Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation
Abstract
:Simple Summary
Abstract
1. Flavonoids—An Introduction
Flavonoid’s Bioavailability and Metabolism
2. Oxidative Stress—An Overview
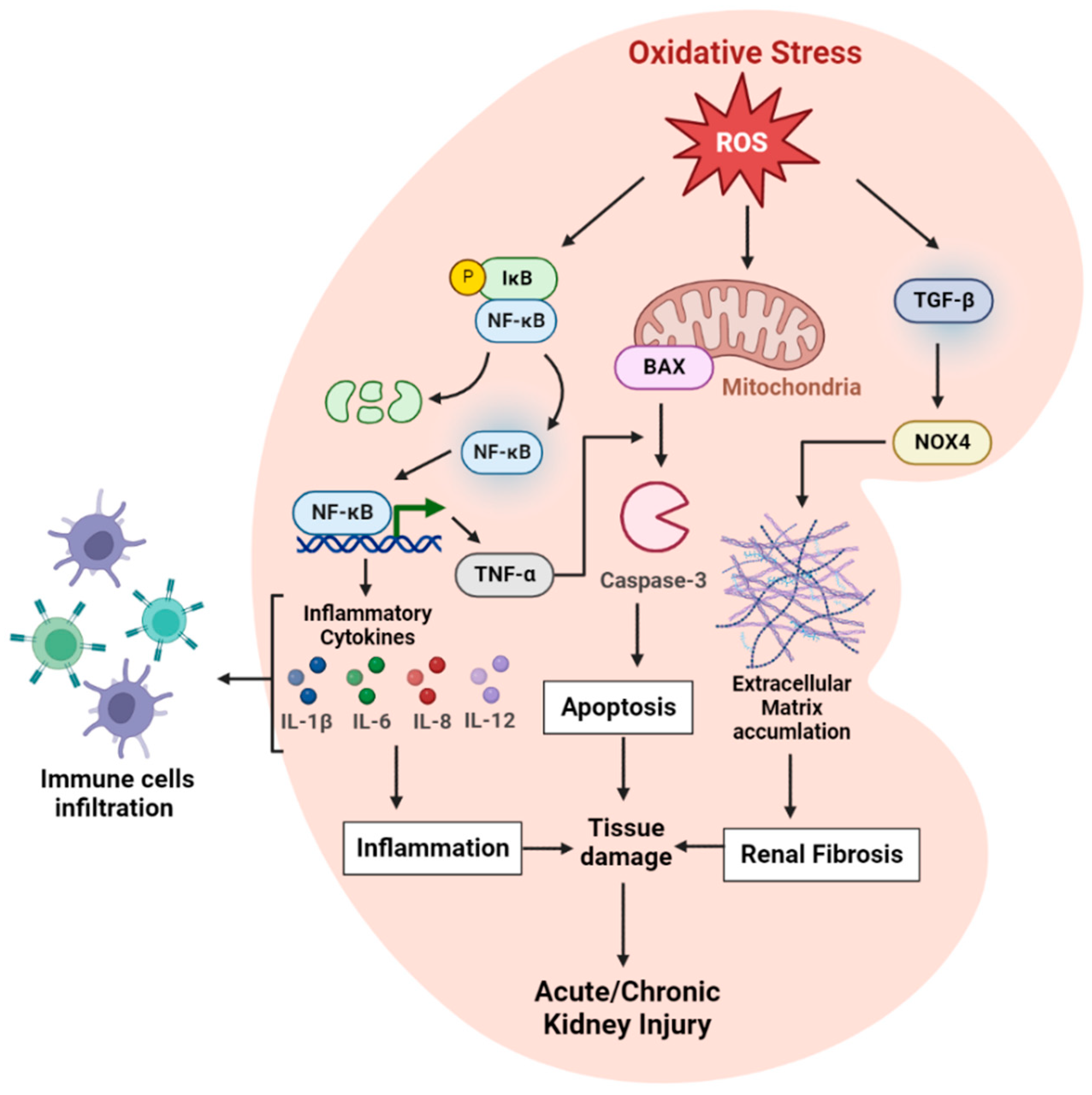
2.1. Oxidative Stress and Inflammatory Markers in Kidney
2.1.1. Role of Toll-Like Receptor
2.1.2. IL-10
2.1.3. IL-6
2.1.4. NF-kB
2.1.5. TNF-α
2.1.6. TGF-β
2.1.7. IL-1β
3. Nephroprotective Effects of Flavonoids
3.1. Flavonoids in Reducing the Kidney Oxidative Stress
3.1.1. Quercetin
3.1.2. Apigenin
3.1.3. Troxerutin
3.1.4. Epigallocatechin-3-gallate (EGCG)
3.1.5. Genistein
3.2. Flavonoids in Preventing DNA Damage in Kidney
3.2.1. Quercetin
3.2.2. Hesperidin
3.2.3. Naringin
3.2.4. Chrysin
3.3. Flavonoids Modulate Inflammatory Markers in Kidney
3.3.1. Kaempferol
3.3.2. Fisetin
3.3.3. Luteolin
3.3.4. Chrysin
3.4. Flavonoids in Inhibiting Cell Death Mechanisms in Kidney
3.4.1. Quercetin
3.4.2. Hesperidin
3.4.3. Naringenin
| Flavonoid Types | Treatment Design/Dose | Factor/Chemical Studied | Type of Study | Subjects Involved | Key Observations | Reference |
|---|---|---|---|---|---|---|
| Quercetin | 20 mg/kg, ip | Ionizing radiation (IR) | In vivo | Sprague–Dawley rats | Significantly reduced the activities of myeloperoxidase and caspase-3, and the levels of 8-OHdG and TNF-α. | [62] |
| 25–50 mg/kg, intragastrical once daily for 75 days. | Lead | In vivo | Wistar rats | Reduced ROS and thiobarbituric acid reactive substances (TBARS) by modulating MAPK and NF-κB signaling pathways. | [63] | |
| 75 mg/kg, oral gavage for 7 days | (NTiO2) nanoparticles | In vivo | Adult female Wistar rats | Normalized the levels of plasma biomarkers and increased the activities of CAT and SOD. Reduced apoptosis and the levels of MDA. | [64] | |
| 5 or 10 mg/kg/day for 21 days | Adenine | In vivo | Male Wistar rats | Reduced serum levels of parathyroid hormone (PTH) and inorganic phosphate, increased serum LDH, urine protein-to-creatinine ratio, urine antioxidants, and IL-8 | [65] | |
| 50 mg/kg/day for 15 days | Ochratoxin | In vivo | Swiss albino mice 2–3 months old | Significantly reduced fecal β-glucuronidase activity and the levels of serum ALP, ALT, AST, LDH, and urea. Reduced DNA damage was detected with a 19.6% decrease in tail length. Reduced macrophage spreading by 5.8%. | [86] | |
| 100 mg/kg by oral gavage for 21 days | Imidacloprid | In vivo | Adult male albino rats | Decreased the concentration of BUN and creatinine. Normalized levels of serum total proteins, globulin, albumin, and A/G ratio. | [87] | |
| 10 mg/kg/day by oral gavage for 70 days | Lead | In vivo | Adult male Wistar rats | Lowered the levels of 8-OHdG, ROS, and GSH/GSSG ratio. Restored the activities of Cu/Zn-SOD, CAT, and GPx, and inhibited apoptosis. | [88] | |
| 25, 50 and 100 mg/kg/day for 28 days | Copper sulfate | In vivo | Male C57BL/6 mice | Inhibited the activities of caspases-3 and−9, reduced the mRNA expression levels of p53 and Bax, and activated the expression of Nrf2 and HO-1 mRNAs. Inhibited the expression of NF-κB, IL-1β, IL-6, and TNF-α, and inhibited mitochondrial apoptosis | [113] | |
| 20 mg/kg/day for 14 days | Carbendazim | In vivo | Adult male Wistar rats | Significantly suppressed the increase in IL-1β, TNF-α, and caspase-3 activity. Reduced the levels of ROS, nitrogen species, and lipid peroxidation. | [115] | |
| 50 mg/kg/daily by oral gavage for 56 days | Cadmium chloride | In vivo | Adult male Wistar rats | Preserved the tubule and glomerulus structure, increased creatinine excretion, reduced the urinary levels of albumin, increased the renal activity of Bcl-2, reduced mRNA levels of CHOP, and the protein levels of Bax, caspase-3, and cleaved caspase-3. It also increased the nuclear activity of SIRT1 and reduced the acetylation of eIf2α and XBP1. Reduced the levels of ROS, TNF, and IL-6. | [116] | |
| Apigenin | 125,250,500 mg/kg/day for 14 days | Doxorubicin | In vivo & in vitro | Male BALB/c mice. NRK-52E, MPC-5, and 4T1 cells | Significantly increased the activity of SOD and GSH levels. Reduced tissue levels of MDA, TNF-α, IL-6, IL-1β, NLRP3, caspase-1, and generation levels of intracellular ROS. Increased cellular viability and reduced apoptosis in both MPC-5 and NRK-52E cells but not 4T1 cells. | [66] |
| Mice: 40 mg/kg for 1 day, ip Cells: 10 μmol/L for 2-and 24-h | MSN | In vivo & In vitro | BALB/c mice and NRK-52E cells | Upregulated the activity of FOXO3a. Increased antioxidant and IkBα levels and reduced ROS accumulation, inhibited the expression of TNF-α and IL-6, and reduced the nuclear translocation of NF-κB. Protected NRK-52E cells from pathological variations and increased cell viability. | [67] | |
| 25 mg/kg/day for 28 days | Nickel oxide nanoparticles | In vivo | Male Wistar rat | Increased renal SOD activity and GSH content and reduced renal MDA levels. Normalized the levels of Ni. Significantly decreased levels of urea, creatinine, and BUN in serum. | [68] | |
| 10 mg/kg for 14 days | Multiwall carbon nanotubes | In vivo | Male Wistar rat | Protected against changes in the activity of mitochondrial SDH, decreased ROS generation, decreased MMP collapse, with a significant reduction in cytochrome c release in kidney mitochondria, and decreased mitochondrial swelling | [70] | |
| 5, 10, 20 mg/kg/day | Gentamicin | In vivo | Male Wistar rat | Increased levels of GR, GPx, SOD, CAT, and GSH. Inhibited changes in serum BUN, creatinine, KIM-1, and NGAL levels. Upregulated Nfe2I2 and Hmox1 mRNA expression. Decreased levels of IL-1β, TNF-α, NFK-β, Bax, and caspase-3. | [71] | |
| Troxerutin | 1, 10, and 100 mg/kg | UUO | In vivo | Male Wistar rats | Increased RBF, SOD, CAT, TAC, GPx activity, and Bcl-2 expression. Decreased serum levels of creatinine, Bax, RVR, MDA, cleaved caspase-3, and TNF-α proteins. | [73] |
| 75 and 150 mg/kg/day, po for 3 days | Cisplatin | In vivo | Male mice | Significantly decrease serum levels of MDA and BUN, and markedly increased the renal levels of SOD and GPx. | [74] | |
| 150 mg/kg/day | Methotrexate | In vivo | Male Wistar rats | Downregulated the expression of HMGB1, RAGE, NF-κB, TNF-α, and COX-2 which lead to the inhibition of HMGB1/RAGE/NF-κB cascade, resulting in a significant reduction in serum levels of BUN, creatinine, and KIM-1. Increased p-AMPK/total AMPK signal and reduced p-mTOR/total mTOR signal. Decreased NOX-1 and lipid peroxidases while restoring levels of SOD, GPx, GSH, and activated Nrf2/HO-1 pathway. | [75] | |
| 100 mg/kg for 20 days | Nickel | In vivo | Wistar Rats | Significantly decreased the levels of lipid peroxidation and increased the levels of enzymatic and non-enzymatic antioxidants. | [76] | |
