infection during heat and drought stress. Tree Physiol. 2021, 42, 523–536. [Google Scholar] [CrossRef]
- Sidahmed, O.A. Incidence of mistletoe (Loranthus spp.) on citrus and guava trees in the central region of the Sudan. In Proceedings of the VIII African Symposium on Horticultural Crops 143, Wed Medani, Sudan, 20–24 March 1983; pp. 417–420. [Google Scholar]
- Knutson, D.M. The influence of urea fertilization on growth of mistle-toed pine seedlings. In Proceedings of the Second International Congress of plant pathology, Minneapolis, MN, USA, 5–12 September 1973; pp. 5–12. [Google Scholar]
- Kulkarni, H.D.; Srimathi, K. Plant pests of sandal (Santalum album L.). My For. 1988, 24, 29–38. [Google Scholar]
- Minko, G.; Fagg, P.C. Control of some mistletoe species on eucalypts by trunk injection with herbicides. Aust. For. 1989, 52, 94–102. [Google Scholar] [CrossRef]
- Brown, A.; Greenham, C. Further investigations in the control of mistletoe by trunk injections. Aust. J. Exp. Agric. 1965, 5, 305–309. [Google Scholar] [CrossRef]
- Brown, A. Mistletoe control on a large scale. J. Aust. Inst. Agric. Sci. 1959, 25, 282–286. [Google Scholar]
- Reid, N.; Fittler, J.; Kar, A.; Storey, A.; Cook, T. Effect of Spray Applications of Several Herbicides on the Mortality of Box Mistletoe (Amyema miquelii) and Host Saplings (Eucalyptus blakelyi and E. melliodora). In Ecosystem Management; University of New England: Armidale, Australia, 2008. [Google Scholar]
- Watson, W.T.; Martinez-Trinidad, T. Strategies and treatments for leafy mistletoe (Phoradendron tomentosum [DC.] Engelm ex. Gray) suppression on cedar elm (Ulmus crassifolia Nutt.). Arboric. Urban For. 2006, 32, 265. [Google Scholar] [CrossRef]
- Quick, C.R. Experimental Herbicidal Control of Dwarfmistletoe on Some California Conifers; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, CA, USA, 1964; Volume 47, 9p. [Google Scholar]
- Livingston, W.; Brenner, M. Ethephon stimulates abscission of eastern dwarf mistletoe aerial shoots on black spruce. Plant Dis. 1983, 67, 909–910. [Google Scholar] [CrossRef]
- Livingston, W.H.; Blanchette, R.A.; Brenner, M.L.; Zuzek, K.J. Effective use of ethylene-releasing agents to prevent spread of eastern dwarf mistletoe on black spruce. Can. J. For. Res. 1985, 15, 872–876. [Google Scholar] [CrossRef]
- Livingston, W.H.; Brenner, M.L.; Blanchette, R.A. Altered concentrations of abscisic acid, indole-3-acetic acid, and zeatin riboside associated eastern dwarf mistletoe infections on black spruce. In Proceedings of the Biology of Dwarf Mistletoes, Symposium Proceedings, Fort Collins, CO, USA, 8 August 1984; Gen. Tech. Rep. RM-111. Hawksworth, F.G., Scharpf, R.F., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1984; pp. 53–60. [Google Scholar]
- Adams, D.H.; Frankel, S.J.; Lichter, J.M. Considerations when using ethephon for supressing dwarf and leafy mistletoe infestations in ornamental landscapes. J. Arboric. 1993, 19, 351–357. [Google Scholar]
- Winder, R.S.; Shamoun, S.F. Forest pathogens: Friend or foe to biodiversity? Can. J. Plant Pathol. 2006, 28, S221–S227. [Google Scholar] [CrossRef]
- Robbins, K.; Johnson, D.W.; Hawksworth, F.G.; Nicholls, T.H. Aerial application of ethephon is ineffective in controlling lodgepole pine dwarf mistletoe. West. J. Appl. For. 1989, 4, 27–28. [Google Scholar] [CrossRef]
- Baker, F.; Knowles, K.; Meyer, T.; French, D. Aerial applications of ethylene-releasing chemicals fail to promote abscission of dwarf mistletoe aerial shoots on Jack pine. For. Chron. 1989, 65, 194–195. [Google Scholar] [CrossRef] [Green Version]
- Weber, H.C.; Forstreuter, W. Parasitismus von Blutenpflanzen; Wissenschaftliche Buchgesellschaft: Darmstadt, Germany, 1993. [Google Scholar]
- Parks, C.A.; Hoffman, J.T. Control of Western Dwarf Mistletoe with the Plant-Growth Regulator Ethephon; US Department of Agriculture, Forest Service, Pacific Northwest Research Station: Berkeley, CA, USA, 1991; Volume 506. [Google Scholar]
- Muir, J.; Geils, B. Management Strategies for Dwarf Mistletoe: Silviculture; Forest Service: Washington, DC, USA; USDA: Washington, DC, USA, 2002; pp. 83–94. [Google Scholar]
- Robinson, D.C.; Geils, B.W.; Muir, J.A. Spatial statistical model for the spread of dwarf mistletoe within and between stands. In Proceedings of the Second Forest Vegetation Simulator Conference, Fort Collins, CO, USA, 12–14 February 2002; Crookston, N.L., Havis, R.N., Eds.; pp. 178–185. [Google Scholar]
- Van Halder, I.; Castagneyrol, B.; Ordóñez, C.; Bravo, F.; del Río, M.; Perrot, L.; Jactel, H. Tree diversity reduces pine infestation by mistletoe. For. Ecol. Manag. 2019, 449, 117470. [Google Scholar] [CrossRef]
- Hanover, J.W. Tree improvement for disease resistance in western United States and Canada. In Breeding Pest-Resistant Trees; Elsevier: Amsterdam, The Netherlands, 1966; pp. 53–56. [Google Scholar]
- Geza, F.; Laszlo, J.; Ildiko, V.; Szilvia, P. Parasitic fungi of European mistletoe (Viscum album L.). Növényvédelem 2009, 45, 178–183. [Google Scholar]
- Scharpf, R.F. Host resistance to dwarf mistletoe. In Proceedings of the Biology of Dwarf Mistletoes, Symposium Proceedings, Fort Collins, CO, USA, 8 August 1984; Hawksworth, F.G., Scharpf, R.F., Eds.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1984; pp. 70–76. [Google Scholar]
- Wyckhuys, K.A.G.; Hughes, A.C.; Buamas, C.; Johnson, A.C.; Vasseur, L.; Reymondin, L.; Deguine, J.P.; Sheil, D. Biological control of an agricultural pest protects tropical forests. Commun. Biol. 2019, 2, 10. [Google Scholar] [CrossRef]
- Nzioki, H.S.; Oyosi, F.; Morris, C.E.; Kaya, E.; Pilgeram, A.L.; Baker, C.S.; Sands, D.C. Striga Biocontrol on a Toothpick: A Readily Deployable and Inexpensive Method for Smallholder Farmers. Front. Plant Sci. 2016, 7, 1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bale, J.; Van Lenteren, J.; Bigler, F. Biological control and sustainable food production. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 761–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charudattan, R. Biological control of weeds by means of plant pathogens: Significance for integrated weed management in modern agro-ecology. BioControl 2001, 46, 229–260. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Geils, B.W. Biotic Associates; Diane Publishing: Washington, DC, USA; Department of Agriculture, Forest Service: Washington, DC, USA, 1996. [Google Scholar]
- Hawksworth, F.G.; Wicker, E.F.; Scharpf, R.F. Fungal Parasites of Dwarf Mistletoes [Arceuthobium, Parasites of Conifers, Biological Control]; Department of Agriculture, Forest Service: Washington, DC, USA, 1977; p. 14. [Google Scholar]
- Kuijt, J. Distribution of Dwarf Mistletoes and Their Fungus Hyperparasites in Western Canada; Department of Northern Affairs and National Resources: Ottawa, ON, Canada, 1963; Volume 186, pp. 134–148. [Google Scholar]
- Stevens, R.E.; Hawksworth, F.G. Insect-dwarf mistletoe associations: An update. In Proceedings of the Symposium: Biology of Dwarf Mistletoes, Fort Collins, CO, USA, 8 August 1984; pp. 94–101. [Google Scholar]
- Stevens, R.E.; Hawksworth, F.G. Insects and Mites Associated with Dwarf Mistletoes; Research Papers; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CA, USA, 1970; p. 12. [Google Scholar]
- Ridings, W. Biological control of stranglervine in citrus—A researcher’s view. Weed Sci. 1986, 34, 31–32. [Google Scholar] [CrossRef]
- Daniel, J.; Templeton, G.; Smith, R.; Fox, W. Biological Control of Northern Jointvetch in Rice by An Endemic Fungal Disease. Weed Sci. 1973, 21, 303–307. [Google Scholar] [CrossRef]
- Makowski, R.M.; Mortensen, K. The first mycoherbicide in Canada: Colletotrichum gloeosporioides f. sp. malvae for round-leaved mallow control. In Proceedings of the First International Weed Control Congress, Melbourne, Australia, 17–21 February 1992; pp. 298–300. [Google Scholar]
- De Jong, M.; Scheepens, P.; Zadoks, J. Risk analysis for biological control: A Dutch case study in biocontrol of Prunus serotina by the fungus Chondrostereum purpureum. Plant Dis. 1990, 74, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Wall, R. Biological control of red alder using stem treatments with the fungus Chondrostereum purpureum. Can. J. For. Res. 1994, 24, 1527–1530. [Google Scholar] [CrossRef]
- Shamoun, S.F. Application of biological control to vegetation management in forestry. In Proceedings of the X International Symposium on Biological Control of Weeds, Bozeman, MO, USA, 4–14 July 1999; pp. 87–96. [Google Scholar]
