Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
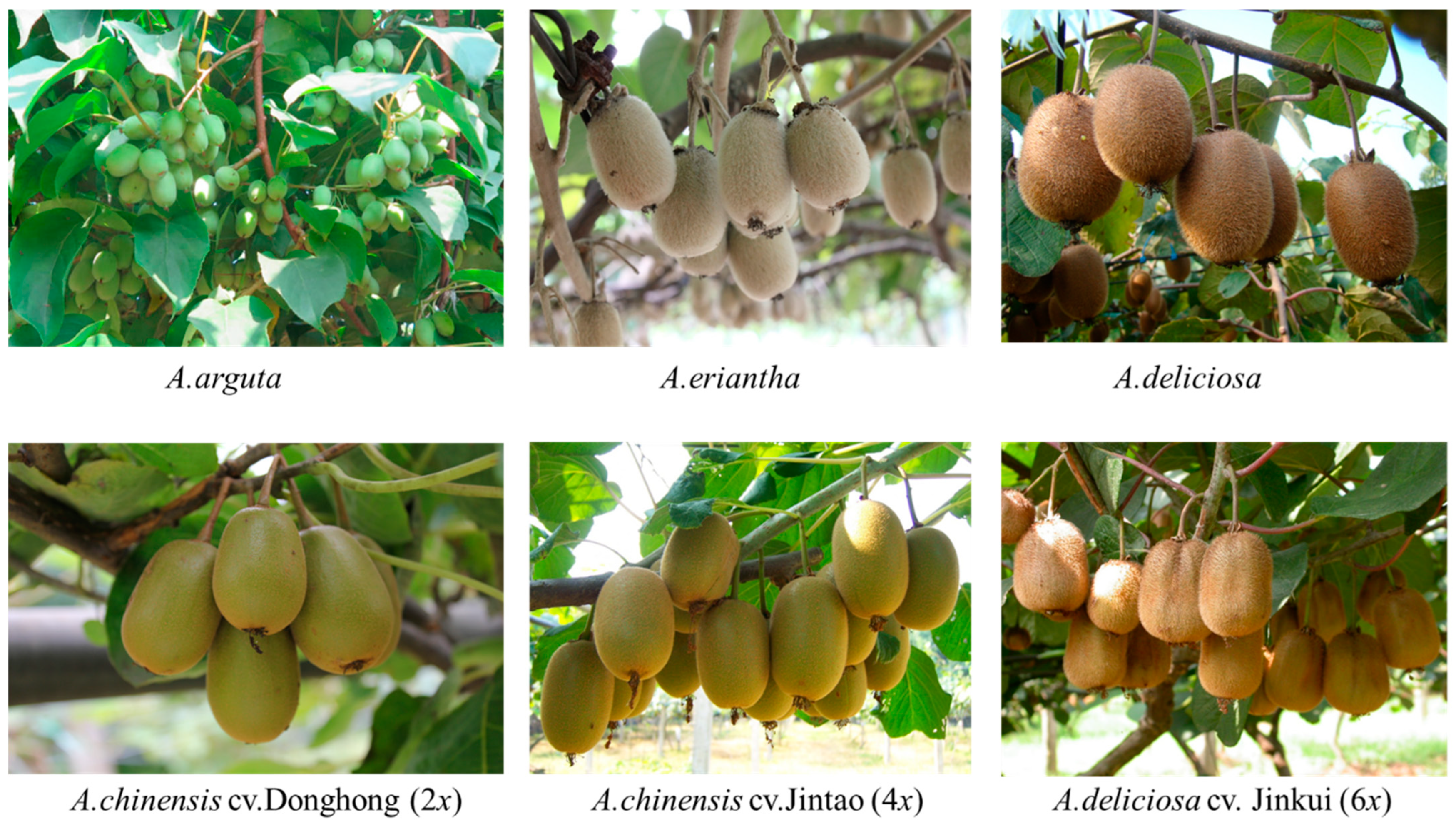
2.1. Plant Material
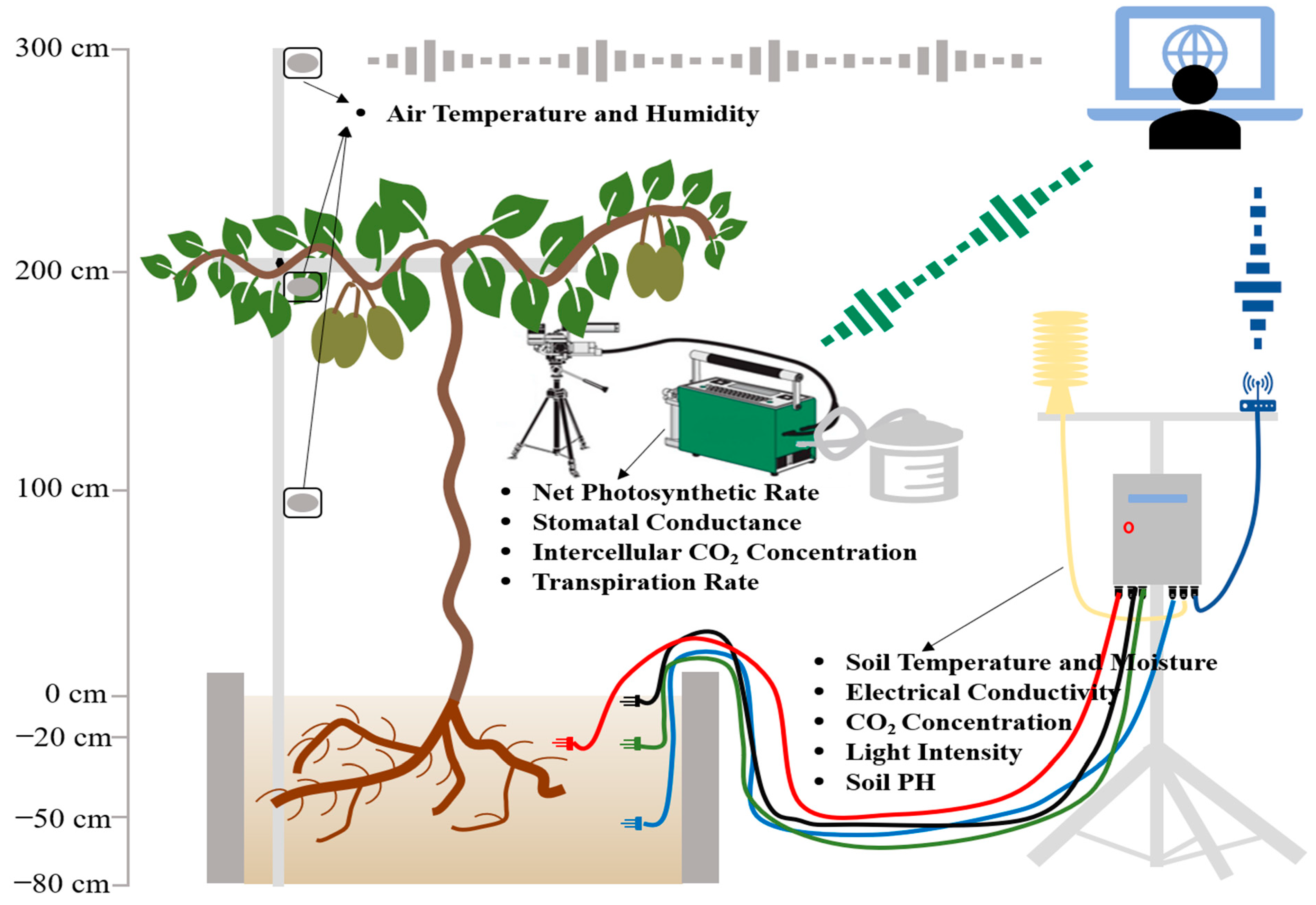
2.2. Measurement of Growth Conditions
2.3. Measurement of Photosynthesis
2.4. Statistical Analysis
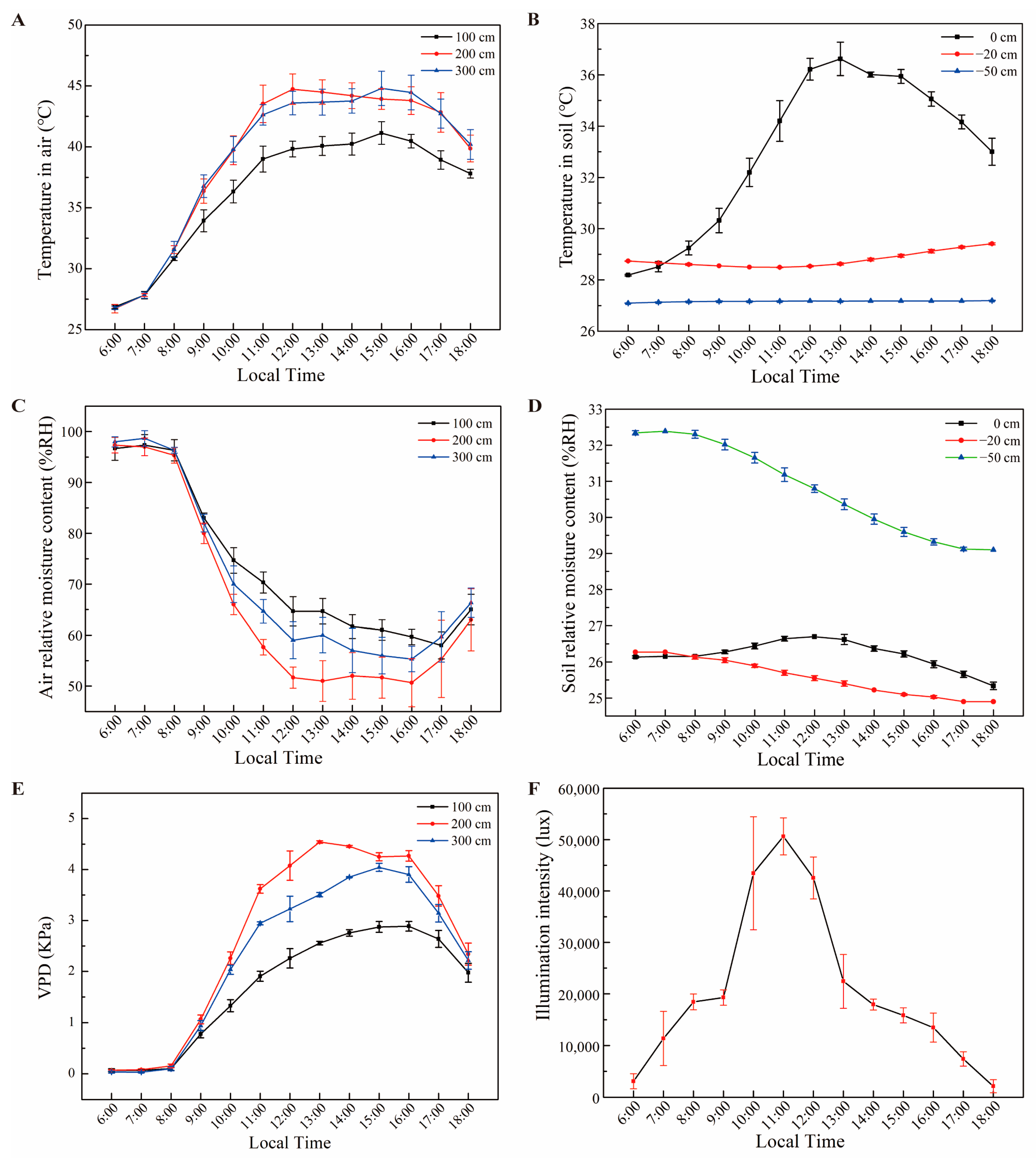
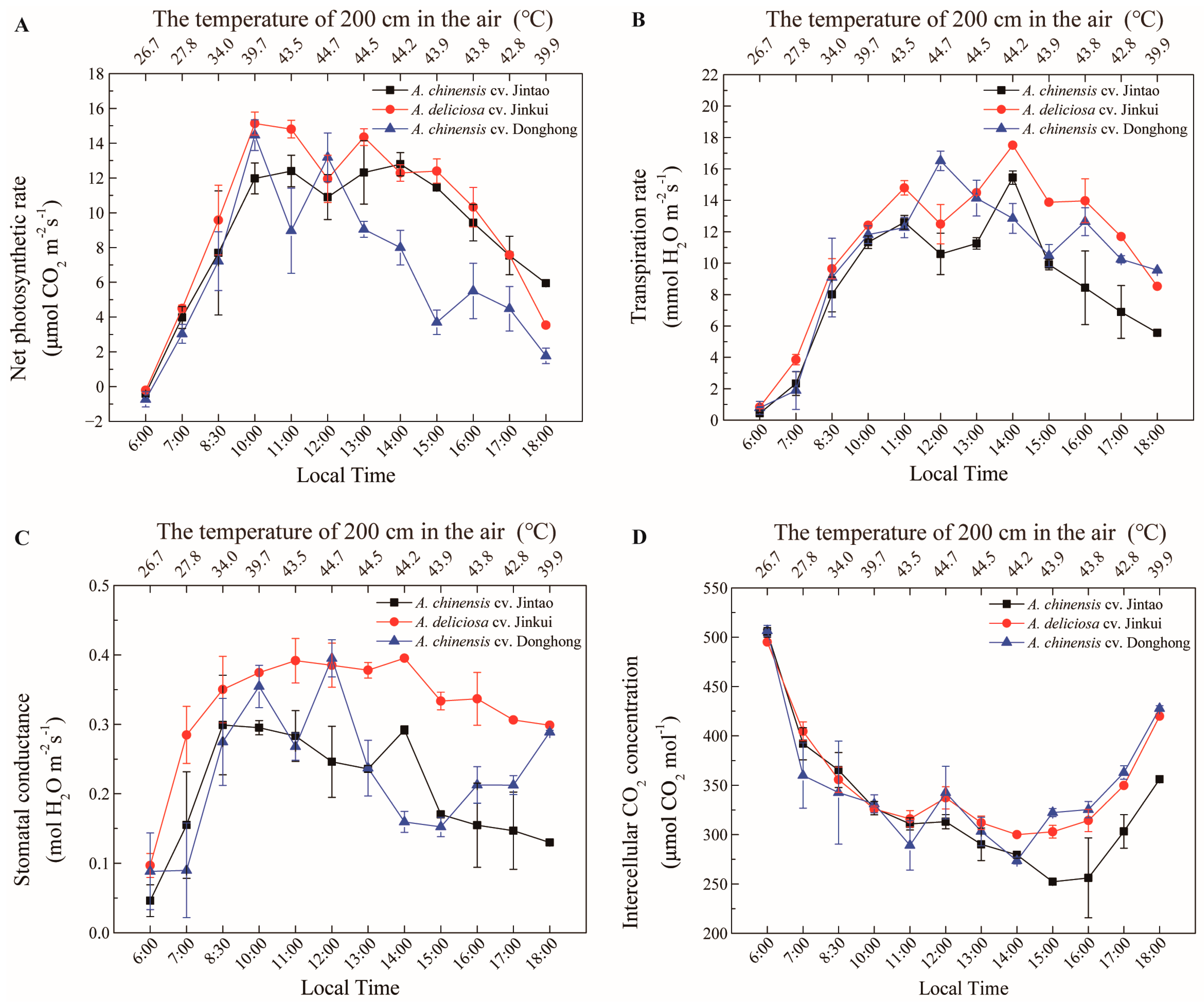