| 150 mg/kg/day for 15 days | Gentamycin | In vivo | Wistar rats | Significantly increased the rate of glomerular filtration and decreased the levels of serum creatinine, BUN, and urinary albumin to creatinine ratio. Decreased KIM-1 protein expression and decreased levels of protein and lipid oxidative modulations. It also increased the total antioxidant capacity and GSH levels. Decreased the expression of TNF-α, IL-6, and -10. | [77] | |
| EGCG | 10 mg/kg/day iv for 35 days | Aluminum oxide nanoparticles | In vivo | Adult male albino rats | Significantly increased GSH concentration, and CAT and SOD activities while decreasing the level of MDA, creatinine, uric acid, and urea. | [78] |
| 40 mg/kg/day for 28 days | Fluoride | In vivo | Male albino Wistar rats | Reduced levels of lipid peroxidation and protein carbonylation. Normalized the expression of Nrf2/Keap1 and its downstream regulatory proteins. Upregulated anti-apoptotic proteins such as Bcl-2 and downregulated Bax, caspase-9, caspase-3, and cytochrome c. Decreased KIM-1 protein expression, NO, TNF-α, IL-6, and NF-κB expression levels. | [79] | |
| 100, 200 mg/kg/day for 112 days | CdCl2 | In vivo | Male Wistar albino rats | Decreased oxidative stress, normalized levels of E-cadherin, and renal enzymatic antioxidant status. Alleviated the over generation of α-SMA, p-Smad3, TGF-β1, and vimentin. Additionally, decreased the production of miR-21 and miR-192, and improved the levels of miR-29a/b/c. | [80] | |
| Genistein | 5–60 µM for 2 h. | Sodium Fluoride | In vitro | Normal kidney epithelial (NKE) cells | Prevented LDH leakage, reduced the percentage of apoptotic cells and maintained intracellular levels of ROS, lipid peroxidation, and GSH: GSSG ratio. Increased the activity of CAT, SOD, GPx, GR, and GST. Decreased the expression levels of the activated forms of caspases -3, -8, and -9. | [82] |
| 40 or 80 mg/kg for 21 days | Renin-angiotensin system (Ras)/Experimental renovascular hypertension | In vivo | Sprague–Dawley rats | Reduced blood pressure, improved renal dysfunction, hypertrophy of the non-clipped kidney (NCK), and atrophy of the clipped kidney (CK). Restored the levels of CAT, SOD, MDA, and the upregulation of AT1R, NADPH, Nox4, and Bax, and downregulation of Bcl2 protein in the CK. Inhibited the overexpression of AT1R, TGF-β1, smad2/3, and p-smad3 in NCK. Reduced serum ACE activity and plasma Ang II. Alleviated renal hypertrophy in NCK through AT1R/TGF-β1/SMAD-dependent signaling pathways and renal atrophy in CK by modulating AT1R/NADPH oxidase/BCL-2/Bax pathways. | [83] | |
| 40 mg/kg/day for 42 days | Adriamycin | In vivo | Adult male Sprague–Dawley rats | Increased CAT and total antioxidant capacity and reduced levels of MDA and protein carbonyl. | [84] | |
| 15 mg/kg in 1mL 1% DMSO via ip. injection | Renal ischemia/reperfusion | In vivo | Adult male Sprague–Dawley rats | Significant reduction in renal injury. Reduced oxidative stress by strengthening the antioxidant system. Decreased levels of MDA, increased activities of SOD, GPx, and CAT, and decreased gene expression levels of TLR4 and TNF-α. | [85] | |
| Hesperidin | 100 and 200 mg/kg for 15 days | Sodium Arsenite | In vivo | Male Sprague–Dawley rats | Reduced levels of 8-OHdG, MDA, urea, creatinine, TNF-α, NF-κB, IL-1β, caspase-3, p53, and IL-6. Increased levels of SOD, GPx, GSH, and CAT. | [89] |
| 50 and 100 mg/kg for 28 days | Chlorpyrifos | In vivo | Male Sprague–Dawley rats | Reduced levels of 8-OHdG and regulated PARP/VEGF genes at biochemical, cellular, and molecular levels. Upregulated Bcl-2 mRNA expression. Alleviated the degenerative and necrotic changes in kidney histology. Reduced PARP-1 activation and the oxidant status by decreasing MDA levels, and increased antioxidant capacity by increasing SOD, CAT, GPx activities, and GSH levels. | [90] | |
| 10 mg/kg/day for 21 days | Acrylamide | In vivo | Male Wistar albino rats | Decreased serum levels of urea and creatinine, OHdG, TNF-α, IL-1β, IL-6, MDA, NO and increased levels of GSH, GSH-Px, CAT, and SOD. | [91] | |
| 25 and 50 mg/kg/day for 7 days, IP | 5-FU | In vivo | Male mice | Decreased levels of MDA, and increased CAT, SOD, GR, and GSH activities. Decreased the expression level of caspase-3 and 8-OHdG. | [117] | |
| 200 mg/kg/day for 28 days. | Cadmium | In vivo | Male Wistar rats | Decreased serum levels of creatinine and urea, improved kidney tissue integrity, and maintained normal levels of cellular antioxidants. Significantly lowered MDA levels, Bax/Bcl2 ratio, and levels of cleaved caspase 3, while significantly increasing the levels of SOD and CAT. | [118] | |
| 100 and 200 mg/kg/day, po, for 10 days | Cyclophosphamide | In vivo | Male albino mice | Prevented the increase in Bax/Bcl2 ratio and inhibited the activation of caspase 3, significantly decreasing levels of serum creatinine and cystatin C, renal MDA, and NO, also, increased the ratio of IL-10/TNF-α. | [119] | |
| Kaempferol | 100 and 200 mg/kg/day, po for 14 days | Cisplatin | In vivo | Male Balb/C mice | Blocked IκBα degradation and NF-κB nuclear translocation and its binding to the DNA. Reduced the levels of IL-12, TNF-α, and MPO. Blocked MAPK cascade. Upregulated Nrf-2/HO-1 levels. | [99] |
| HK-2 cells: 10, 20, 40 μM Mice: 25, 50 mg/kg | Calcium oxalate crystal (CaOx) | In vitro & In vivo | HK-2 cells and Male C57BL/6 mice | Reduced CaOx crystal deposition in renal tubules and adhesion of crystals to HK-2 cells. Suppressed Nox2 by regulating the expression of AR in vitro and in vivo, decreased the levels of MDA, ROS, and H2O2 in renal tissue, and increased the levels of GSH and SOD. Significantly increased the mRNA levels of IL-4, IL-10, and Arg1 and reduced the mRNA levels of TNF-α, IL-1β, and IL-6. | [100] | |
| 200 mg/kg for 20 days | Doxorubicin | In vivo | Adult male and female rats | Significantly decreased final body weights, levels of urine volume, rate of urinary flow, urinary Cr, and CrCl. Significantly increased mRNA and the total protein levels of MDA, TNF-α, ROS, and IL-6. However, significantly increased the levels of GSH and SOD. Increased nuclear levels of Nrf2 with a parallel decrease in NF-κB p65. | [101] | |
| Naringin | 50 and 100 mg/kg/day for 7 days | Cyclophosphamide | In vivo | Male Wistar rats | Decreased serum toxicity markers, regulated inflammation (TNF-α, NF-κB, IL-6, IL-1β), regulated apoptosis and autophagy (caspase-3, LC3B), regulated oxidative DNA damage (8-OHdG), and decreased serum toxicity markers. | [93] |
| 100 mg/kg/day for 14 days | 5-FU | In vivo | Male adult Sprague–Dawley rats | Decreased the weight of kidneys, significantly increased GSH levels, significantly decreased serum levels of BUN, LDH, and creatinine, TNF-α, IL-6, IL-1α. | [94] | |
| Chrysin | 25 and 50 mg/kg/day for 7 days | Lead acetate (PbAc) | In vivo | Sprague–Dawley rats | Reduced levels of urea, creatinine, lipid peroxidation, 8-OHdG, NF-κB, IL-33, TNF-α, PGE-2, iNOS, and Cox-2. Increased the levels of SOD, CAT, GSH, GPx, AQP-1, and nephrine. It also decreased the levels of lead, iron, copper, zinc, and sodium, and increased the contents of calcium and potassium in renal tissue. | [95] |
