Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biological Evaluation
2.2.1. Total Phenolic Content
2.2.2. HPLC-ESI-TOF-MS Analysis
2.2.3. Extraction of Pomegranate Peel Powder Water-Soluble Fraction (PPPwsf)
2.2.4. Cell Cultures and Treatment
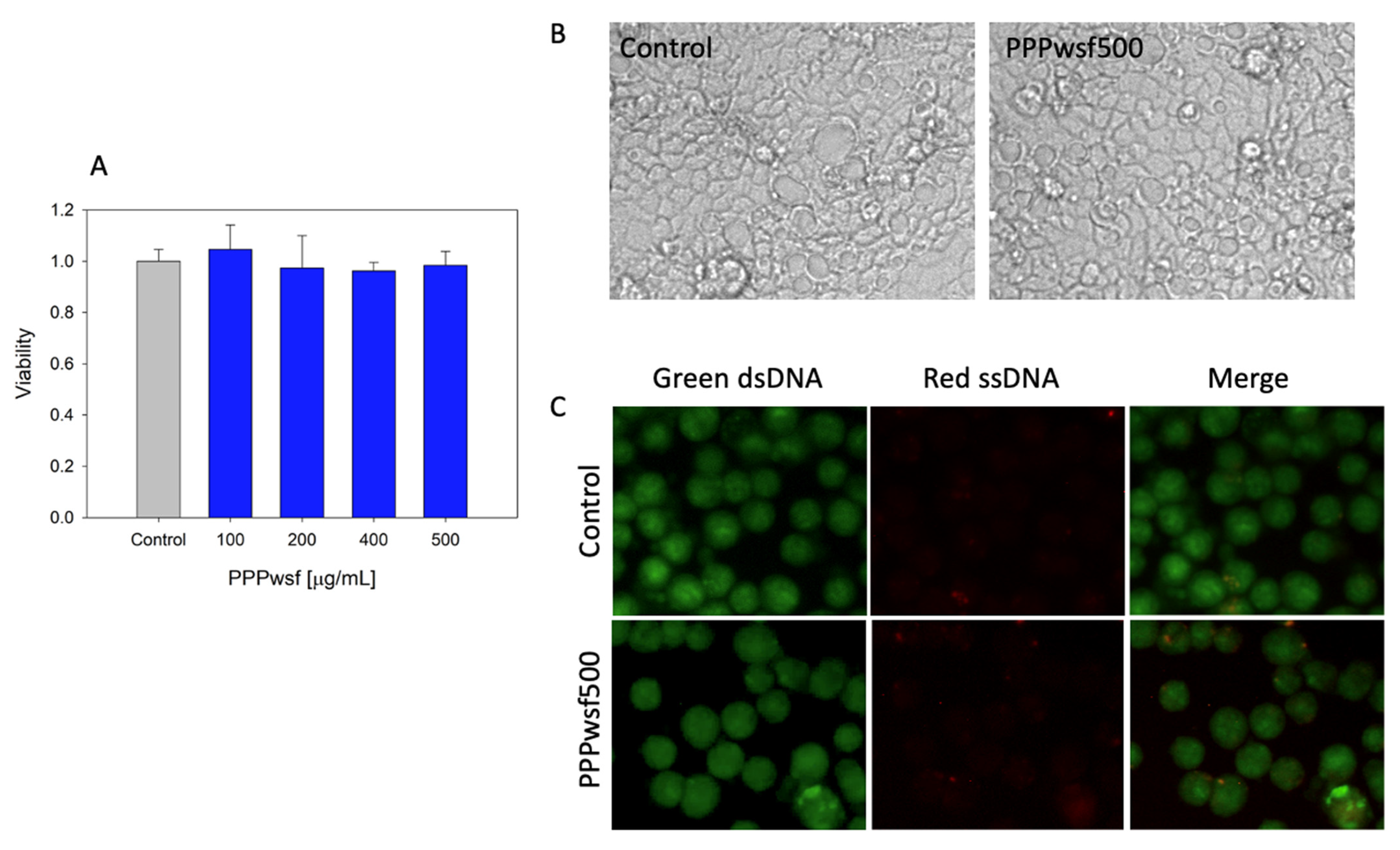
2.2.5. Cell Viability and Morphology
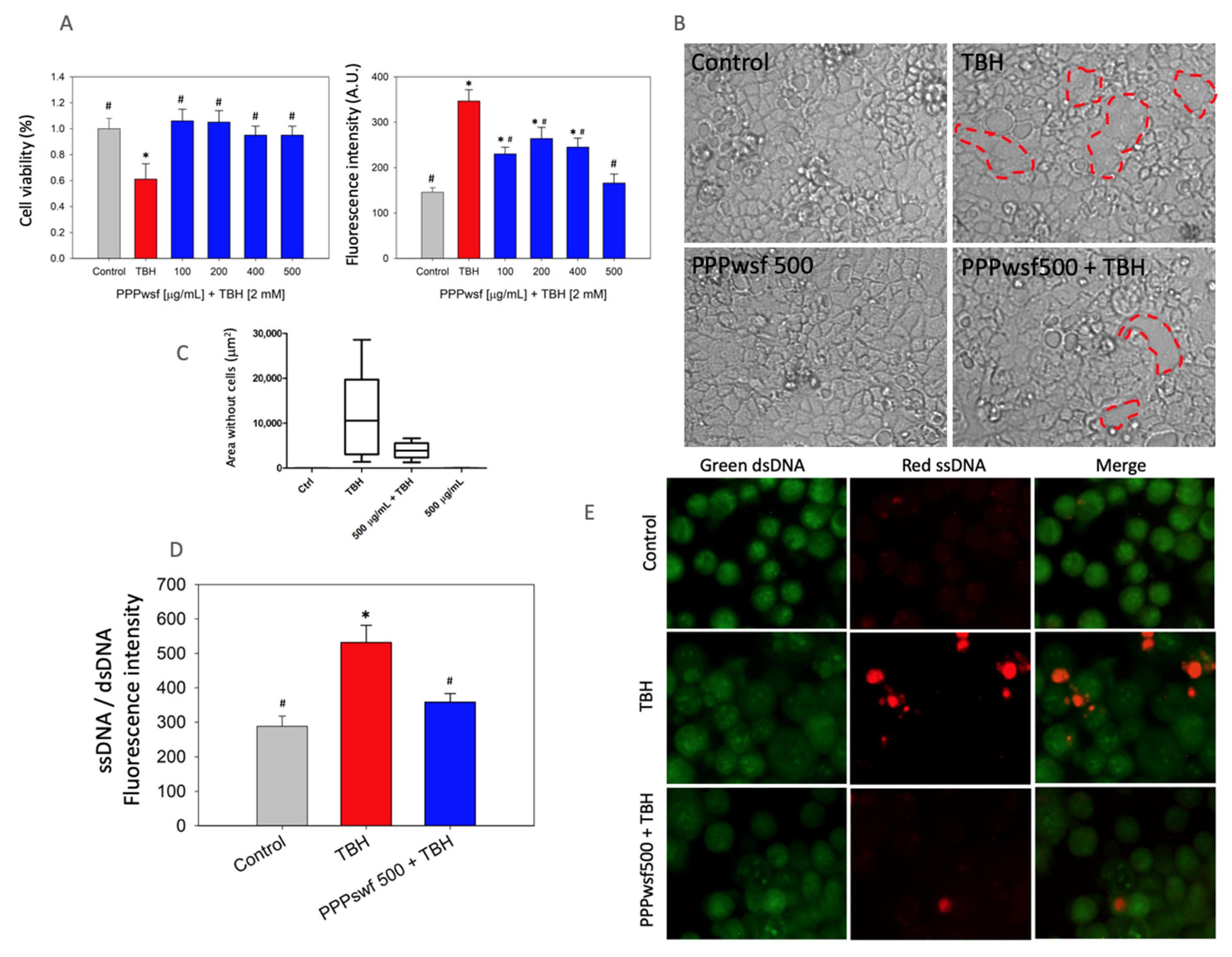