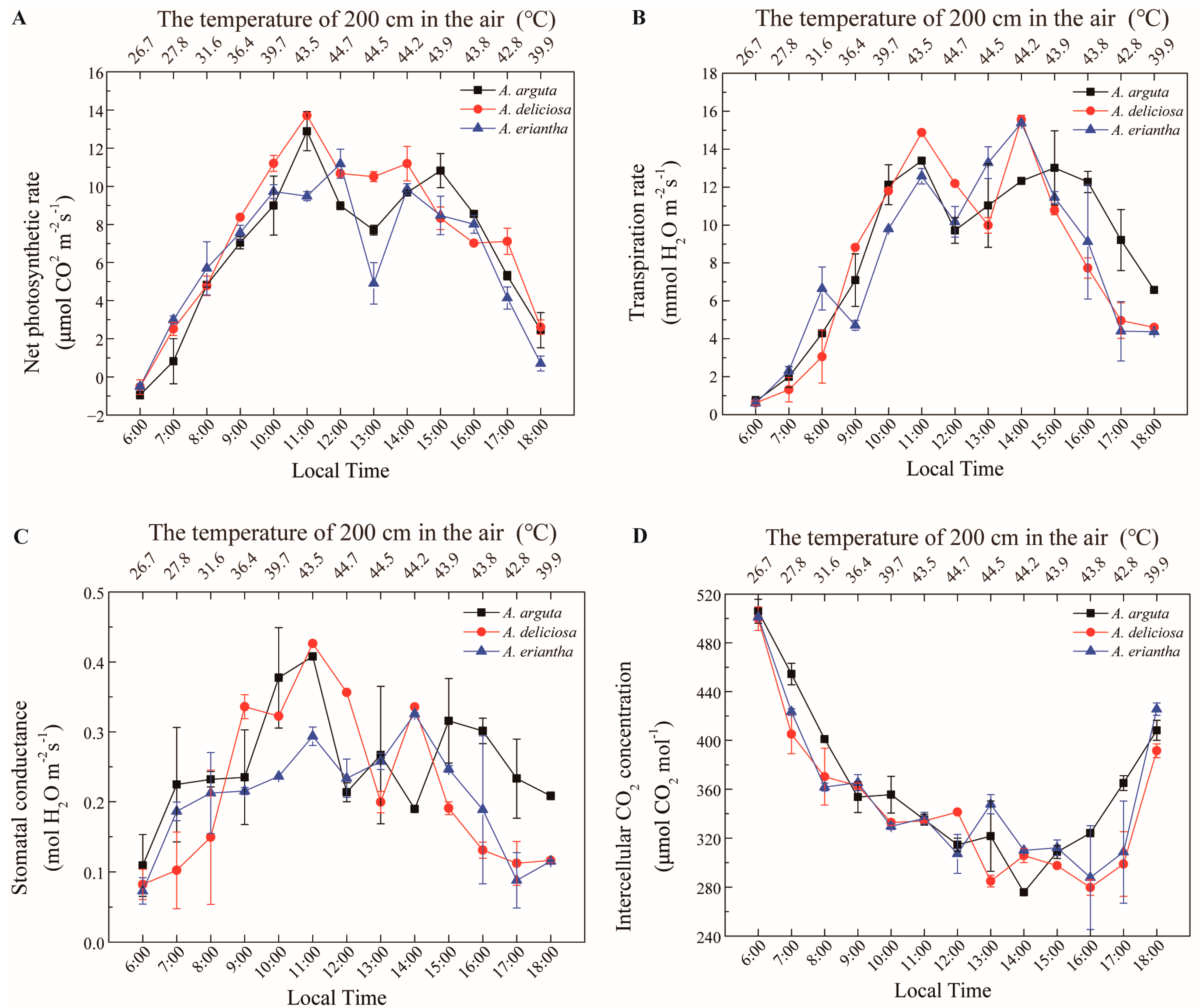
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef] [PubMed]
- Fraire-Velázquez, S.; Balderas-Hernández, V.E. Abiotic stress in plants and metabolic responses. In Abiotic Stress-Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; InTech Open Science: New York, NY, USA, 2013; pp. 25–48. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Janni, M.; Gullì, M.; Maestri, E.; Marmiroli, M.; Valliyodan, B.; Nguyen, H.T.; Marmiroli, N. Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J. Exp. Bot. 2010, 71, 3780–3802. [Google Scholar] [CrossRef]
- Schoffl, F.; Prandl, R.; Reindl, A. Molecular responses to heat stress. In Molecular Responses to Cold, Drought, Heat and Salt Stress in Higher Plants; Shinozaki, K., Yamaguchi-Shinozaki, K., Eds.; R.G. Landes Co.: Austin, TX, USA, 1999; pp. 81–98. [Google Scholar]
- Schwarz, D.; Thompson, A.J.; Kläring, H.P. Guidelines to use tomato in experiments with a controlled environment. Front. Plant Sci. 2014, 5, 625. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Anandhi, A. Temperature based indicators to develop adaptive responses for crop production in Florida, USA. Ecol. Indic. 2020, 121, 107064. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Cornic, G. Effects of Temperature on Photosynthesis. 2021. Encyclopédie de l’Environnement. Available online: http://www.encyclopedie-environnement.org/?p=13467 (accessed on 10 February 2022).