| 50 mg/kg/day for 30 days | Arsenic | In vivo | Rats | Increased levels of SOD, CAT, GSH, and GST. Reduced levels of urea, creatinine, urobilinogen, KIM-1, neutrophil gelatinase-associated lipocalin (NGAL), NF-κB, IL-1β, TNF-α, IL-6, Cox-2, TBARS and ROS. | [96] | |
| 25 and 50 mg/kg/day for 14 days | Cisplatin | In vivo | Male Wistar rats | Significantly restored membrane integrity and XO activity, significantly elevated CAT, GSH, GPx, GST, and GR activities. Decreased the activity of BUN. Prevented the elevation of creatinine levels. Protective changes in the morphology of tubular epithelial cells, tubules, and glomeruli were also observed. | [97] | |
| 25 or 50 mg/kg/day for 6 days | Paracetamol | In vivo | Male Sprague–Dawley rats | Decreased the levels of serum creatinine, urea, and MDA. Significantly increased the levels of antioxidant enzymes including GPx, SOD, CAT, and GSH while it significantly decreased the levels of inflammatory markers such as TNF-α, IL-1β, and IL-33. A significant decrease in the apoptotic marker Caspase-3 was also observed. The autophagic marker LC3B was significantly decreased. | [110] | |
| 25 or 50 mg/kg/day for 7 days | Cyclophosphamide | In vivo | Male Wistar rats | Decreased the levels of creatinine, urea, MDA, and hepatorenal deterioration. Improved the activities of antioxidant enzymes including CAT, SOD, GSH, and GPx. Reduced alterations in levels of NF-κB, IL-1β, IL-6, TNF-α, iNOS, COX-2, Bcl-2, Bax and LC3B. | [112] | |
| Fisetin | 1.25 and 2.5 mg/kg/day, ip for 7 days. | Cisplatin | In vivo | Male Sprague–Dawley rats | Restored the levels of creatinine, BUN, and histopathological alterations. Reduced the degradation and phosphorylation of IκBα, and blocked the nuclear translocation of NF-κB, which decreased the activities of TNF-α, iNOS, and MPO. Impaired the translocation o cytochrome c from the mitochondria to the cytosol which decreased the expression of Bax, cleaved caspases -3 and -9, and p53, and prevented the decrease in the levels of Bcl-2. Significantly lowered the mRNA expression of NOX2/gp91phox, NOX4/RENOX, and the activity of NADPH oxidase enzyme. | [102] |
| 100 mg/kg/day for 3 days | Lipopolysaccharide | In vivo | Male C57BL/6J mice | Decreased levels of serum BUN and creatinine, decreased the injury markers KIM-1 and NGAL, inhibited renal expression of IL-1β, IL-6, TNF-α, COX-2, iNOS, and HMGB1. Reduced TUNEL (+) apoptotic cells and inhibited Bcl-2, Bax, and activated caspase-3. inhibited Src-mediated NF-κB p65 and MAPK signaling pathways. | [103] | |
| 50 or 100 mg/kg/day | Potassium oxonate and adenine | In vivo | Male C57BL/6J mice | Improved renal function, decreased urinary albumin: creatinine ratio, preserved tissue architecture. Reduced the expression of kidney urate transporters including organic anion transporter 1 (OAT1), organic anion transporter 3 (OAT3), urate transporter 1 (URAT1), and ATP binding cassette subfamily G member 2 (ABCG2). Mitigated the secretion of TNF-α, IL-6, and MCP-1. Restore the expression of alpha-smooth muscle actin (α-SMA), fibronectin, and collagen I. reduced the abnormal activation of STAT3 and TGF-β signaling. | [104] | |
| Luteolin | 50 mg/kg, orally for 7 days | PbAc | In vivo | Male Wistar rats | Activated the Nrf2/ARE signaling pathway. Increased the expression of CAT, SOD, GPx, and GR. Decreased the levels of serum creatinine and urea, and decreased the expression of IL-1β, TNF-α, and NO. Upregulated the mRNA expression of Nfe212 and Homx1. | [107] |
| 100 and 200 mg/kg/day for 28 days | Bisphenol A | In vivo | Adult male Wistar rats | Reduced levels of serum creatinine, uric acid, and BUN, and decreased the generation of IL-1β, IL-6, and TNF-α. Inhibited DNA damage and reduced lipid peroxidation. Augmented the expression of Nrf2 and HO-1 by modulating the Nrf2/ARE/HO-1 pathway. | [123] | |
| 40 mg/kg for 3 days | Lipopolysaccharide | In vivo | Male ICR mice | Decreased levels of BUN and serum creatinine, reduced tubular necrosis, NF-κB, TNF-α, IL-1β, cleaved caspase-3, ICAM-1 expression, and MCP-1. | [108] | |
| 80 mg/kg/day for 14 days. | HgCl2 | In vivo | Male Wistar rats | Reduced the formation of MDA. Increased the level of GSH. Inhibited the activation of NF-κB. Reduced the accumulation of mercury in the kidneys, increase nuclear translocation of Nrf2 and the resulting protein expression of HO-1 and nicotinamide adenine dinucleotide phosphatase: quinone-acceptor 1 (NQO1). | [109] | |
| Naringenin | 20, 40, and 80 mg/kg, | Methotrexate | In vivo | Male rats | Reduced the expression levels of creatinine, urea, NO, MDA, IL-6, TNF-α, and active caspase-3 in the renal tissue. Exerted an anti-apoptotic effect by modulating NF-κB, p53, Bcl-2, Bax, and caspase-3. Significantly increased the expression levels of CAT, SOD, GSH, GPx, GR, and GSH. | [120] |
| 20 and 40 mg/kg/day, orally for 3 days | CCl4 | In vivo | Male Wistar rats | Improved kidney tissue architecture. Decreased creatinine, urea, and uric acid levels. Increased the expression of Bcl-2. Significantly changed serum metabolic profiling including an increase in stearic acid, palmitic acid, lauric acid, and myristic acid, and a decrease in the levels of alanine, lactic acid, tryptophan, glucose, and glucosamine. | [121] |
4. Pharmacokinetics and Nephroprotective Effects of Flavonoids
5. Flavonoid’s Clinical Advancements in Renal Therapy
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Vijesh, V.V.; Amararathna, D.I.M.; Lakshmi, C.A.; Anbarasu, K.; Kumar, D.R.N.; Ethiraj, K.R.; Kumar, R.A.; Rupasinghe, H.P.V. Mechanism of Action of Flavonoids in Prevention of Inflammation- Associated Skin Cancer. Curr. Med. Chem. 2016, 23, 3697–3716. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef] [PubMed]
- George, V.C.; Dellaire, G.; Rupasinghe, H.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef]
- Vazhappilly, C.G.; Amararathna, M.; Cyril, A.C.; Linger, R.; Matar, R.; Merheb, M.; Ramadan, W.S.; Radhakrishnan, R.; Rupasinghe, H.V. Current methodologies to refine bioavailability, delivery, and therapeutic efficacy of plant flavonoids in cancer treatment. J. Nutr. Biochem. 2021, 94, 108623. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Rupasinghe, H.P.V. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef] [PubMed]
- Viskupičová, J.; Ondrejovič, M.; Šturdík, E. Bioavailability and metabolism of flavonoids. J. Food Nutr. Res. 2008, 47, 151–162. [Google Scholar]