- Shamoun, S. Implementation of weed biocontrol in forest vegetation management for conifer production. In Handbook of Sustainable Weed Management; Singh, H.P., Batish, D.R., Kohli, R.K., Eds.; Howarth Press, Inc.: Binghamton, NY, USA, 2006; pp. 475–506. [Google Scholar]
- Morris, M.; Wood, A.; Den Breeyen, A. Development and registration of a fungal inoculant to prevent regrowth of cut wattle tree stumps in South Africa and brief overview of other bioherbicide projects currenlty in progress. In Proceedings of the Fourth International Bioherbicide Workshop Programme and Abstracts, Glasgow, UK, 6–7 August 1998; University of Strathclyde: Strathclyde, UK, 1998; p. 15. [Google Scholar]
- Beilharz, V. Fungal pathogens of mistletoes in Victoria. Vic. Nat. 1997, 114, 162–163. [Google Scholar]
- Shamoun, S.F.; Ramsfield, T.D.; Van Der Kamp, B.J. Biological control approach for management of dwarf mistletoes. N. Z. J. For. Sci. 2003, 33, 373–384. [Google Scholar]
- Deeks, S.J.; Shamoun, S.F.; Punja, Z.K. In vitro germination and development of western hemlock dwarf mistletoe. Plant Cell Tissue Organ Cult. 2001, 66, 97–105. [Google Scholar] [CrossRef]
- Funk, A.; Smith, R.; Baranyay, J. Canker of dwarf mistletoe swellings on western hemlock caused by Nectria funkeliana var. macrospora. Can. J. For. Res. 1973, 3, 71–74. [Google Scholar] [CrossRef]
- Rietman, L.M.; van der Kamp, B.J.; Shamoun, S.F. Assessment of Neonectria neomacrospora (anamorph Cylindrocarpon cylindroides) as an inundative biocontrol agent against hemlock dwarf mistletoe. Can. J. Plant Pathol. 2005, 27, 603–609. [Google Scholar] [CrossRef]
- Scharpf, R.F. Dwarf Mistletoe on Red Fir: Infection and Control in Understory Stands; US Department of Agriculture, Pacific Southwest Forest and Range: Berkeley, CA, USA, 1969; Volume 50. [Google Scholar]
- Scharpf, R.F.; Bynum, H. Cytospora Canker of True Firs; Forest Service, US Department of Agriculture: Berkeley, CA, USA, 1975; Volume 146. [Google Scholar]
- Mark, W.R.; Hawksworth, F.G.; Oshima, N. Resin disease: A new disease of lodgepole pine dwarf mistletoe. Can. J. For. Res. 1976, 6, 415–424. [Google Scholar] [CrossRef]
- Kotan, R.; Okutucu, A.; Ala Görmez, A.; Karagoz, K.; Dadasoglu, F.; Karaman, I.; Hasanekoglu, I.; Kordali, Ş. Parasitic bacteria and fungi on common mistletoe (Viscum album L.) and their potential application in biocontrol. J. Phytopathol. 2013, 161, 165–171. [Google Scholar] [CrossRef]
- Ulukapı, M.; Erdoğdu, M. Aureobasidium Harposporum: A Potential Biocontrol Agent of Viscum Album. In Proceedings of the WITAM-2016, Kershehill, Turkey, 28 September–2 October 2016; p. 103. [Google Scholar]
- Mandeel, Q.; Hasan, A.; Al-Nafea, H.; Abbas, H. Antibacterial activity of extracts from selected marine algae in Bahrain. Arab Gulf J. Sci. Res. 2010, 28, 147–162. [Google Scholar] [CrossRef]
- Galal, H.; Salem, W.; Nasr El-Deen, F. Biological control of some pathogenic fungi using marine algae. Res. J. Microbiol 2011, 6, 645–657. [Google Scholar] [CrossRef] [Green Version]
- Shah, M.A.; Khan, A.A. Use of diatomaceous earth for the management of stored-product pests. Int. J. Pest Manag. 2014, 60, 100–113. [Google Scholar] [CrossRef]
- El-Ansary, M.S.M.; Hamouda, R.A. Biocontrol of root-knot nematode infected banana plants by some marine algae. Russ. J. Mar. Biol. 2014, 40, 140–146. [Google Scholar] [CrossRef]
- Nabaei, N.; Mehrvar, A.; Saber, M.; Bagheri, M. Efficacy of entomopathogenic fungi in combination with diatomaceous earth against Callosobruchus maculatus (Coleoptera: Bruchidae). Acta Entomol. Sin. 2012, 55, 1282–1288. [Google Scholar]
- Athanassiou, C.G.; Palyvos, N.E. Laboratory evaluation of two diatomaceous earth formulations against Blattisocius keegani Fox (Mesostigmata, Ascidae) and Cheyletus malaccensis oudemans (Prostigmata, Cheyletidae). Biol. Control 2006, 38, 350–355. [Google Scholar] [CrossRef]
- Solomon, J.; Newsome, L.; Filer, T. Discovery and observations of a stem-boring weevil (Myrmex sp.) a potentially useful biocontrol of mistletoe. J. Miss. Acad. Sci. 1984, 29, 7–11. [Google Scholar]
- Van Dyke, E.C. New Rhynchophora (Coleoptera) from western North America. Pan-Pac. Entomol. 1930, 6, 149–165. [Google Scholar]
- Sleeper, E.L. New genera and species of Curculionidae with a new species of Anthribidae (Coleoptera). New Curculionidae 1953, 53, 113–120. [Google Scholar]
- Vanin, S.A.; Guerra, T.J. A remarkable new species of flesh-fly mimicking weevil (Coleoptera: Curculionidae: Conoderinae) from Southeastern Brazil. Zootaxa 2012, 3413, 55–63. [Google Scholar] [CrossRef]
- Guerra, T.J. Evasive mimicry: Too beetle, or not too beetle? Ecology 2019, 100, e02773. [Google Scholar] [CrossRef] [PubMed]
- Baloch, G.; Mohyuddin, A. The phytophagous fauna of a mistletoe (Loranthus longiflorus Desr.: Loranthaceae) in West Pakistan. Weed Res. 1969, 9, 62–64. [Google Scholar] [CrossRef]
- Mushtaque, M.; Baloch, G. Possibilities of biological control of mistletoes, Loranthus spp., using oligophagous insects from Pakistan. Entomophaga 1979, 24, 73–81. [Google Scholar] [CrossRef]
- Jendek, E.; Poláková, J. Host Plants of World Agrilus (Coleoptera, Buprestidae), 1st ed.; Springer: Cham, Germany, 2014; Volume 706. [Google Scholar]
- Królik, R. Agrilus kutahyanus n. sp. from Turkey (Coleoptera: Buprestidae). Genus 2002, 13, 25–31. [Google Scholar]
- Lastuvka, Z. Morphology and biology of clearwing moths Synanthedon loranthi (Kr.) and Synanthedon cephiformis (O.) (Lepidoptera, Sesiidae). Acta Univ. Agric. Fac. Agron 1983, 31, 143–158. [Google Scholar]
- Senn, P.; Kowalczyk, J. Nowe stanowiska Ditula angustiorana (Haworth, 1811)(Lepidoptera: Tortricidae) oraz pierwsze stwierdzenia samców tego gatunku w Polsce. Wiadomości Entomol. 2018, 37, 125–126. [Google Scholar]
- Buszko, J.; Nowacki, J.; Entomologiczne, P.T. A Distributional Checklist of the Lepidoptera of Poland; Polish Entomological Society: Poznan, Poland, 2017. [Google Scholar]
- Reichholf, J.H. Aspekte der Biologie und Fragen zur Verbreitung des Mistelwicklers Celypha woodiana Barrett, 1882, in Bayern. Atalanta 2008, 39, 379–383. [Google Scholar]
- Briggs, J. Mistletoe (Viscum album): A brief review of its local status with recent observations on its insects associations and conservation problems. In Proceedings of the Cotteswold Naturalists’ Field Club, Cotteswold, UK, 12 September 2011; pp. 181–193. [Google Scholar]
- Lazaro-Gonzalez, A.; Hodar, J.A.; Zamora, R. Do the arthropod communities on a parasitic plant and its hosts differ? Eur. J. Entomol. 2017, 114, 215. [Google Scholar] [CrossRef] [Green Version]
- Rubiales, D.; Fernández-Aparicio, M. First report of cottony-cushion scale (Icerya purchasi) on red berried mistletoe (Viscum cruciatum). Entomol. Res. 2009, 39, 95–96. [Google Scholar] [CrossRef]
- Chávez-Salcedo, L.F.; Queijeiro-Bolaños, M.E.; López-Gómez, V.; Cano-Santana, Z.; Mejía-Recamier, B.E.; Mojica-Guzmán, A. Contrasting arthropod communities associated with dwarf mistletoes Arceuthobium globosum and A. vaginatum and their host Pinus hartwegii. J. For. Res. 2018, 29, 1351–1364. [Google Scholar] [CrossRef]
- Tong, J.; Ren, W. Preliminary studies on the disease cycle of Arceuthobium chinense. J. Yunnan For. Coll. 1980, 1, 19–25. [Google Scholar]
- Krasylenko, Y.; Těšitel, J.; Ceccantini, G.; Oliveira-da-Silva, M.; Dvořák, V.; Steele, D.; Sosnovsky, Y.; Piwowarczyk, R.; Watson, D.M.; Teixeira-Costa, L. Parasites on parasites: Hyper-, epi-, and autoparasitism among flowering plants. Am. J. Bot. 2021, 108, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Calvin, C.L.; Wilson, C.A. Epiparasitism in Phoradendron durangense and P. falcatum (Viscaceae). Aliso A J. Syst. Evol. Bot. 2009, 27, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Glatzel, G.; Balasubramaniam, S. Mineral nutrition of mistletoes: General concepts. In Proceedings of the 4th International Symposium on Parasitic Flowering Plants, Marburg, Germany, 10 August 1987; pp. 263–276. [Google Scholar]
- Wiens, D.; Calvin, C. Epiparasitism in mistletoes. Gold. Bough (R. Bot. Gard. Kew) 1987, 9, 3–5. [Google Scholar]
- Downey, P.O. An inventory of host species for each aerial mistletoe species (Loranthaceae and Viscaceae) in Australia. Cunninghamia 1998, 5, 685–720. [Google Scholar]
- Moss, J.S.L. Mistletoe/hostplant associations of the Barakula, Columboola and Gurulmundi region, south-east Queensland. Qld. Nat. 1998, 36, 73–87. [Google Scholar]
- Eastwood, K. A Guide to Australia’s Possums and Gliders. Available online: https://www.australiangeographic.com.au/topics/wildlife/2019/07/a-guide-to-all-27-species-of-australias-possums-and-gliders/ (accessed on 22 October 2022).