- Lorenz, R.; Stalhandske, Z.; Fischer, E.M. Detection of a climate change signal in extreme heat, heat stress, and cold in Europe from observations. Geophys. Res. Lett. 2019, 46, 8363–8374. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Govindaraj, M.; Pattanashetti, S.K.; Patne, N.; Kanatti, A.A. Breeding cultivars for heat stress tolerance in staple food crops. In Next Generation Plant Breeding; Çiftçi, Y.Ö., Ed.; InTechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Berry, J.; Björkmann, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Wang, W.X.; Vinocur, B.; Shoseyov, O.; Altman, A. Biotechnology of plant osmotic stress tolerance: Physiological and molecular considerations. Acta Hort. 2001, 560, 285–292. [Google Scholar] [CrossRef]
- Yan, K.; Chen, P.; Shao, H.; Zhang, L.; Xu, G. Effects of Short-Term High Temperature on Photosynthesis and Photosystem II Performance in Sorghum. J. Agron. Crop Sci. 2011, 197, 400–408. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of photosynthesis by heat stress: The activation state of Rubisco as a limiting factor in photosynthesis. Physiol. Plant. 2004, 120, 179–186. [Google Scholar] [CrossRef]
- Satpal, D.; Kaur, J.; Bhadariya, V.; Sharma, K. Actinidia deliciosa (Kiwi fruit): A comprehensive review on the nutritional composition, health benefits, traditional utilization, and commercialization. J. Food Process. Preserv. 2021, 45, e15588. [Google Scholar] [CrossRef]
- Zhong, C.; Huang, W.; Wang, Z.; Li, L.; Li, D.; Zhang, Q.; Zhao, T.; Zhang, P. The breeding progress and development status of the kiwifruit industry in China. Acta Hortic. 2022, 1332, 445–454. [Google Scholar] [CrossRef]
- Bardi, L.; Nari, L.; Morone, C.; Faga, M.G.; Malusà, E. Possible Role of High Temperature and Soil Biological Fertility on Kiwifruit Early Decline Syndrome. Front. Agron. 2020, 2, 580659. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Hirayama, T.; Shinozaki, K. Research on plant abiotic stress responses in the post-genome era: Past, present and future. Plant J. 2010, 61, 1041–1052. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine Responses to Heat Stress and Global Warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, J.G.; Green, T.G.A.; Curtis, J.I. Canopy photosynthesis and respiration of kiwifruit (Actinidiu deliciosa var. deliciosa) vines growing in the field. Tree Physiol. 1992, 10, 327–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soar, C.J.; Collins, M.J.; Sadras, V.O. Irrigated Shiraz vines (Vitis vinifera) upregulate gas exchange and maintain berry growth in response to short spells of high maximum temperature in the field. Funct. Plant Biol. 2009, 36, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Orellana, A.; Silva, D.I.; Bastías, R.M.; Bambach, N.; Aburto, F. Late-season plastic covering delays the occurrence of severe water stress and improves intrinsic water use efficiency and fruit quality in kiwifruit vines. Agric. Water Manag. 2021, 249, 106795. [Google Scholar] [CrossRef]
- Savé, R.; Olivella, C.; Biel, C.; Adillón, J.; Rabella, R. Seasonal patterns of water relationships, photosynthetic pigments and morphology of Actinidia deliciosa plants of the Hayward and Tomuri cultivars. Agronomie 1994, 14, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Judd, M.J.; McAneney, K.J.; Wilson, K.S. Influence of water stress on kiwifruit growth. Irrig. Sci. 1989, 10, 303–311. [Google Scholar] [CrossRef]