- Nguyen, T.T.H.; Moon, Y.-H.; Ryu, Y.-B.; Kim, Y.-M.; Nam, S.-H.; Kim, M.-S.; Kimura, A.; Kim, D. The influence of flavonoid compounds on the in vitro inhibition study of a human fibroblast collagenase catalytic domain expressed in E. coli. Enzym. Microb. Technol. 2013, 52, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Lotito, S.B.; Zhang, W.-J.; Yang, C.S.; Crozier, A.; Frei, B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free. Radic. Biol. Med. 2011, 51, 454–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Reyes, D.; Morales, A.; Prieto, M. Transit and Metabolic Pathways of Quercetin in Tubular Cells: Involvement of Its Antioxidant Properties in the Kidney. Antioxidants 2021, 10, 909. [Google Scholar] [CrossRef]
- Irska, I.; Paszkiewicz, S.; Pawlikowska, D.; Dryzek, J.; Linares, A.; Nogales, A.; Ezquerra, T.A.; Piesowicz, E. Relaxation behaviour and free volume of bio-based Poly(trimethylene terephthalate)-block-poly(caprolactone) copolymers as revealed by Broadband Dielectric and Positron Annihilation Lifetime Spectroscopies. Polymer 2021, 229, 123949. [Google Scholar] [CrossRef]
- Oliveira, E.; Watson, D.G.; Grant, M.H. Metabolism of quercetin and kaempferol by rat hepatocytes and the identification of flavonoid glycosides in human plasma. Xenobiotica 2002, 32, 279–287. [Google Scholar] [CrossRef]
- Mullen, W.; Graf, B.A.; Caldwell, S.T.; Hartley, R.C.; Duthie, G.G.; Edwards, C.A.; Lean, M.E.J.; Crozier, A. Determination of Flavonol Metabolites in Plasma and Tissues of Rats by HPLC−Radiocounting and Tandem Mass Spectrometry Following Oral Ingestion of [2-14C]Quercetin-4‘-glucoside. J. Agric. Food Chem. 2002, 50, 6902–6909. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K.A.; Day, A.J.; Needs, P.W.; Mellon, F.A.; O’Brien, N.M.; Williamson, G. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: The role of human β-glucuronidase, sulfotransferase, catechol-O-methyltransferase and multi-resistant protein 2 (MRP2) in flavonoid metabolism. Biochem. Pharmacol. 2003, 65, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Thangasamy, T.; Sittadjody, S.; Burd, R. Quercetin: A Potential Complementary and Alternative Cancer Therapy. In Complementary and Alternative Therapies and the Aging Population; Watson, R.R., Ed.; Elsevier: New York, NY, USA, 2009; pp. 563–584. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Taherkhani, S.; Suzuki, K.; Ruhee, R. A Brief Overview of Oxidative Stress in Adipose Tissue with a Therapeutic Approach to Taking Antioxidant Supplements. Antioxidants 2021, 10, 594. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reac-tive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-M.; Ren, G.-L.; Gao, L.; Yang, Q.; Li, H.-D.; Wu, W.-F.; Huang, C.; Zhang, L.; Lv, X.-W.; Li, J. NADPH oxidase 4 promotes cisplatin-induced acute kidney injury via ROS-mediated programmed cell death and inflammation. Lab. Investig. 2017, 98, 63–78. [Google Scholar] [CrossRef] [Green Version]
- Afsar, T.; Razak, S.; Almajwal, A.; Al-Disi, D. Doxorubicin-induced alterations in kidney functioning, oxidative stress, DNA damage, and renal tissue morphology; Improvement by Acacia hydaspica tannin-rich ethyl acetate fraction. Saudi J. Biol. Sci. 2020, 27, 2251–2260. [Google Scholar] [CrossRef] [PubMed]
- Ikewuchi, C.C.; Ifeanacho, M.O.; Ikewuchi, J.C. Moderation of doxorubicin-induced nephrotoxicity in Wistar rats by aqueous leaf-extracts of Chromolaena odorata and Tridax procumbens. Porto Biomed. J. 2021, 6, e129. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Wei, W.; Fan, X.; Ci, X. Farrerol Attenuates Cisplatin-Induced Nephrotoxicity by Inhibiting the Reactive Oxygen Species-Mediated Oxidation, Inflammation, and Apoptotic Signaling Pathways. Front. Physiol. 2019, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, K.; Gadanec, L.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Mitazaki, S.; Hashimoto, M.; Matsuhashi, Y.; Honma, S.; Suto, M.; Kato, N.; Nakagawasai, O.; Tan-No, K.; Hiraiwa, K.; Yoshida, M.; et al. Interleukin-6 modulates oxidative stress produced during the development of cisplatin nephrotoxicity. Life Sci. 2013, 92, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. TLR4 Signaling Mediates Inflammation and Tissue Injury in Nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade-Silva, M.; Cenedeze, M.A.; Perandini, L.A.; Felizardo, R.J.F.; Watanabe, I.K.M.; Agudelo, J.S.H.; Castoldi, A.; Gonçalves, G.M.; Origassa, C.S.T.; Semedo, P.; et al. TLR2 and TLR4 play opposite role in autophagy associated with cisplatin-induced acute kidney injury. Clin. Sci. 2018, 132, 1725–1739. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Ren, L.; Kundu, S.; Gamon, A.; Tyagi, S.C.; Sen, U. Toll-like Receptor 4 Deficiency Reduces Oxidative Stress and Macrophage Mediated Inflammation in Hypertensive Kidney. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.C.; Butter, L.M.; Pulskens, W.P.C.; Teske, G.J.D.; Claessen, N.; Van Der Poll, T.; Florquin, S. The Role of Toll-Like Receptor 2 in Inflammation and Fibrosis during Progressive Renal Injury. PLoS ONE 2009, 4, e5704. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, R.; Xie, J.; Xiong, H.; He, J.C.; Chen, N. Interleukin-10 deficiency aggravates kidney inflammation and fibrosis in the unilateral ureteral obstruction mouse model. Lab. Investig. 2013, 93, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinuani, I.; Beberashvili, I.; Averbukh, Z.; Sandbank, J. Role of IL-10 in the progression of kidney disease. World J. Transplant. 2013, 3, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chun, S.Y.; Lee, E.; Kim, B.; Yoon, B.; Gil, H.; Han, M.-H.; Ha, Y.-S.; Lee, J.N.; Kwon, T.G.; et al. IL-10 Deficiency Aggravates Renal Inflammation, Fibrosis and Functional Failure in High-Fat Dieted Obese Mice. Tissue Eng. Regen. Med. 2021, 18, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Nozaki, Y.; Murao, Y.; Yano, T.; Ri, J.; Niki, K.; Kinoshita, K.; Funauchi, M.; Matsumura, I. Protective effect and mechanism of IL-10 on renal ischemia–reperfusion injury. Lab. Investig. 2019, 99, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Pecoits-Filho, R.; Heimbürger, O.; Bárány, P.; Suliman, M.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am. J. Kidney Dis. 2003, 41, 1212–1218. [Google Scholar] [CrossRef]
- Dennen, P.; Altmann, C.; Kaufman, J.; Klein, C.L.; Andres-Hernando, A.; Ahuja, N.H.; Edelstein, C.L.; Cadnapaphornchai, M.A.; Keniston, A.; Faubel, S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit. Care 2010, 14, R181. [Google Scholar] [CrossRef] [Green Version]
- Nechemia-Arbely, Y.; Barkan, D.; Pizov, G.; Shriki, A.; Rose-John, S.; Galun, E.; Axelrod, J.H. IL-6/IL-6R Axis Plays a Critical Role in Acute Kidney Injury. J. Am. Soc. Nephrol. 2008, 19, 1106–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitazaki, S.; Kato, N.; Suto, M.; Hiraiwa, K.; Abe, S. Interleukin-6 deficiency accelerates cisplatin-induced acute renal failure but not systemic injury. Toxicology 2009, 265, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Lingappan, K. NF-κB in oxidative stress. Curr. Opin. Toxicol. 2017, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Singla, S.K.; Puri, V.; Puri, S. The Restrained Expression of NF-kB in Renal Tissue Ameliorates Folic Acid Induced Acute Kidney Injury in Mice. PLoS ONE 2015, 10, e115947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, H.; Nakagawa, K.; Morita, S.; Shinoda, K.; Mizuno, R.; Kikuchi, E.; Miyajima, A.; Umezawa, K.; Oya, M. Effect of a Novel Nuclear Factor-κB Activation Inhibitor on Renal Ischemia-Reperfusion Injury. Transplantation 2013, 96, 863–870. [Google Scholar] [CrossRef]