- Sessions, L.A.; Rance, C.; Grant, A.; Kelly, D. Possum (Trichosurus vulpecula) control benefits native beech mistletoes (Loranthaceae). N. Z. J. Ecol. 2001, 25, 27–33. [Google Scholar]
- Reid, N. Control of mistletoes by possums and fire: A review of the evidence. Vic. Nat. 1997, 114, 149–158. [Google Scholar]
- Kar, A.K. Methods to Manage Mistletoe—A Landholder’s Guide. 2008. Available online: https://www.snelandcare.org.au/linkedfiles/MethodsManageMistletoe.pdf (accessed on 8 October 2022).
- Room, P. The constitution and natural history of the fauna of the mistletoe Tapinanthus bangwensis (Engl. & K. Krause) growing on cocoa in Ghana. J. Anim. Ecol. 1972, 41, 519–535. [Google Scholar]
- Patrick, B.H.; Dugdale, J.S. Mistletoe moths-New Zealand’s loranthaceous mistletoes. In Proceedings of the A Workshop Hosted by Threatened Species Unit, Department of Conservation, Cass, Wellington, New Zealand, 17–20 July 1995; p. 125. [Google Scholar]
- Wicker, E.F.; Shaw, C.G. Fungal parasites of dwarf mistletoes. Mycologia 1968, 60, 372–383. [Google Scholar] [CrossRef]
- Nguyen, J.; Fernandez, V.; Pontrelli, S.; Sauer, U.; Ackermann, M.; Stocker, R. A distinct growth physiology enhances bacterial growth under rapid nutrient fluctuations. Nat. Commun. 2021, 12, 3662. [Google Scholar] [CrossRef] [PubMed]
- Burman, E.; Bengtsson-Palme, J. Microbial Community Interactions Are Sensitive to Small Changes in Temperature. Front. Microbiol. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.E. Studies of fruitflies associated with mistletoe in Australia and Pakistan with notes and decriptions on genera related to Perilampsis Bezzi (Diptera: Tephritidae). Beiträge Zur Entomol. Contrib. Entomol. 1967, 17, 127–149. [Google Scholar]
- Garbelotto, M.; Lowell, N.; Chen, I.Y.; Osmundson, T.W. Evidence for inhibition of a fungal biocontrol agent by a plant microbiome. J. Plant Pathol. 2019, 101, 457–466. [Google Scholar] [CrossRef]
- Perry, E. Broadleaf Mistletoe in Landscape Trees; University of California: Berkeley, CA, USA, 1995; p. 14. [Google Scholar]
- Rubiales, D. Parasitic plants, wild relatives and the nature of resistance. New Phytol. 2003, 160, 459–461. [Google Scholar] [CrossRef]
- Aly, R.; Dubey, N.K. Weed Management for Parasitic Weeds. In Recent Advances in Weed Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 315–345. [Google Scholar]
- Hawksworth, F.G. Biological factors of dwarf mistletoe in relation to control. In Proceedings of the Symposium on Dwarf Mistletoes Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; Gen. Tech. Rep. PSW-31. pp. 5–15. [Google Scholar]
- Roth, L.F. Genetic control of dwarf mistletoe. In Proceedings of the Symposium on Dwarf Mistletoes Control through Forest Management, Berkeley, CA, USA, 11–13 April 1978; pp. 69–72. [Google Scholar]
- Scharpf, R.F.; Roth, L.F. Resistance of Ponderosa Pine to Western Dwarf Mistletoe in Central Oregon; Res. Pap. PSW-RP-208; US Department of Agriculture, Pacific Southwest Forest and Range: Albany, CA, USA, 1992; Volume 208, 9p. [Google Scholar]
- Roth, L.F. Resistance of ponderosa pine to dwarf mistletoe. Silvae Genet. 1974, 23, 116–120. [Google Scholar]
- Arruda, R.; Carvalho, L.N.; Del-Claro, K. Host specificity of a Brazilian mistletoe, Struthanthus aff. polyanthus (Loranthaceae), in Cerrado Tropical Savanna. Flora-Morphol. Distrib. Funct. Ecol. Plants 2006, 201, 127–134. [Google Scholar] [CrossRef]
- Atsatt, P. Host-parasite interactions in higher plants. In Physiological Plant Ecology III; Springer: Berlin/Heidelberg, Germany, 1983; Volume 12/C, pp. 519–535. [Google Scholar]
- Dean, W.; Midgley, J.; Stock, W. The distribution of mistletoes in South Africa: Patterns of species richness and host choice. J. Biogeogr. 1994, 21, 503–510. [Google Scholar] [CrossRef]
- Downey, P.O.; Gill, A.M.; Banks, J.C. The Influence of Host Attributes on Mistletoe Colonization: An Example from Mulligan’s Flat Nature Reserve, A.C.T. Vic. Nat. 1997, 114, 105–111. [Google Scholar]
- Ghosh, S.K.; Balasundaram, M.; Mohamed, A.M. Studies in Host Parasite Relationship of Phanerogamic Parasites on Teak and Their Possible Control; Kerela Forest Reseach Institute: Kerala, India, 1984; p. 21. [Google Scholar]
- Halbritter, D.A.; Willett, D.S.; Gordon, J.M.; Stelinski, L.L.; Daniels, J.C. Behavioral Evidence for Host Transitions in Plant, Plant Parasite, and Insect Interactions. Environ. Entomol. 2018, 47, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.J.; Fuentes, E.R.; Cortes, I.; Liberona, F.; Costa, V. Tristerix tetrandrus (Loranthaceae) and its host-plants in the Chilean matorral: Patterns and mechanisms. Oecologia 1986, 69, 202–206. [Google Scholar] [CrossRef]
- Norton, D.A.; Carpenter, M.A. Mistletoes as parasites: Host specificity and speciation. Trends Ecol. Evol. 1998, 13, 101–105. [Google Scholar] [CrossRef]
- Okubamichael, D.Y.; Griffiths, M.E.; Ward, D. Host specificity in parasitic plants—Perspectives from mistletoes. AoB Plants 2016, 8, plw069. [Google Scholar] [CrossRef]
- Rödl, T.; Ward, D. Host recognition in a desert mistletoe: Early stages of development are influenced by substrate and host origin. Funct. Ecol. 2002, 16, 128–134. [Google Scholar] [CrossRef]
- Tainter, F.; French, D. The role of wound periderm in the resistance of eastern larch and jack pine to dwarf mistletoe. Can. J. Bot. 1971, 49, 501–504. [Google Scholar] [CrossRef]
- Smith, R.; Wass, E.; Meagher, M. Evidence of resistance to hemlock dwarf mistletoe (Arceuthobium tsugense) in western hemlock (Tsuga heterophylla) clones. Eur. J. For. Pathol. 1993, 23, 163–170. [Google Scholar] [CrossRef]
- Roeser, J., Jr. The importance of seed source and the possibilities of forest tree breeding. J. For. 1926, 24, 38–51. [Google Scholar] [CrossRef]
- Bates, C.G. Better seeds, better trees. J. For. 1927, 25, 130–144. [Google Scholar] [CrossRef]
- Hawksworth, F.G.; Edminster, C.B. Carlos Bates’ Dwarf Mistletoe Resistant Ponderosa Pines: A Postscript after Half a Century; USDA Forest Service, Rocky Mountain Forest and Range Experiment Station: Berkeley, CA, USA, 1981; Volume 412, p. 4. [Google Scholar]
- Roth, L.F. Dwarf mistletoe damage to small ponderosa pines. For. Sci. 1971, 17, 373–380. [Google Scholar] [CrossRef]