- Jo, Y.S.; Cho, H.S.; Park, M.Y.; Bang, G.P.; Kim, W.S. Drought stress tolerance of Actinidia arguta and Actinidia eriantha. Acta Hortic. 2008, 773, 283–287. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Q.; Haoru, T. Variation on photosynthetic performance in kiwifruit seedling during drought stress and rewatering. Adv. Biol. Sci. Res. 2018, 5, 56–59. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Tao, H.B.; Tian, B.J.; Sheng, D.C.; Xu, C.C.; Zhou, H.M.; Huang, S.B.; Wang, P. Flowering dynamics, pollen, and pistil contribution to grain yield in response to high temperature during maize flowering. Environ. Exp. Bot. 2019, 158, 80–88. [Google Scholar] [CrossRef]
- Srivastava, S.; Pathak, A.D.; Gupta, P.S.; Shrivastava, A.K.; Srivastava, A.K. Hydrogen peroxide scavenging enzymes impart tolerance to high temperature induced oxidative stress in sugarcane. J. Environ. Biol. 2012, 33, 657–661. [Google Scholar]
- Evans, J.R. Improving Photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McElrone, A.J.; Choat, B.; Gambetta, G.A.; Brodersen, C.R. Water Uptake and Transport in Vascular Plants. Nat. Educ. Knowl. 2013, 4, 6. [Google Scholar]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, A.E.; Perez Pena, J.E.; Gonzalez, C.V.; Prieto, J.A. Syrah and Grenache (Vitis vinifera) revealed different strategies to cope with high temperature. Aust. J. Grape Wine Res. 2022, 28, 383–394. [Google Scholar] [CrossRef]
- Galat Giorgi, E.; Sadras, V.O.; Keller, M.; Perez Pena, J.E. Interactive effects of high temperature and water deficit on Malbec grapevines. Aust. J. Grape Wine Res. 2019, 25, 345–356. [Google Scholar] [CrossRef]
- Dolan, L. Plant Evolution: An Ancient Mechanism Protects Plants and Algae from Heat Stress. Curr. Biol. 2020, 30, 277–278. [Google Scholar] [CrossRef]
- Monte, I.; Kneeshaw, S.; Franco-Zorrilla, J.M.; Chini, A.; Zamarreño, A.M.; García-Mina, J.M.; Solano, R. An ancient COI1-independent function for reactive electrophilic oxylipins in thermotolerance. Curr. Biol. 2020, 30, 962–971. [Google Scholar] [CrossRef]
- Zhao, X.; Nishimura, Y.; Fukumoto, Y.; Li, J. Effect of high temperature on active oxygen species, senescence and photosynthetic properties in cucumber leaves. Environ. Exp. Bot. 2011, 70, 212–216. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Xie, X.; Khaldun, A.B.M.; Atak, A.; Zhong, C.; Li, D. Effect of light on growth and chlorophyll development in kiwifruit ex vitro and in vitro. Sci. Hortic. 2022, 291, 110599. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xie, X.; Liu, X.; Cheng, C.; Guo, W.; Zhong, C.; Atak, A. Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.). Biology 2022, 11, 1686. https://doi.org/10.3390/biology11111686
Li D, Xie X, Liu X, Cheng C, Guo W, Zhong C, Atak A. Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.). Biology. 2022; 11(11):1686. https://doi.org/10.3390/biology11111686
Chicago/Turabian StyleLi, Dawei, Xiaodong Xie, Xiaoying Liu, Chang Cheng, Wen Guo, Caihong Zhong, and Arif Atak. 2022. "Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.)" Biology 11, no. 11: 1686. https://doi.org/10.3390/biology11111686
APA StyleLi, D., Xie, X., Liu, X., Cheng, C., Guo, W., Zhong, C., & Atak, A. (2022). Effects of Short-Term High Temperature on Gas Exchange in Kiwifruits (Actinidia spp.). Biology, 11(11), 1686. https://doi.org/10.3390/biology11111686




Arif_ATAK.jpg)