- Fearn, A.; Situmorang, G.R.; Fox, C.; Oakley, F.; Howarth, R.; Wilson, C.L.; Kiosia, A.; Robson, M.G.; Mann, D.A.; Moles, A.; et al. The NF-κB1 is a key regulator of acute but not chronic renal injury. Cell Death Dis. 2017, 8, e2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therrien, F.J.; Agharazii, M.; Lebel, M.; Larivière, R. Neutralization of Tumor Necrosis Factor-Alpha Reduces Renal Fibrosis and Hypertension in Rats with Renal Failure. Am. J. Nephrol. 2012, 36, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Azushima, K.; Yamaji, T.; Urate, S.; Suzuki, T.; Abe, E.; Tanaka, S.; Tsukamoto, S.; Kamimura, D.; Kinguchi, S.; et al. Effects of tumor necrosis factor-α inhibition on kidney fibrosis and inflammation in a mouse model of aristolochic acid nephropathy. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Gai, Z.; Kullak-Ublick, G.A.; Liu, Z. TNF-α Deficiency Prevents Renal Inflammation and Oxidative Stress in Obese Mice. Kidney Blood Press. Res. 2017, 42, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Gao, L.; Hu, X.-W.; Wang, J.-N.; Zhang, Y.; Dong, Y.-H.; Lan, H.Y.; Meng, X.-M. Smad3-Targeted Therapy Protects against Cisplatin-Induced AKI by Attenuating Programmed Cell Death and Inflammation via a NOX4-Dependent Mechanism. Kidney Dis. 2021, 7, 372–390. [Google Scholar] [CrossRef]
- Kilari, S.; Yang, B.; Sharma, A.; McCall, D.L.; Misra, S. Increased transforming growth factor beta (TGF-β) and pSMAD3 signaling in a Murine Model for Contrast Induced Kidney Injury. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Meng, X.-M.; Huang, X.R.; Xiao, J.; Chen, H.-Y.; Zhong, X.; Chung, A.C.; Lan, H.Y. Diverse roles of TGF-β receptor II in renal fibrosis and inflammation in vivo and in vitro. J. Pathol. 2012, 227, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J.; Lech, M. NOD-like and Toll-like receptors or inflammasomes contribute to kidney disease in a canonical and a non-canonical manner. Kidney Int. 2013, 84, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, G.; Darisipudi, M.N.; Anders, H.-J. Canonical and non-canonical effects of the NLRP3 inflammasome in kidney inflammation and fibrosis. Nephrol. Dial. Transplant. 2013, 29, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kher, A.; Meldrum, K.K.; Hile, K.L.; Wang, M.; Tsai, B.M.; Turrentine, M.W.; Brown, J.W.; Meldrum, D.R. Aprotinin improves kidney function and decreases tubular cell apoptosis and proapoptotic signaling after renal ischemia-reperfusion. J. Thorac. Cardiovasc. Surg. 2005, 130, 662–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulay, S.R.; Kulkarni, O.P.; Rupanagudi, K.V.; Migliorini, A.; Darisipudi, M.N.; Vilaysane, A.; Muruve, D.; Shi, Y.; Munro, F.; Liapis, H.; et al. Calcium oxalate crystals induce renal inflammation by NLRP3-mediated IL-1β secretion. J. Clin. Investig. 2012, 123, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Privratsky, J.R.; Zhang, J.; Lu, X.; Rudemiller, N.; Wei, Q.; Yu, Y.-R.; Gunn, M.D.; Crowley, S.D. Interleukin 1 receptor (IL-1R1) activation exacerbates toxin-induced acute kidney injury. Am. J. Physiol. Physiol. 2018, 315, F682–F691. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.; Abo-Ghalia, M.H.; Moustafa, G.; Al-Omar, M.A.; Nossier, E.S.; Elsayed, E.A. Design, synthesis and docking studies of novel macrocyclic pentapeptides as anticancer multi-targeted kinase inhibitors. Molecules 2018, 23, 2416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, F.; Romecín, P.; Guillen, A.I.G.; Wangesteen, R.; Vargas-Tendero, P.; Paredes, M.D.; Atucha, N.M.; García-Estañ, J. Flavonoids in Kidney Health and Disease. Front. Physiol. 2018, 9, 394. [Google Scholar] [CrossRef] [Green Version]
- Rahman, S.; Mathew, S.; Nair, P.; Ramadan, W.S.; Vazhappilly, C.G. Health benefits of cyanidin-3-glucoside as a potent modulator of Nrf2-mediated oxidative stress. Inflammopharmacology 2021, 29, 907–923. [Google Scholar] [CrossRef] [PubMed]
- Achouri, M.; Alti, A.; Derdour, M.; Laborie, S.; Roose, P. Smart fog computing for efficient situations management in smart health environments. J. Inf. Commun. Technol. 2018, 17, 537–567. [Google Scholar] [CrossRef]
- Özyurt, H.; Çevik, O.; Özgen, Z.; Özden, A.S.; Çadırcı, S.; Elmas, M.A.; Ercan, F.; Gören, M.Z.; Şener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Sun, Y.-Z.; Sun, J.-M.; Ma, J.-Q.; Cheng, C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim. Biophys. Acta BBA—Gen. Subj. 2012, 1820, 1693–1703. [Google Scholar] [CrossRef]
- Alidadi, H.; Khorsandi, L.; Shirani, M. Effects of Quercetin on Tubular Cell Apoptosis and Kidney Damage in Rats Induced by Titanium Dioxide Nanoparticles. Malays. J. Med. Sci. 2018, 25, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Song, Y.; Liang, Y.-N.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. J. Pharmacol. Exp. Ther. 2018, 24, 4760–4766. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin ameliorates doxorubicin-induced renal injury via inhibition of oxidative stress and inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Xie, M.; Li, S.; Zhang, J.; Zhou, J. Apigenin Attenuates Mesoporous Silica Nanoparticles-Induced Nephrotoxicity by Activating FOXO3a. Biol. Trace Element Res. 2021, 200, 2793–2806. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.-M.; Mansour, A.B.; Attia, S.A. The potential protective role of apigenin against oxidative damage induced by nickel oxide nanoparticles in liver and kidney of male Wistar rat, Rattus norvegicus. Environ. Sci. Pollut. Res. 2021, 28, 27577–27592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Althagafi, H.A.; Alharthi, F.; Albrakati, A.; Alsharif, K.F.; Theyab, A.; Kassab, R.B.; Mufti, A.H.; Algahtani, M.; Oyouni, A.A.A.; et al. Apigenin attenuates molecular, biochemical, and histopathological changes associated with renal impairments induced by gentamicin exposure in rats. Environ. Sci. Pollut. Res. 2022, 29, 65276–65288. [Google Scholar] [CrossRef] [PubMed]
- Panat, N.A.; Maurya, D.K.; Ghaskadbi, S.S.; Sandur, S.K. Troxerutin, a plant flavonoid, protects cells against oxidative stress-induced cell death through radical scavenging mechanism. Food Chem. 2016, 194, 32–45. [Google Scholar] [CrossRef]