- Roth, L.F. Juvenile Susceptibility of Ponderosa Pine. Phytopathology 1974, 64, 689–692. [Google Scholar] [CrossRef]
- Harrington, T.; Wingfield, M. Diseases and the ecology of indigenous and exotic pines. In Ecology and Biogeography of Pinus; Richardson, D., Ed.; Cambridge University Press: Cambridge, UK, 1998; pp. 1–33. [Google Scholar]
- Okubamichael, D.Y. Host Specificity of the Hemiparasitic Mistletoe, Agelanthus natalitius. Master’s Thesis, University of KwaZulu-Natal, Pietermaritzburg, South Africa, 2009. [Google Scholar]
- Skrypnik, L.; Maslennikov, P.; Feduraev, P.; Pungin, A.; Belov, N. Ecological and Landscape Factors Affecting the Spread of European Mistletoe (Viscum album L.) in Urban Areas (A Case Study of the Kaliningrad City, Russia). Plants 2020, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Zaroug, M.S.; Zahran, E.B.; Abbasher, A.A. Distribution And Host Range of Mistletoe (Tapinanthus globiferus) (A. Rich.) Van Tieghan) Along the Blue Nile Banks in Central Sudan. Int. J. Sci. Technol. Res. 2014, 3, 1–5. [Google Scholar]
- Ladley, J.J.; Kelly, D.; Robertson, A.W. Explosive flowering, nectar production, breeding systems, and pollinators of New Zealand mistletoes (Loranthaceae). N. Z. J. Bot. 1997, 35, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Lavorel, S.; Smith, M.S.; Reid, N. Spread of mistletoes (Amyema preissii) in fragmented Australian woodlands: A simulation study. Landsc. Ecol. 1999, 14, 147–160. [Google Scholar] [CrossRef]
- Yoder, J.I. Parasitic plant responses to host plant signals: A model for subterranean plant–plant interactions. Curr. Opin. Plant Biol. 1999, 2, 65–70. [Google Scholar] [CrossRef]
- Tomilov, A.; Tomilova, N.; Shin, D.H.; Jamison, D.; Torres, M.; Reagan, R.; McGray, H.; Horning, T.; Truong, R.; Nava, A. Chemical signalling between plants. In Chemical Ecology: From Gene to Ecosystem; Springer: Dordrecht, The Netherlands, 2006; Volume 16, pp. 55–69. [Google Scholar]
- Yan, Z. Resistance to haustorial development of two mistletoes, Amyema preissii (Miq.) Tieghem and Lysiana exocarpi (Behr.) Tieghem ssp. exocarpi (Loranthaceae), on host and nonhost species. Int. J. Plant Sci. 1993, 154, 386–394. [Google Scholar] [CrossRef]
- Lech, P.; Żółciak, A.; Hildebrand, R. Occurrence of European Mistletoe (Viscum album L.) on Forest Trees in Poland and Its Dynamics of Spread in the Period 2008–2018. Forests 2020, 11, 83. [Google Scholar] [CrossRef]
- Nickrent, D.; Musselman, L. Introduction to parasitic flowering plants. Plant Health Instr. 2004. [Google Scholar] [CrossRef]
- Overton, J.M. Dispersal and infection in mistletoe metapopulations. J. Ecol. 1994, 82, 711–723. [Google Scholar] [CrossRef]
- Amico, G.; Aizen, M.A. Mistletoe seed dispersal by a marsupial. Nature 2000, 408, 929–930. [Google Scholar] [CrossRef]
- Lira-Noriega, A.; Toro-Núñez, O.; Oaks, J.R.; Mort, M.E. The roles of history and ecology in chloroplast phylogeographic patterns of the bird-dispersed plant parasite Phoradendron californicum (Viscaceae) in the Sonoran Desert. Am. J. Bot. 2015, 102, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Crespo, M.; Ornelas, J.; Martén-Rodríguez, S.; González-Rodríguez, A.; Lara, C. Reproductive biology and nectar production of the Mexican endemic P. sittacanthus auriculatus (Loranthaceae), a hummingbird-pollinated mistletoe. Plant Biol. 2016, 18, 73–83. [Google Scholar] [CrossRef]
- Burgess, V.J.; Kelly, D.; Robertson, A.W.; Ladley, J.J. Positive effects of forest edges on plant reproduction: Literature review and a case study of bee visitation to flowers of Peraxilla tetrapetala (Loranthaceae). N. Z. J. Ecol. 2006, 30, 179–190. [Google Scholar]
- Kelly, D.; Ladley, J.J.; Robertson, A.W.; Crowfoot, L. Flower predation by Zelleria maculata (Lepidoptera) on Peraxilla mistletoes: Effects of latitude and fragmentation, and impact on fruit set. N. Z. J. Ecol. 2008, 32, 186–196. [Google Scholar]
- MacRaild, L.M.; Radford, J.Q.; Bennett, A.F. Non-linear effects of landscape properties on mistletoe parasitism in fragmented agricultural landscapes. Landsc. Ecol. 2010, 25, 395–406. [Google Scholar] [CrossRef]
- Bowen, M.E.; McAlpine, C.A.; House, A.P.; Smith, G.C. Agricultural landscape modification increases the abundance of an important food resource: Mistletoes, birds and brigalow. Biol. Conserv. 2009, 142, 122–133. [Google Scholar] [CrossRef]
- Reid, N.; Stafford Smith, M.; Yan, Z. Ecology and population biology of mistletoes. In Forest Canopies; Lowman, M.D., Nadkarn, N.M., Eds.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 285–310. [Google Scholar]
- Norton, D.A.; Ladley, J.J. Establishment and early growth of Alepis flavida in relation to Nothofagus solandri branch size. N. Z. J. Bot. 1998, 36, 213–217. [Google Scholar] [CrossRef]
- Aukema, J.E.; Del Rio, C.M. Variation in mistletoe seed deposition: Effects of intra-and interspecific host characteristics. Ecography 2002, 25, 139–144. [Google Scholar] [CrossRef]
- Baena-Díaz, F.; Ramírez-Barahona, S.; Ornelas, J.F. Hybridization and differential introgression associated with environmental shifts in a mistletoe species complex. Sci. Rep. 2018, 8, 5591. [Google Scholar] [CrossRef] [PubMed]
- Azpeitia, F.; Lara, C. Reproductive biology and pollination of the parasitic plant Psittacanthus calyculatus (Loranthaceae) in central México1. J. Torrey Bot. Soc. 2006, 133, 429–438. [Google Scholar] [CrossRef]
- Ramírez, M.M.; Ornelas, J.F. Polinización y producción de néctar de Psittacanthus schiedeanus (Loranthaceae) en el centro de Veracruz, México. Boletín De La Soc. Botánica De México 2010, 87, 61–67. [Google Scholar]
- Guerra, T.; Galetto, L.; Silva, W. Nectar secretion dynamic links pollinator behavior to consequences for plant reproductive success in the ornithophilous mistletoe Psittacanthus robustus. Plant Biol. 2014, 16, 956–966. [Google Scholar] [CrossRef]
- Belchior, M.M.; Camarota, F.; Antiqueira, P.A.P.; Neves, F.S. A neotropical mistletoe influences herbivory of its host plant by driving changes in the associated insect community. Sci. Nat. 2022, 109, 27. [Google Scholar] [CrossRef]
- Ollerton, J.; Stott, A.; Allnutt, E.; Shove, S.; Taylor, C.; Lamborn, E. Pollination niche overlap between a parasitic plant and its host. Oecologia 2007, 151, 473–485. [Google Scholar] [CrossRef]