2.2.6. PPPwsf Effect against ROS
2.2.7. Genotoxicity Assay
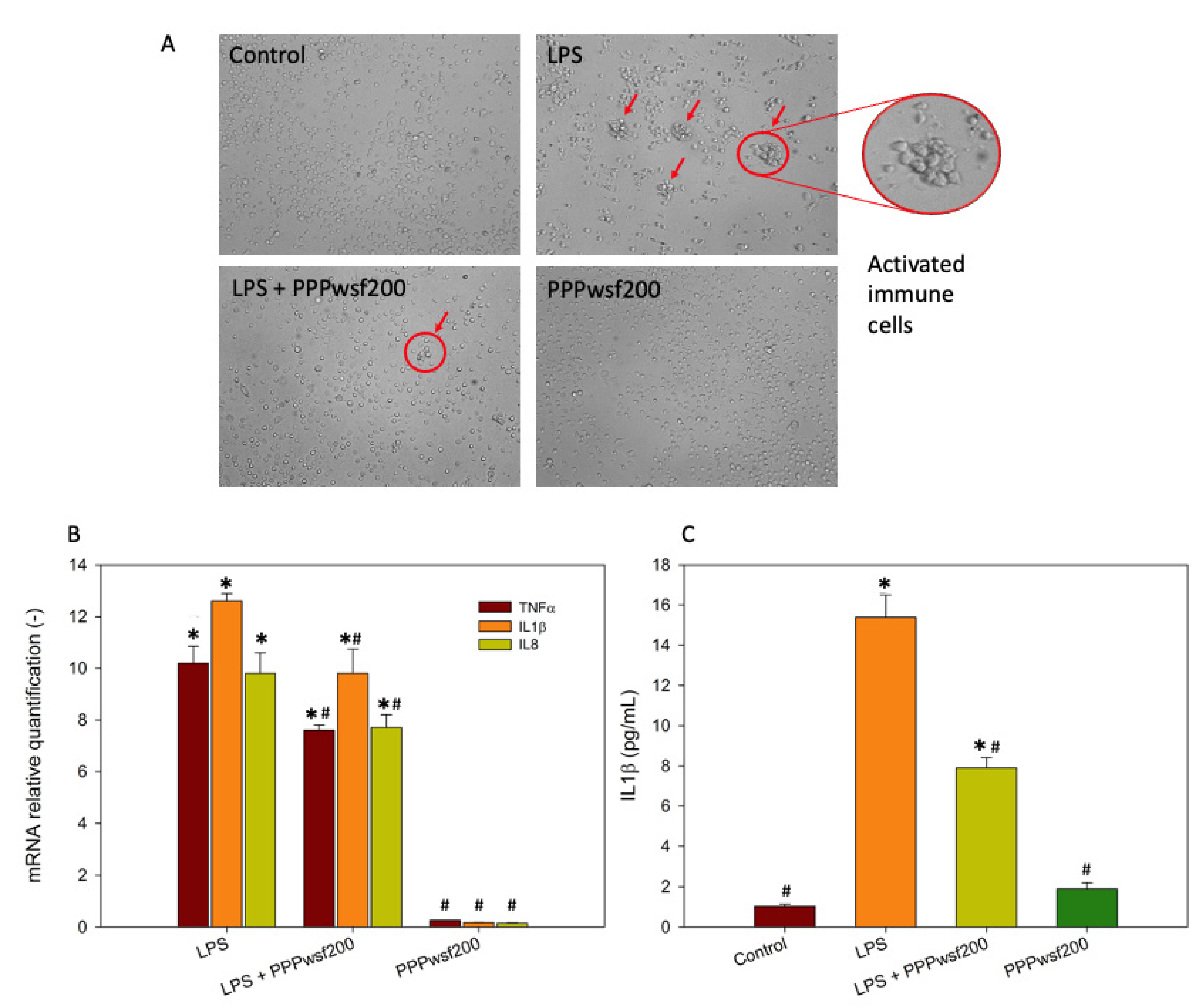
2.2.8. Immunological Tests
2.2.9. Quantitative Real-Time qPCR
2.2.10. Statistical Analysis