- Kaeidi, A.; Taghipour, Z.; Allahtavakoli, M.; Fatemi, I.; Hakimizadeh, E.; Hassanshahi, J. Ameliorating effect of troxerutin in unilateral ureteral obstruction induced renal oxidative stress, inflammation, and apoptosis in male rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Dehnamaki, F.; Karimi, A.; Pilevarian, A.A.; Fatemi, I.; Hakimizadeh, E.; Kaeidi, A.; Allahtavakoli, M.; Rahmani, M.R.; Khademalhosseini, M.; Bazmandegan, G. Treatment with troxerutin protects against cisplatin-induced kidney injury in mice. Acta Chir. Belg. 2018, 119, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; El-Aal, S.A.A.; Eid, A.H.; Arafa, E.-S.A.; Mahmoud, A.M.; Ashour, A.M. Targeting inflammation, autophagy, and apoptosis by troxerutin attenuates methotrexate-induced renal injury in rats. Int. Immunopharmacol. 2022, 103, 108284. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, P.; Ramakrishnan, R.; Amudha, K.; Jalaludeen, A.M.; Sagaran, G.K.; Babu, F.R.; Pari, L. Beneficial Protec-tive Effect of Troxerutin on Nickel-Induced Renal Dysfunction in Wistar Rats. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Arab, H.H.; Maghrabi, I.A. Troxerutin down-regulates KIM-1, modulates p38 MAPK signaling, and enhances renal regenerative capacity in a rat model of gentamycin-induced acute kidney injury. Food Funct. 2018, 9, 6632–6642. [Google Scholar] [CrossRef] [PubMed]
- El Fattah, M.E.A.; AbdelGawad, M.R.; El Boughdady, B.A.E. The protective role of Epigallocatechin gallate (EGCG) on oxidative stress in normal and treated rats with aluminum oxide nanoparticles. Int. J. Adv. Biochem. Res. 2018, 2, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Thangapandiyan, S.; Miltonprabu, S. Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: Role of Nrf2/HO-1 signaling. Toxicol. Rep. 2014, 1, 12–30. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Du, L.; Li, J.; Song, H. Epigallocatechin-3-gallate attenuates cadmium-induced chronic renal injury and fibrosis. Food Chem. Toxicol. 2016, 96, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Vazhappilly, C.G. Recent pharmacological advances on genistein in clinical trials. EXCLI J. 2020, 19, 1120–1123. [Google Scholar] [PubMed]
- Sadhukhan, P.; Saha, S.; Sil, P.C. Anti-Oxidative Effect of Genistein and Mangiferin on Sodium Fluoride Induced Oxi-dative Insult of Renal Cells: A Comparative Study Abstract. Biomark. J. 2016, 2, 1:2. [Google Scholar] [CrossRef]
- Poasakate, A.; Maneesai, P.; Potue, P.; Bunbupha, S.; Tong-Un, T.; Settheetham-Ishida, W.; Khamseekaew, J.; Pakdeechote, P. Genistein alleviates renin-angiotensin system mediated vascular and kidney alterations in renovascular hypertensive rats. Biomed. Pharmacother. 2022, 146, 112601. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, M.H.; Sadria, R.; Djalali, M.; Derakhshanian, H.; Hosseinzadeh, P.; Zarei, M.; Azizi, G.; Sedaghat, R.; Mirshafiey, A. Soy protein and genistein improves renal antioxidant status in experimental nephrotic syndrome. Nefrologia 2014, 34, 483–490. [Google Scholar] [CrossRef]
- Gholampour, F.; Mohammadi, Z.; Karimi, Z.; Owji, S.M. Protective effect of genistein in a rat model of ischemic acute kidney injury. Gene 2020, 753, 144789. [Google Scholar] [CrossRef]
- Oršolić, N.; Jembrek, M.J.; Terzić, S. Honey and quercetin reduce ochratoxin A-induced DNA damage in the liver and the kidney through the modulation of intestinal microflora. Food Agric. Immunol. 2017, 28, 812–833. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.M.S.; El-Ela, F.I.A.; Abdel-Aziz, A.M. Investigating the potential protective effects of natural product quercetin against imidacloprid-induced biochemical toxicity and DNA damage in adults rats. Toxicol. Rep. 2019, 6, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Ma, J.-Q.; Sun, Y.-Z. Quercetin protects the rat kidney against oxidative stress-mediated DNA damage and apoptosis induced by lead. Environ. Toxicol. Pharmacol. 2010, 30, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Turk, E.; Kandemir, F.M.; Yildirim, S.; Caglayan, C.; Kucukler, S.; Kuzu, M. Protective Effect of Hesperidin on Sodium Arsenite-Induced Nephrotoxicity and Hepatotoxicity in Rats. Biol. Trace Element Res. 2018, 189, 95–108. [Google Scholar] [CrossRef]
- Küçükler, S.; Çomaklı, S.; Özdemir, S.; Çağlayan, C.; Kandemir, F.M. Hesperidin protects against the chlorpyrifos-induced chronic hepato-renal toxicity in rats associated with oxidative stress, inflammation, apoptosis, autophagy, and up-regulation of PARP-1/VEGF. Environ. Toxicol. 2021, 36, 1600–1617. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Ela, F.I.A.; Alshahrani, F.K.; Bin-Jumah, M.; Al-Zharani, M.; Almutairi, B.; Alyousif, M.S.; Bungau, S.; Aleya, L.; Alkahtani, S. Protective effects of thymoquinone against acrylamide-induced liver, kidney and brain oxidative damage in rats. Environ. Sci. Pollut. Res. 2020, 27, 37709–37717. [Google Scholar] [CrossRef]
- Gelen, V.; Yıldırım, S.; Şengül, E.; Çınar, A.; Çelebi, F.; Küçükkalem, M.; Gök, M. Naringin attenuates oxidative stress, inflammation, apoptosis, and oxidative DNA damage in acrylamide-induced nephrotoxicity in rats. Asian Pac. J. Trop. Biomed. 2022, 12, 223. [Google Scholar] [CrossRef]
- Caglayan, C.; Temel, Y.; Kandemir, F.M.; Yildirim, S.; Kucukler, S. Naringin protects against cyclophosphamide-induced hepatotoxicity and nephrotoxicity through modulation of oxidative stress, inflammation, apoptosis, autophagy, and DNA damage. Environ. Sci. Pollut. Res. 2018, 25, 20968–20984. [Google Scholar] [CrossRef] [PubMed]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Atila, G. The protective effects of naringin against 5-fluorouracil-ınduced hepatotoxicity and nephrotoxicity in rats. Iran J. Basic Med. Sci 2018, 21, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Kucukler, S.; Benzer, F.; Yildirim, S.; Gur, C.; Kandemir, F.M.; Bengu, A.S.; Ayna, A.; Caglayan, C.; Dortbudak, M.B. Protective Effects of Chrysin Against Oxidative Stress and Inflammation Induced by Lead Acetate in Rat Kidneys: A Biochemical and Histopathological Approach. Biol. Trace Element Res. 2020, 199, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Jabeen, F.; Ashraf, A.; Imran, M.; Ehsan, N.; Samad, A.; Saleemi, M.K.; Iqbal, J. Evaluation of possible protective role of Chrysin against arsenic-induced nephrotoxicity in rats. Toxin Rev. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Sultana, S.; Verma, K.; Khan, R. Nephroprotective efficacy of chrysin against cisplatin-induced toxicity via attenuation of oxidative stress. J. Pharm. Pharmacol. 2012, 64, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Shi, H.; Liu, D. Chrysin protects against renal ischemia reperfusion induced tubular cell apoptosis and inflammation in mice. Exp. Ther. Med. 2019, 17, 2256–2262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sun, W.; Sun, X.; Wang, Y.; Zhou, M. Kaempferol ameliorates Cisplatin induced nephrotoxicity by modulating oxidative stress, inflammation and apoptosis via ERK and NF-κB pathways. AMB Express 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Yuan, P.; Sun, X.; Liu, X.; Hutterer, G.; Pummer, K.; Hager, B.; Ye, Z.; Chen, Z. Kaempferol alleviates calcium oxalate crystal-induced renal injury and crystal deposition via regulation of the AR/NOX2 signaling pathway. Phytomedicine 2021, 86, 153555. [Google Scholar] [CrossRef] [PubMed]