- Kuijt, J. Monograph of Psittacanthus (Loranthaceae). Syst. Bot. Monogr. 2009, 86, 1–362. [Google Scholar]
- Amico, G.C.; Vidal-Russell, R.; Garcia, M.A.; Nickrent, D.L. Evolutionary history of the South American mistletoe Tripodanthus (Loranthaceae) using nuclear and plastid markers. Syst. Bot. 2012, 37, 218–225. [Google Scholar] [CrossRef]
- Ornelas, J.F.; Gándara, E.; Vásquez-Aguilar, A.A.; Ramírez-Barahona, S.; Ortiz-Rodriguez, A.E.; González, C.; Saules, M.T.M.; Ruiz-Sanchez, E. A mistletoe tale: Postglacial invasion of Psittacanthus schiedeanus (Loranthaceae) to Mesoamerican cloud forests revealed by molecular data and species distribution modeling. BMC Evol. Biol. 2016, 16, 78. [Google Scholar] [CrossRef] [Green Version]
- Reid, N. Dispersal of misteltoes by honeyeaters and flowerpeckers: Components of seed dispersal quality. Ecology 1989, 70, 137–145. [Google Scholar] [CrossRef]
- Godschalk, S. Feeding behaviour of avian dispersers of mistletoe fruit in the Loskop Dam Nature Reserve, South Africa. Afr. Zool. 1985, 20, 136–146. [Google Scholar] [CrossRef]
- Van Ommeren, R.J.; Whitham, T.G. Changes in interactions between juniper and mistletoe mediated by shared avian frugivores: Parasitism to potential mutualism. Oecologia 2002, 130, 281–288. [Google Scholar] [CrossRef] [PubMed]
- García, D.; Rodríguez-Cabal, M.A.; Amico, G.C. Seed dispersal by a frugivorous marsupial shapes the spatial scale of a mistletoe population. J. Ecol. 2009, 97, 217–229. [Google Scholar] [CrossRef]
- Rodriguez-Cabal, M.A.; Branch, L.C. Influence of habitat factors on the distribution and abundance of a marsupial seed disperser. J. Mammal. 2011, 92, 1245–1252. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.M.; Rivarola, M.D.; Amico, G.; Carlo, T.A. Neighborhood effects on seed dispersal by frugivores: Testing theory with a mistletoe–marsupial system in Patagonia. Ecology 2012, 93, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Arriaga, L.; Franco, M.; Sarukhan, J. Identification of natural groups of trees in uneven-aged forests using multivariate methods. J. Ecol. 1988, 76, 1092–1100. [Google Scholar] [CrossRef]
- Donohue, K. The spatial demography of mistletoe parasitism on a Yemeni Acacia. Int. J. Plant Sci. 1995, 156, 816–823. [Google Scholar] [CrossRef]
- Gairola, S.; Bhatt, A.; Govender, Y.; Baijnath, H.; Procheş, Ş.; Ramdhani, S. Incidence and intensity of tree infestation by the mistletoe Erianthemum dregei (Eckl. & Zeyh.) V. Tieghem in Durban, South Africa. Urban For. Urban Green. 2013, 12, 315–322. [Google Scholar] [CrossRef]
- Teixeira-Costa, L.; Coelho, F.M.; Ceccantini, G.C.T. Comparative phenology of mistletoes shows effect of different host species and temporal niche partitioning. Botany 2017, 95, 271–282. [Google Scholar] [CrossRef]
- Yule, K.M.; Bronstein, J.L. Reproductive ecology of a parasitic plant differs by host species: Vector interactions and the maintenance of host races. Oecologia 2018, 186, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuadra-Valdés, J.; Vizentin-Bugoni, J.; Fontúrbel, F.E. An exotic magnet plant alters pollinator abundance and behavior: A field test with a native mistletoe. Biol. Invasions 2021, 23, 2515–2525. [Google Scholar] [CrossRef]
- Watson, D.M. Parasitic plants as facilitators: More Dryad than Dracula? J. Ecol. 2009, 97, 1151–1159. [Google Scholar] [CrossRef]
- Watson, D.M. Disproportionate declines in ground-foraging insectivorous birds after mistletoe removal. PLoS ONE 2015, 10, e0142992. [Google Scholar] [CrossRef]
- Hódar, J.A.; Lázaro-González, A.; Zamora, R. Beneath the mistletoe: Parasitized trees host a more diverse herbaceous vegetation and are more visited by rabbits. Ann. For. Sci. 2018, 75, 77. [Google Scholar] [CrossRef] [Green Version]
- Mellado, A.; Hobby, A.; Lázaro-González, A.; Watson, D.M. Hemiparasites drive heterogeneity in litter arthropods: Implications for woodland insectivorous birds. Austral Ecol. 2019, 44, 777–785. [Google Scholar] [CrossRef]
- Lázaro-González, A.; Hódar, J.A.; Zamora, R. Mistletoe generates non-trophic and trait-mediated indirect interactions through a shared host of herbivore consumers. Ecosphere 2019, 10, e02564. [Google Scholar] [CrossRef]
- Ferrenberg, S. Dwarf Mistletoe Infection Interacts with Tree Growth Rate to Produce Opposing Direct and Indirect Effects on Resin Duct Defenses in Lodgepole Pine. Forests 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Zamora, R.; Mellado, A. Identifying the abiotic and biotic drivers behind the elevational distribution shift of a parasitic plant. Plant Biol. 2019, 21, 307–317. [Google Scholar] [CrossRef]
- Lawson, D.A.; Rands, S.A. The effects of rainfall on plant–pollinator interactions. Arthropod-Plant Interact. 2019, 13, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Cubero, J.; Hernández, L. Breeding faba bean (Vicia faba L.) for resistance to Orobanche crenata Forsk. Options Méditerranéennes 1991, 10, 51–57. [Google Scholar]
- Ringnes, D.; Stover, P.; Scharpf, R. Dwarf mistletoe resistance in ponderosa pine: Selection and testing protocols. In Proceedings of the Congresos y Jornadas-Junta de Andalucia (Espana), Cordoba, Spain, 16–18 April 1996; pp. 690–696. [Google Scholar]
- Haussmann, B.; Hess, D.; Reddy, B.; Mukuru, S.; Seetharama, N.; Kayentao, M.; Welz, H.; Geiger, H. QTL for Striga resistance in sorghum populations derived from IS 9830 and N 13. In Proceedings of the Breeding for Striga Resistance in Cereals: Proceedings of a Workshop, IITA, Ibadan, Nigeria, 18–20 August 1999; pp. 18–20. [Google Scholar]
- Mohamed, A.; Rich, P.; Housley, T.; Ejeta, G. In vitro techniques for studying mechanisms of Striga resistance in sorghum. In Proceedings of the 7th International Parasitic Weed Symposium, Nantes, France, 3–8 June 2001; pp. 96–100. [Google Scholar]
- Omanya, G.O.; Haussmann, B.I.G.; Hess, D.E.; Reddy, B.V.S.; Kayentao, M.; Welz, H.G.; Geiger, H.H. Utility of indirect and direct selection traits for improving Striga resistance in two sorghum recombinant inbred populations. Field Crops Res. 2004, 89, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Liu, B.; Guo, Q.; Song, L.; Chen, L.; Wang, C. Construction of a haustorium development associated SSH library in Thesium chinense and analysis of specific ESTs included by Imperata cylindrica. Biochem. Syst. Ecol. 2016, 64, 46–52. [Google Scholar] [CrossRef]
- Ranjan, A.; Ichihashi, Y.; Farhi, M.; Zumstein, K.; Townsley, B.; David-Schwartz, R.; Sinha, N.R. De novo assembly and characterization of the transcriptome of the parasitic weed dodder identifies genes associated with plant parasitism. Plant Physiol. 2014, 166, 1186–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichihashi, Y.; Kusano, M.; Kobayashi, M.; Suetsugu, K.; Yoshida, S.; Wakatake, T.; Kumaishi, K.; Shibata, A.; Saito, K.; Shirasu, K.J.P.; et al. Transcriptomic and metabolomic reprogramming from roots to haustoria in the parasitic plant, Thesium chinense. Plant Cell Physiol. 2018, 59, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Zhou, W.; Li, T.; Tian, C.J.B.g. De novo assembly and transcriptome characterization of spruce dwarf mistletoe Arceuthobium sichuanense uncovers gene expression profiling associated with plant development. BMC Genom. 2016, 17, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Abdul Wahid, H. Identification of Dwarf Mistletoe Resistant Genes in Ziarat Junipers (Juniperus excelsa M. Bieb). Ph.D. Thesis, University of Baluchistan, Quetta, Pakistan, 2019. [Google Scholar]
- Abdul Wahid, H.; Barozai, M.Y.K.; Din, M. Identification and characterization of dwarf mistletoe responding genes in Ziarat juniper tree (Juniperus excelsa M. Bieb) through suppression subtractive hybridization and deep sequencing. Trees 2019, 33, 1027–1039. [Google Scholar] [CrossRef]
- Kuang, J.; Wang, Y.; Mao, K.; Milne, R.; Wang, M.; Miao, N. Transcriptome Profiling of a Common Mistletoe Species Parasitizing Four Typical Host Species in Urban Southwest China. Genes 2022, 13, 1173. [Google Scholar] [CrossRef]
- Dan, M.W.; Filiz, G. Plant Genome Editing and its Applications in Cereals. In Genetic Engineering; Farrukh, J., Ed.; IntechOpen: Rijeka, Croatia, 2016; p. 4. [Google Scholar]
- Mandal, K.; Boro, P.; Chattopadhyay, S. Micro-RNA based gene regulation: A potential way for crop improvements. Plant Gene 2021, 27, 100312. [Google Scholar] [CrossRef]
- Yu, H.; Li, J. Breeding future crops to feed the world through de novo domestication. Nat. Commun. 2022, 13, 1171. [Google Scholar] [CrossRef] [PubMed]
- Van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic modification to improve disease resistance in crops. New Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaidi, S.S.-e.-A.; Mahas, A.; Vanderschuren, H.; Mahfouz, M.M. Engineering crops of the future: CRISPR approaches to develop climate-resilient and disease-resistant plants. Genome Biol. 2020, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.R.; Timko, M.P.; Yoder, J.I.; Axtell, M.J.; Westwood, J.H. Molecular Dialog Between Parasitic Plants and Their Hosts. Annu. Rev. Phytopathol. 2019, 57, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; de Pamphilis, C.W.; Das, M.; Fernández-Aparicio, M.; Honaas, L.A.; Timko, M.P.; Wafula, E.K.; Wickett, N.J.; Yoder, J.I. The Parasitic Plant Genome Project: New Tools for Understanding the Biology of Orobanche and Striga. Weed Sci. 2017, 60, 295–306. [Google Scholar] [CrossRef]
- Neale, D.B.; Kremer, A. Forest tree genomics: Growing resources and applications. Nat. Rev. Genet. 2011, 12, 111. [Google Scholar] [CrossRef]
- Muleo, R.; Morgante, M.; Velasco, R.; Cavallini, A.; Perrotta, G.; Baldoni, L. Olive tree genomic. In Olive Germplasm–The Olive Cultivation, Table Olive and Olive Oil Industry in Italy, 1st ed.; Muzzalupo, I., Ed.; Intechopen: Rijeka, Croatia, 2012; pp. 133–148. [Google Scholar]
- Diningrat, D.; Widiyanto, S.; Pancoro, A.; Shim, D.; Panchangam, B.; Zembower, N.; Carlson, J.E. Transcriptome of teak (Tectona grandis, Lf) in vegetative to generative stages development. J. Plant Sci. 2015, 10, 1. [Google Scholar] [CrossRef]
- Schnell, R.J.; Priyadarshan, P. Genomics of Tree Crops; Springer Science & Business Media; Springer: New York, NY, USA, 2012. [Google Scholar]
- Gmitter, F.G.; Chen, C.; Machado, M.A.; De Souza, A.A.; Ollitrault, P.; Froehlicher, Y.; Shimizu, T. Citrus genomics. Tree Genet. Genomes 2012, 8, 611–626. [Google Scholar] [CrossRef] [Green Version]
- Arias, R.S.; Borrone, J.W.; Tondo, C.L.; Kuhn, D.N.; Irish, B.M.; Schnell, R.J. Genomics of tropical fruit tree crops. In Genomics of Tree Crops; Springer: Berlin/Heidelberg, Germany, 2012; pp. 209–239. [Google Scholar]
- Marler, M.; Pedersen, D.; Mitchell-Olds, T.; Callaway, R. A polymerase chain reaction method for detecting dwarf mistletoe infection in Douglas-fir and western larch. Can. J. For. Res. 1999, 29, 1317–1321. [Google Scholar] [CrossRef]
- Petri, C.; Burgos, L. Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Res. 2005, 14, 15–26. [Google Scholar] [CrossRef]
- Atsatt, P.R. On the evolution of leaf resemblance between mistletoes and their hosts. In Proceedings of the 2nd Symposium on Parasitic Weeds, Raleigh, NC, USA, 16–19 July 1979. [Google Scholar]
- Lev-Yadun, S. Does chemical aposematic (warning) signaling occur between host plants and their potential parasitic plants? Plant Signal. Behav. 2013, 8, e24907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, S.; Cui, S.; Ichihashi, Y.; Shirasu, K. The Haustorium, a Specialized Invasive Organ in Parasitic Plants. Annu. Rev. Plant Biol. 2016, 67, 643–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.; Lynn, D.G. The haustorium and the chemistry of host recognition in parasitic angiosperms. J. Chem. Ecol. 1986, 12, 561–579. [Google Scholar] [CrossRef]
- Sallé, G.L. Germination and Establishment of Viscum album. In The Biology of Mistletoes; Calder, D., Bernhardt, P., Eds.; Academic Press: London, UK, 1983. [Google Scholar]
- Bandaranayake, P.C.G.; Yoder, J.I. Haustorium Initiation and Early Development. In Parasitic Orobanchaceae: Parasitic Mechanisms and Control Strategies; Joel, D.M., Gressel, J., Musselman, L.J., Eds.; Springer: Berlin Heidelberg, Germany, 2013; pp. 61–74. [Google Scholar]