- Alagal, R.I.; AlFaris, N.A.; Alshammari, G.M.; Altamimi, J.Z.; AlMousa, L.A.; Yahya, M.A. Kaempferol attenuates doxorubicin-mediated nephropathy in rats by activating SIRT1 signaling. J. Funct. Foods 2021, 89, 104918. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kalvala, A.K.; Koneru, M.; Kumar, J.M.; Kuncha, M.; Rachamalla, S.S.; Sistla, R. Ameliorative Effect of Fisetin on Cisplatin-Induced Nephrotoxicity in Rats via Modulation of NF-κB Activation and Antioxidant Defence. PLoS ONE 2014, 9, e105070. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Guo, F.; Tao, S.; Huang, R.; Ma, L.; Fu, P. Flavonoid fisetin alleviates kidney inflammation and apoptosis via inhibiting Src-mediated NF-κB p65 and MAPK signaling pathways in septic AKI mice. Biomed. Pharmacother. 2019, 122, 109772. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Tao, S.; Guo, F.; Wang, B.; Yang, L.; Ma, L.; Fu, P. Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling. Phytomedicine 2021, 87, 153552. [Google Scholar] [CrossRef] [PubMed]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a Bioflavonoid Inhibits Colorectal Cancer through Modulation of Multiple Signaling Pathways: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- George, V.C.; Kumar, D.R.N.; Suresh, P.K.; Kumar, S.; Kumar, R.A. Comparative Studies to Evaluate Relative in vitro Potency of Luteolin in Inducing Cell Cycle Arrest and Apoptosis in HaCaT and A375 Cells. Asian Pac. J. Cancer Prev. 2013, 14, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albarakati, A.J.A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Moneim, A.E.A. Luteolin protects against lead acetate-induced nephrotoxicity through antioxidant, anti-inflammatory, anti-apoptotic, and Nrf2/HO-1 signaling pathways. Mol. Biol. Rep. 2020, 47, 2591–2603. [Google Scholar] [CrossRef]
- Xin, S.-B.; Yan, H.; Ma, J.; Sun, Q.; Shen, L. Protective Effects of Luteolin on Lipopolysaccharide-Induced Acute Renal Injury in Mice. J. Pharmacol. Exp. Ther. 2016, 22, 5173–5180. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Liu, B.; Lu, J.; Li, S.; Baiyun, R.; Lv, Y.; Lu, Q.; Zhang, Z. Dietary luteolin protects against HgCl2-induced renal injury via activation of Nrf2-mediated signaling in rat. J. Inorg. Biochem. 2018, 179, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, F.M.; Kucukler, S.; Eldutar, E.; Caglayan, C.; Gülçin, I. Chrysin Protects Rat Kidney from Paracetamol-Induced Oxidative Stress, Inflammation, Apoptosis, and Autophagy: A Multi-Biomarker Approach. Sci. Pharm. 2017, 85, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, B.H.; Al Za’’Abi, M.; Adham, S.A.; Yasin, J.; Nemmar, A.; Schupp, N. Therapeutic Effect of Chrysin on Adenine-Induced Chronic Kidney Disease in Rats. Cell. Physiol. Biochem. 2016, 38, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Temel, Y.; Kucukler, S.; Yıldırım, S.; Caglayan, C.; Kandemir, F.M. Protective effect of chrysin on cyclophosphamide-induced hepatotoxicity and nephrotoxicity via the inhibition of oxidative stress, inflammation, and apoptosis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 325–337. [Google Scholar] [CrossRef]
- Peng, X.; Dai, C.; Zhang, M.; Das Gupta, S. Molecular Mechanisms Underlying Protective Role of Quercetin on Copper Sulfate-Induced Nephrotoxicity in Mice. Front. Vet. Sci. 2021, 7, 586033. [Google Scholar] [CrossRef] [PubMed]
- Bethesda, M.D.; National Center for Biotechnology Information. PubChem Compound Summary for CID 25429, Car-Bendazim. National Library of Medicine (US). Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carbenda (accessed on 23 September 2022).
- Owumi, S.E.; O. Nwozo, S.; Najophe, E.S. Quercetin abates induction of hepatic and renal oxidative damage, inflammation, and apoptosis in carbendazim-treated rats. Toxicol. Res. Appl. 2019, 3, 239784731984952. [Google Scholar] [CrossRef] [Green Version]
- Alshammari, G.M.; Al-Qahtani, W.H.; AlFaris, N.A.; Albekairi, N.A.; Alqahtani, S.; Eid, R.; Yagoub, A.E.A.; Al-Harbi, L.N.; Yahya, M.A. Quercetin alleviates cadmium chloride-induced renal damage in rats by suppressing endoplasmic reticulum stress through SIRT1-dependent deacetylation of Xbp-1s and eIF2α. Biomed. Pharmacother. 2021, 141, 111862. [Google Scholar] [CrossRef]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Senturk, E.; Tekin, S.; Kükürt, A. The protective effects of hesperidin and curcumin on 5-fluorouracil–induced nephrotoxicity in mice. Environ. Sci. Pollut. Res. 2021, 28, 47046–47055. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.; Khalaf, M.; Mohamed, W. Hesperidin and eugenol attenuate cadmium-induced nephrotoxicity via regulation of oxidative stress, Bax/Bcl2 and cleaved caspase 3 expression. Turk. J. Biochem. 2020, 45, 767–775. [Google Scholar] [CrossRef]
- Fouad, A.A.; Abdel-Gaber, S.; Abdelghany, M.I. Hesperidin opposes the negative impact of cyclophosphamide on mice kidneys. Drug Chem. Toxicol. 2019, 44, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Alzahrani, A.M.; Alfwuaires, M.; Abdel-Moneim, A.M.; Khalil, M. Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed. Pharmacother. 2021, 143, 112180. [Google Scholar] [CrossRef] [PubMed]
- Ammar, N.M.; Hassan, H.A.; Abdallah, H.M.I.; Afifi, S.M.; Elgamal, A.M.; Farrag, A.R.H.; El-Gendy, A.E.-N.G.; Farag, M.A.; Elshamy, A.I. Protective Effects of Naringenin from Citrus sinensis (var. Valencia) Peels against CCl4-Induced Hepatic and Renal Injuries in Rats Assessed by Metabolomics, Histological and Biochemical Analyses. Nutrients 2022, 14, 841. [Google Scholar] [CrossRef] [PubMed]
- Uckun, Z.; Guzel, S.; Canacankatan, N.; Yalaza, C.; Kibar, D.; Yilmaz, B.C. Potential protective effects of naringenin against vancomycin-induced nephrotoxicity via reduction on apoptotic and oxidative stress markers in rats. Drug Chem. Toxicol. 2018, 43, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sita, G.J.A.; Gowthami, M.; Srikanth, G.; Krishna, M.M.; Sireesha, K.R.; Sajjarao, M.; Nagarjuna, K.; Nagarjuna, M.; Chinnaboina, G.K.; Mishra, A.; et al. Protective role of luteolin against bisphenol A-induced renal toxicity through suppressing oxidative stress, inflammation, and upregulating Nrf2/ARE/HO-1 pathway. IUBMB Life 2019, 71, 1041–1047. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Baicalin: A Review. Brain Sci. 2018, 8, 104. [Google Scholar] [CrossRef] [Green Version]
- Yang, M.; Kan, L.; Wu, L.; Zhu, Y.; Wang, Q. Effect of baicalin on renal function in patients with diabetic nephropathy and its therapeutic mechanism. Exp. Ther. Med. 2019, 17, 2071–2076. [Google Scholar] [CrossRef] [Green Version]