- Saucet, S.B.; Shirasu, K. Molecular Parasitic Plant-Host Interactions. PLoS Pathog. 2016, 12, e1005978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokla, A.; Melnyk, C.W. Developing a thief: Haustoria formation in parasitic plants. Dev. Biol. 2018, 442, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Keyes, W.J.; Palmer, A.G.; Erbil, W.K.; Taylor, J.V.; Apkarian, R.P.; Weeks, E.R.; Lynn, D.G. Semagenesis and the parasitic angiosperm Striga asiatica. Plant J. Cell Mol. Biol. 2007, 51, 707–716. [Google Scholar] [CrossRef]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Runyon, J.B.; Mescher, M.C.; De Moraes, C.M. Plant defenses against parasitic plants show similarities to those induced by herbivores and pathogens. Plant Signal. Behavior. 2010, 5, 929–931. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Reyes, P.; Pérez-López, G.; Maldonado-López, Y.; González-Rodríguez, A. Effects of herbivory and mistletoe infection by Psittacanthus calyculatus on nutritional quality and chemical defense of Quercus deserticola along Mexican forest fragments. Plant Ecol. 2017, 218, 687–697. [Google Scholar] [CrossRef]
- Hu, B.; Sakakibara, H.; Takebayashi, Y.; Peters, F.S.; Schumacher, J.; Eiblmeier, M.; Arab, L.; Kreuzwieser, J.; Polle, A.; Rennenberg, H. Mistletoe infestation mediates alteration of the phytohormone profile and anti-oxidative metabolism in bark and wood of its host Pinus sylvestris. Tree Physiol. 2017, 37, 676–691. [Google Scholar] [CrossRef] [Green Version]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallé, G.L.; Armillotta, A.; Frochot, H. Mechanisms of resistance of four cultivars of poplar against Viscum album L. In Proceedings of the 3rd International Symposium of Parasitic Weeds, Aleppo, Syria, 7–10 May 1984; Parker, C., Musselman, L.J., Polhill, R.M., Wilson, A.K., Eds.; [Google Scholar]
- Bhat, K.A.; Akhtar, S.; Dar, N.A.; Bhat, M.I.; Bhat, F.A.; Rizwan, R.; Horielov, O.; Krasylenko, Y. Mistletoe Eradicator—A Novel Tool for Simultaneous Mechanical and Chemical Control of Mistletoe. J. Vis. Exp. 2022, 181, e63455. [Google Scholar] [CrossRef]
- Schrader-Patton, C.; Grulke, N.; Bienz, C. Assessment of Ponderosa Pine Vigor Using Four-Band Aerial Imagery in South Central Oregon: Crown Objects to Landscapes. Forests 2021, 12, 612. [Google Scholar] [CrossRef]
- Barbedo, J. A Review on the Use of Unmanned Aerial Vehicles and Imaging Sensors for Monitoring and Assessing Plant Stresses. Drones 2019, 3, 40. [Google Scholar] [CrossRef] [Green Version]
- Duarte, A.; Borralho, N.; Cabral, P.; Caetano, M. Recent Advances in Forest Insect Pests and Diseases Monitoring Using UAV-Based Data: A Systematic Review. Forests 2022, 13, 911. [Google Scholar] [CrossRef]
- Thapa, S. Detection and Mapping of Incidence of Viscum album in Pinus sylvestris Forest of Southern French Alpe Using Satellite and Airborne Optical Imagery. Master’s Thesis, University of Twente, Enskode, The Netherlands, 2013. [Google Scholar]
- Barbosa, J.; Sebastián-González, E.; Asner, G.; Knapp, D.; Anderson, C.; Martin, R.; Dirzo, R. Hemiparasite-host plant interactions in a fragmented landscape assessed via imaging spectroscopy and LiDAR. Ecol. Appl. 2016, 26, 55–66. [Google Scholar] [CrossRef]
- Lawley, V.; Lewis, M.; Clarke, K.; Ostendorf, B. Site-based and remote sensing methods for monitoring indicators of vegetation condition: An Australian review. Ecol. Indic. 2016, 60, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Windmuller-Campione, M.A.; Moser, R.L. Remote and Seasonal Field Detection of Eastern Spruce Dwarf Mistletoe in Northern Minnesota; Minnesota Forestry Research Notes; Department of Forest Resources, University of Minnesota: St. Paul, MN, USA, 2022; Volume 315. [Google Scholar]
- Mejia-Zuluaga, P.A.; Dozal, L.; Valdiviezo-N., J.C. Genetic Programming Approach for the Detection of Mistletoe Based on UAV Multispectral Imagery in the Conservation Area of Mexico City. Remote Sens. 2022, 14, 801. [Google Scholar] [CrossRef]
- Pernar, R.; Bajic, M.; Ancic, M.; Seletkovic, A.; Idzojtic, M. Detection of mistletoe in digital colour infrared images of infested fir trees. Period. Biol. 2007, 109, 67–75. [Google Scholar]
- Sabrina, F.; Sohail, S.; Thakur, S.; Azad, S.; Wasimi, S. Use of deep learning approach on UAV imagery to detect mistletoe infestation. In Proceedings of the 2020 IEEE Region 10 Symposium (TENSYMP), Dhaka, Bangladesh, 5–7 June 2020; pp. 556–559. [Google Scholar]
- Ančić, M.; Pernar, R.; Bajić, M.; Seletković, A.; Kolić, J. Detecting mistletoe infestation on Silver fir using hyperspectral images. Iforest-Biogeosci. For. 2014, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Maes, W.H.; Huete, A.R.; Avino, M.; Boer, M.M.; Dehaan, R.; Pendall, E.; Griebel, A.; Steppe, K. Can UAV-based infrared thermography be used to study plant-parasite interactions between mistletoe and eucalypt trees? Remote Sens. 2018, 10, 2062. [Google Scholar] [CrossRef] [Green Version]
- Xiong, D.; Huang, H.; Wang, Z.; Li, Z.; Tian, C. Assessment of dwarf mistletoe (Arceuthobium sichuanense) infection in spruce trees by using hyperspectral data. For. Pathol. 2021, 51, e12669. [Google Scholar] [CrossRef]
- Miraki, M.; Sohrabi, H.; Fatehi, P.; Kneubuehler, M. Detection of mistletoe infected trees using UAV high spatial resolution images. J. Plant Dis. Prot. 2021, 128, 1679–1689. [Google Scholar] [CrossRef]
- León-Bañuelos, L.A.; Endara-Agramont, A.R.; Gómez-Demetrio, W.; Martínez-García, C.G.; Gabino Nava-Bernal, E. Identification of Arceuthobium globosum using unmanned aerial vehicle images in a high mountain forest of central Mexico. J. For. Res. 2020, 31, 1759–1771. [Google Scholar] [CrossRef]
- Hamamouch, N.; Westwood, J.H.; Banner, I.; Cramer, C.L.; Gepstein, S.; Aly, R. A peptide from insects protects transgenic tobacco from a parasitic weed. Transgenic Res. 2005, 14, 227–236. [Google Scholar] [CrossRef]
- Aly, R.; Dina, P.; Guy, A. Expression of sarcotoxin IA gene via a root-specific tob promoter enhanced host resistance against parasitic weeds in tomato plants. Plant Cell Rep. 2006, 25, 297–303. [Google Scholar] [CrossRef]
- Aly, R.; Cholakh, H.; Joel, D.M.; Leibman, D.; Steinitz, B.; Zelcer, A.; Naglis, A.; Yarden, O.; Gal-On, A. Gene silencing of mannose 6-phosphate reductase in the parasitic weed Orobanche aegyptiaca through the production of homologous dsRNA sequences in the host plant. Plant Biotechnol. J. 2009, 7, 487–498. [Google Scholar] [CrossRef]