- Borges, C.M.; Papadimitriou, A.; Duarte, D.A.; de Faria, J.M.L.; de Faria, J.B.L. The use of green tea polyphenols for treating residual albuminuria in diabetic nephropathy: A double-blind randomised clinical trial. Sci. Rep. 2016, 6, 28282. [Google Scholar] [CrossRef] [Green Version]
- Silveira, M.; Teles, F.; Melo, E.; Borges, V.; Miranda, F.; Dutra, F.; Berretta, A.; Cezar, R.; Silva, J.; Santos, H.; et al. P1574effects of brazilian green propolis extract (epp-af) on inflammation in hemodialysis patients. Nephrol. Dial. Transplant. 2020, 35, gfaa142.P1574. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Xue, J.; Gu, Y.; Lin, S. Meta-analysis of the clinical value of Astragalus membranaceus in diabetic nephropathy. J. Ethnopharmacol. 2010, 133, 412–419. [Google Scholar] [CrossRef]
- Milosavljevic, I.; Jakovljevic, V.; Petrovic, D.; Draginic, N.; Jeremic, J.; Mitrovic, M.; Zivkovic, V.; Srejovic, I.; Stojic, V.; Bolevich, S.; et al. Standardized Aronia melanocarpa extract regulates redox status in patients receiving hemodialysis with anemia. Mol. Cell. Biochem. 2021, 476, 4167–4175. [Google Scholar] [CrossRef]
- Fanti, P.; Asmis, R.; Stephenson, T.J.; Sawaya, B.P.; Franke, A.A. Positive effect of dietary soy in ESRD patients with systemic inflammation—Correlation between blood levels of the soy isoflavones and the acute-phase reactants. Nephrol. Dial. Transplant. 2006, 21, 2239–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Li, P.; Xing, C.-Y.; Zhao, J.-Y.; He, Y.-N.; Wang, J.-Q.; Wu, X.-F.; Liu, Z.-S.; Zhang, A.-P.; Lin, H.-L.; et al. Efficacy and Safety of Abelmoschus manihot for Primary Glomerular Disease: A Prospective, Multicenter Randomized Controlled Clinical Trial. Am. J. Kidney Dis. 2014, 64, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Okuda, M.; Horikoshi, S.; Matsumoto, M.; Tanimoto, M.; Yasui, H.; Tomino, Y. Beneficial effect of Astragalus membranaceus on estimated glomerular filtration rate in patients with progressive chronic kidney disease. Hong Kong J. Nephrol. 2012, 14, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Silveira, M.A.D.; Teles, F.; Berretta, A.A.; Sanches, T.R.; Rodrigues, C.E.; Seguro, A.C.; Andrade, L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]


| Type of Flavonoid/Plant | Treatment Design/Dose | Type of Study | Subjects Studied | Key Observations | Reference |
|---|---|---|---|---|---|
| Baicalein extract from the Scutellaria baicalensis herb | 800 mg every 8 h (×3/day) for 6 months | Random controlled study | 95 men and women with diabetic nephropathy 46–64 years old 23.4 ≤ BMI ≤ 26.1 | A significant decrease in the 24 h urinary albumin, urinary β2-MG, and UAER. A significant increase in SOD and GSH levels and a decrease in the levels of NF-κB, TGFβ1, and VEGF were observed in the treatment group. The increase in BUN and serum creatinine was also significantly reduced at both doses (25 and 50 mg/kg baicalein). It also prevented the increase in the relative weight of the kidney and body’s weight loss. | [125] |
| EGCG from green tea | 800 mg/day for 12 weeks | Randomized, double-blind study | 47 patients with diabetic nephropathy Age ≥ 18 UACR > 40 mg/g | Urine Albumin to Creatinine Ratio (UACR) levels were reduced compared to median baseline value. No significant change in blood pressure, BMI, HbA1c, eGFR, or serum CRP was observed. The mean TNFα, and serum DKK-1 levels were reduced. | [126] |
| Standardized Aronia melanocarpa extract (SAE) | 400 mg of polyphenols/30 mL/day for 30 days | Clinical trial | 30 patients with chronic kidney disease on dialysis treatment | Increase in the levels of hemoglobin, haptoglobin, and LDH levels. Significant decrease in iron, and ferritin, and superoxide anion radical levels, and a decrease in nitrite levels were observed. No significant change in hydrogen peroxide level was observed. The CAT activity was increased, and GSH level was reduced. No significant changes in C-reactive protein, leukocytes, and TNF-α after treatment was observed. | [129] |
| Aglycone (Daidzein, Genistein) from soy protein | 26 mg/non-dialysis day and 54 mg/dialysis session for 8 weeks | Randomized, double-blind study | 32 patients with end-stage renal disease on chronic hemodialysis (HD) CRP > 10.0 mg/L | Increase in blood isoflavone levels 5-to10-folds. Serum isoflavone levels correlated positively with the variation of albumin and insulin-like growth factor-1 A trend towards lower levels of CRP was observed. | [130] |
| Abelmoschus Manihot (A Manihot) | 2.5 g/×3/day for 24 weeks | Randomized, controlled, clinical trial | 417 patients with glomerular disease | The 24-h proteinuria level considerably dropped (p < 0.001) upon treatment. No significant difference in eGFR after 24-week treatment was observed. No significant change in serum creatinine levels was observed after treatment. Change in SBP showed a significant difference between the A Manihot and combined treatment groups. | [131] |
| Astragalus membranaceus (A membranaceus) | 2.5 g/×2/day for 3 months | Clinical Trial | 35 patients with CKD stages 4 & 5 | BUN Levels were increased, Levels of eGFR were increased in CKD stage 4 patients, with no significant change in CKD stage 5. | [132] |
| Brazilian green propolis | 500 mg/day for 12 months | Randomized, double-blind, study | 32 CKD patients 18-90 years old | Levels of proteinuria, UACR, and Urinary monocyte chemoattractant protein-1 significantly decreased. No change in eGFR was observed | [133] |
| 250 mg/day, in capsules | Prospective trial, open-label 9-week crossover study | 37 patients with end-stage CKD on HD (×3/week) 43.4 ≤ Age ≤ 73.8 | Reduced inflammation and serum levels of high-sensitivity c-reactive protein (HsCRP). | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsawaf, S.; Alnuaimi, F.; Afzal, S.; Thomas, R.M.; Chelakkot, A.L.; Ramadan, W.S.; Hodeify, R.; Matar, R.; Merheb, M.; Siddiqui, S.S.; et al. Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology 2022, 11, 1717. https://doi.org/10.3390/biology11121717
Alsawaf S, Alnuaimi F, Afzal S, Thomas RM, Chelakkot AL, Ramadan WS, Hodeify R, Matar R, Merheb M, Siddiqui SS, et al. Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology. 2022; 11(12):1717. https://doi.org/10.3390/biology11121717
Chicago/Turabian StyleAlsawaf, Seba, Fatema Alnuaimi, Saba Afzal, Rinku Mariam Thomas, Ayshwarya Lakshmi Chelakkot, Wafaa S. Ramadan, Rawad Hodeify, Rachel Matar, Maxime Merheb, Shoib Sarwar Siddiqui, and et al. 2022. "Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation" Biology 11, no. 12: 1717. https://doi.org/10.3390/biology11121717
APA StyleAlsawaf, S., Alnuaimi, F., Afzal, S., Thomas, R. M., Chelakkot, A. L., Ramadan, W. S., Hodeify, R., Matar, R., Merheb, M., Siddiqui, S. S., & Vazhappilly, C. G. (2022). Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology, 11(12), 1717. https://doi.org/10.3390/biology11121717