- Alakonya, A.; Kumar, R.; Koenig, D.; Kimura, S.; Townsley, B.; Runo, S.; Garces, H.M.; Kang, J.; Yanez, A.; David-Schwartz, R.; et al. Interspecific RNA interference of SHOOT MERISTEMLESS-like disrupts Cuscuta pentagona plant parasitism. Plant Cell 2012, 24, 3153–3166. [Google Scholar] [CrossRef] [Green Version]
- Stanivuković, Z.; Milenković, M.; Karadžić, D.; Mihajlović, L. The mistletoe (Viscum album L.)-a problem in fir (Abies alba Mill.) forests in Serbia and the Republic of Srpska [Bosnia and Herzegovina]. In Proceedings of the Serbian Forestry Congress, Belgrade, Serbia, 11–13 November 2010. [Google Scholar]
- Idžojtić, M.; Glavaš, M.; Zebec, M.; Pernar, R.; Kušan, Ž.; List, Đ.; Grahovac-Tremski, M. Intensity of Infection with Yellow Mistletoe and White-berried Mistletoe on the Area of the Forest Administrations Zagreb and Koprivnica. Šumarski List 2008, 132, 107–114. [Google Scholar]
- Struwe, I.; Gertsson, C.; Coulianos, C. Insects monophagous on mistletoe (Viscum album L.) newly discovered in Sweden: Cacopsylla visci (Curtis, 1835)(Hemiptera, Psyllidae) and Pinalitus viscicola (Puton, 1888) (Hemiptera, Miridae). Entomol. Tidskr. 2009, 130, 155–160. [Google Scholar]
- Parker, A. Growth of Wallrothiella arceuthobii on artificial media. Can. J. Bot. 1970, 48, 837–838. [Google Scholar] [CrossRef]
- Ramsfield, T.; Shamoun, S.; van der Kamp, B. Infection of Arceuthobium americanum by Colletotrichum gloeosporioides and its potential for inundative biological control. For. Pathol. 2005, 35, 332–338. [Google Scholar] [CrossRef]
- Askew, S.E.; Shamoun, S.F.; Van Der Kamp, B.J. An in vitro method for screening Colletotrichum gloeosporioides as a biological control agent for western hemlock dwarf mistletoe. For. Pathol. 2009, 39, 279–288. [Google Scholar] [CrossRef]
- Askew, S.E.; Shamoun, S.F.; van der Kamp, B.J. Assessment of Colletotrichum gloeosporioides as a biological control agent for management of hemlock dwarf mistletoe (Arceuthobium tsugense). For. Pathol. 2011, 41, 444–452. [Google Scholar] [CrossRef]
- Parmeter, J.; Hood, J.; Scharpf, R. Colletotrichum blight of dwarf mistletoe. Phytopathology 1959, 49, 812–815. [Google Scholar]
- Shamoun, S. Development of biological control strategy for management of dwarf mistletoes. In Proceedings of the 45th Western International Forest Disease Work Conference, Prince George, Victoria, BC, Canada, 15–19 September 1997; Sturrock, R., Ed.; Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 1997; pp. 36–42. [Google Scholar]
- Stojanović, S. The investigation of Sphaeropsis visci (Salm.) Sacc. and Colletotrichum gloeosporoides (Sacc.) Penz., parasite on European mistletoe (Viscum album ssp. typicum Beck). Zaštita Bilja 1989, 40, 493–503. [Google Scholar]
- Deeks, S.J.; Shamoun, S.F.; Punja, Z.K. Histopathology of callus and germinating seeds of Arceuthobium tsugense subsp. tsugense infected by Cylindrocarpon cylindroides and Colletotrichum gloeosporioides. Int. J. Plant Sci. 2002, 163, 765–773. [Google Scholar] [CrossRef]
- Hawksworth, F.G. Biological Control of the Mistletoes. In Proceedings of the Fifteenth Congress of the International Union of Forestry Research Organizations, Gainesville, FL, USA, 14–20 March 1971; Biological Control of Forest Diseases. Nordin, V.J., Ed.; Canadian Forestry Service: Ottawa, ON, Canada, 1972; pp. 83–92. [Google Scholar]
- Karadžić, D.; Lazarev, V.S.; Milenković, M. The most significant parasitic and saprophytic fungi on common mistletoe (Viscum album L) and their potential application in biocontrol. Glas. Šumarskog Fak. 2004, 89, 115–126. [Google Scholar] [CrossRef]
- Byler, J.; Cobb, F.W., Jr. The occurrence and pathogenicity of Nectria fuckeliana on dwarf mistletoe in California. Can. J. Bot. 1972, 50, 1162. [Google Scholar] [CrossRef]
- Varga, I.; Baltazár, T.; Pejchal, M. Optimisation of growing conditions of European mistletoe hyperparasitic fungus (phaeobotryosphaeria visci): Effect of different media and antibiotics/optimalizácia rastových podmienok Phaeobotryosphaeria visci, hyperparazitickej huby imela bieleho: Vplyv rôznych živných médií a antibiotík. Acta Hortic. Et Reg. 2013, 16, 44–47. [Google Scholar]
- Varga, I.; Baltazár, T.; Apró, M.; Poczai, P.; Hyvönen, J. Optimizing conditions for sporulation of European mistletoe hyperparasitic fungus (Phaeobotryosphaeria visci): Effect of light and different media. J. Agric. Sci. 2012, 50, 60–66. [Google Scholar]
- Brandenburger, W. Parasitische Pilze an Gefäßpflanzen in Europa; Gustav Fischer Verlag: Stuttgart, Germany, 1985. [Google Scholar]
- Chen, J.; Liu, X.; Jia, H.; Zhu, W. First report of leaf-spot disease caused by Sphaeropsis visci on Asian mistletoe [Viscum coloratum (Kom.) Nakai] in China. J. For. Res. 2018, 29, 1769–1774. [Google Scholar] [CrossRef]
- Haque, M.; Badshah, K. Loranthus parasitism-a challenge to the development of economic tree resource in the Rawalpindi East Region. Pak. J. For. 1984, 34, 101–109. [Google Scholar]
- Holecová, M.; Hollá, K.; Šebestová, M. Poznámky k rozšíreniu Ixapion Variegatum (Wencker, 1864) (Coleoptera, Curculionoidea, Apionidae) na území borskej nížiny (Jz Slovensko). Folia Faun. Slovaca 2016, 21, 9–12. [Google Scholar]

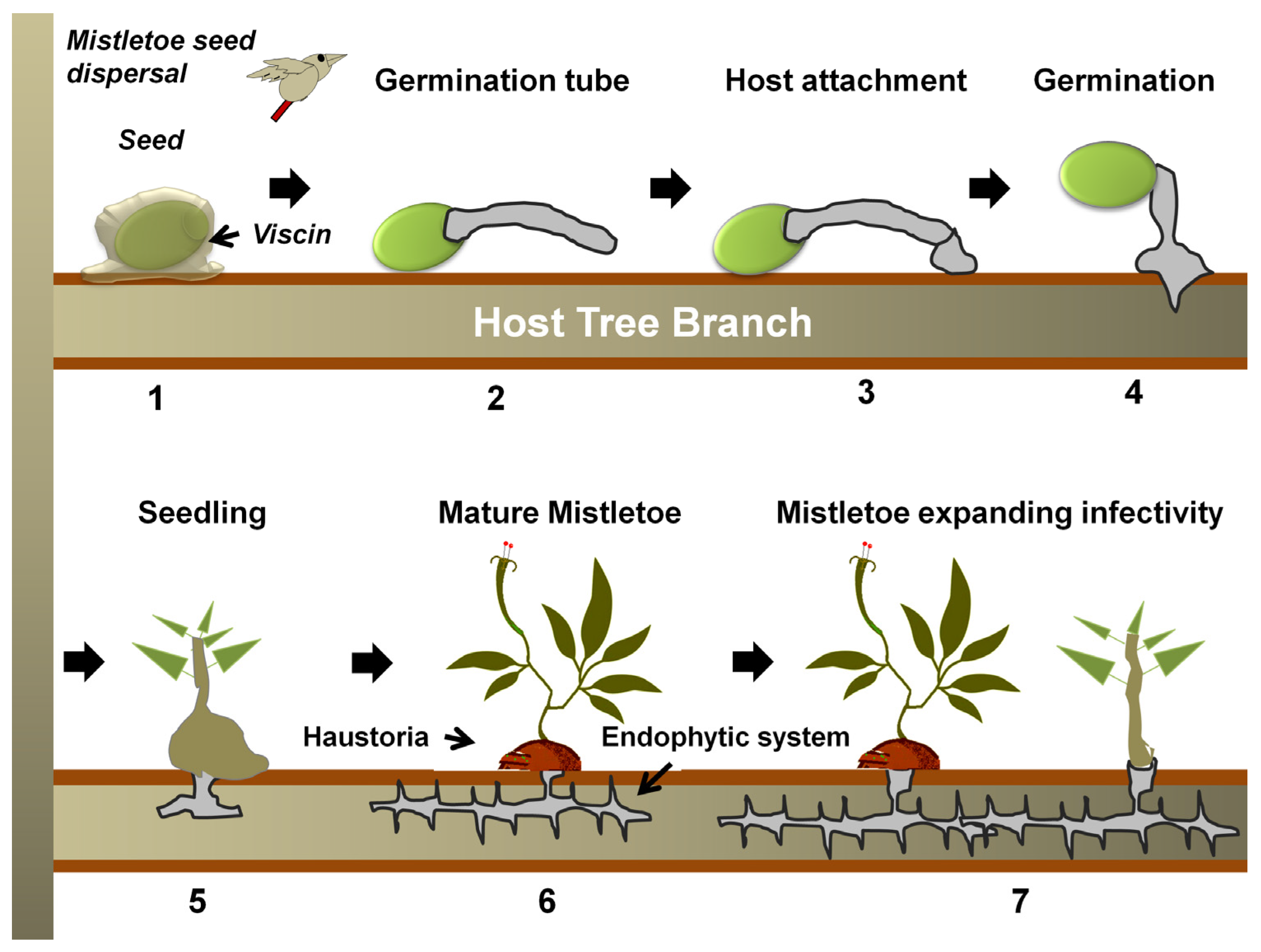

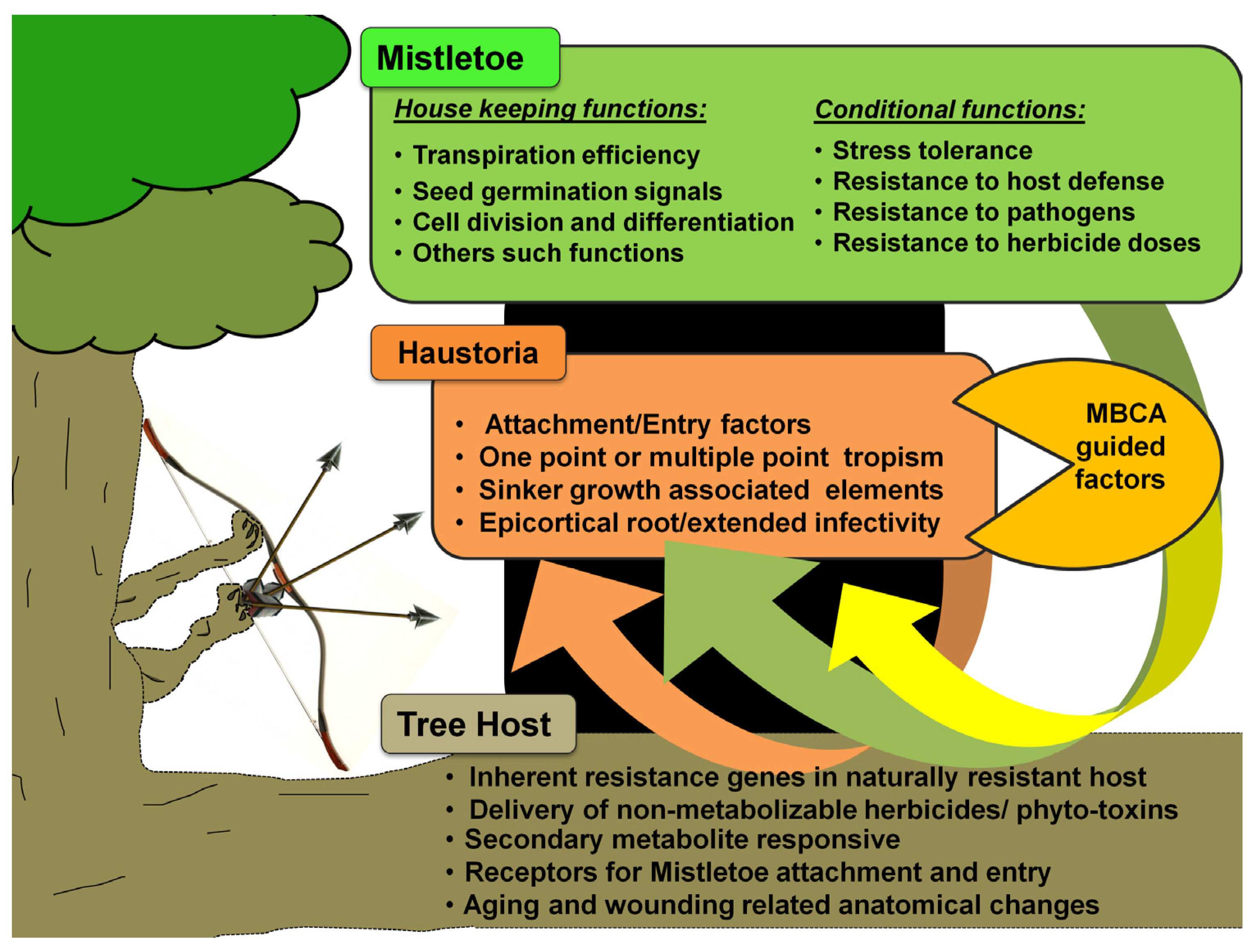

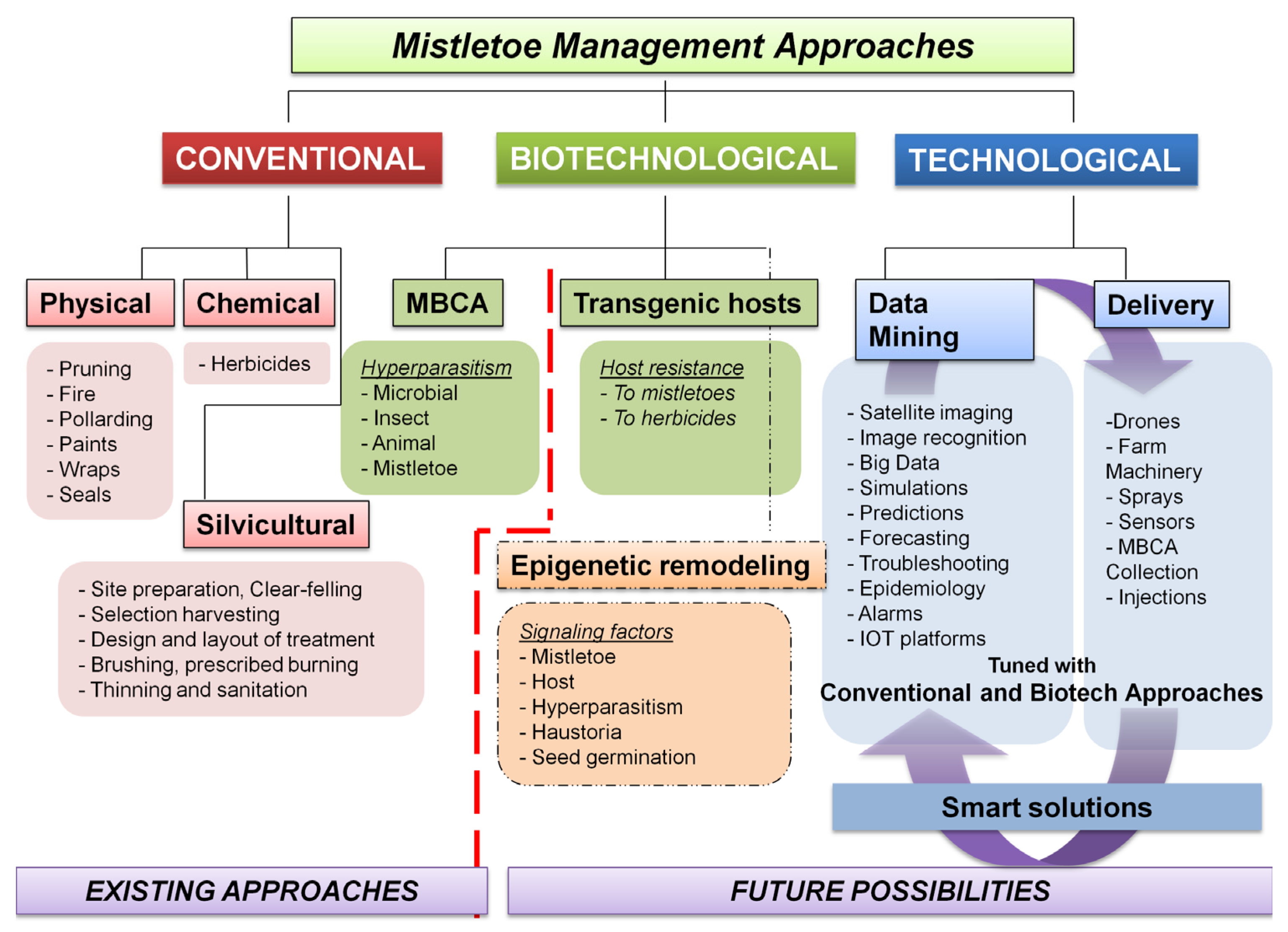
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudgal, G.; Kaur, J.; Chand, K.; Parashar, M.; Dhar, S.K.; Singh, G.B.; Gururani, M.A. Mitigating the Mistletoe Menace: Biotechnological and Smart Management Approaches. Biology 2022, 11, 1645. https://doi.org/10.3390/biology11111645
Mudgal G, Kaur J, Chand K, Parashar M, Dhar SK, Singh GB, Gururani MA. Mitigating the Mistletoe Menace: Biotechnological and Smart Management Approaches. Biology. 2022; 11(11):1645. https://doi.org/10.3390/biology11111645
Chicago/Turabian StyleMudgal, Gaurav, Jaspreet Kaur, Kartar Chand, Manisha Parashar, Sanjoy K. Dhar, Gajendra B. Singh, and Mayank A. Gururani. 2022. "Mitigating the Mistletoe Menace: Biotechnological and Smart Management Approaches" Biology 11, no. 11: 1645. https://doi.org/10.3390/biology11111645
APA StyleMudgal, G., Kaur, J., Chand, K., Parashar, M., Dhar, S. K., Singh, G. B., & Gururani, M. A. (2022). Mitigating the Mistletoe Menace: Biotechnological and Smart Management Approaches. Biology, 11(11), 1645. https://doi.org/10.3390/biology11111645