2.3. Biscuit Dough Preparation and Characterization
2.3.1. Biscuits Composition and Preparation
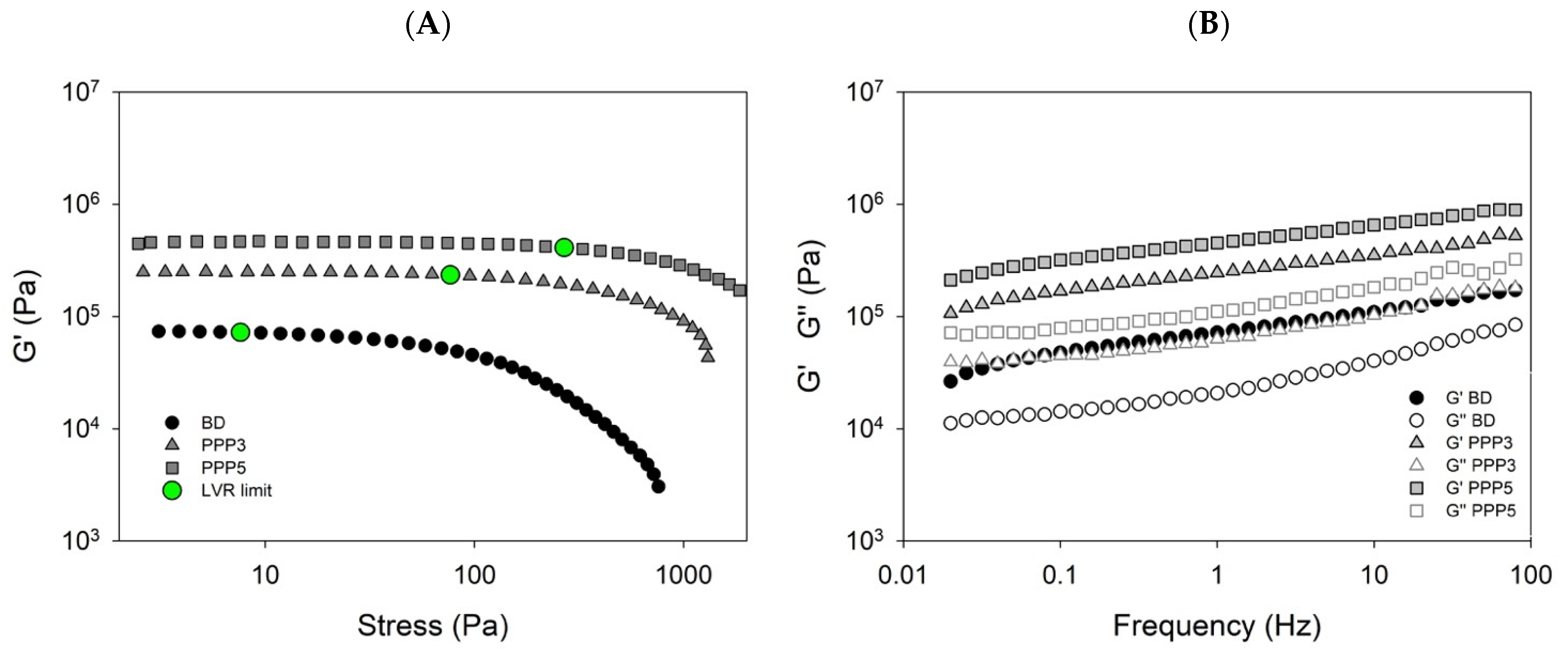
2.3.2. Rheological Measurements
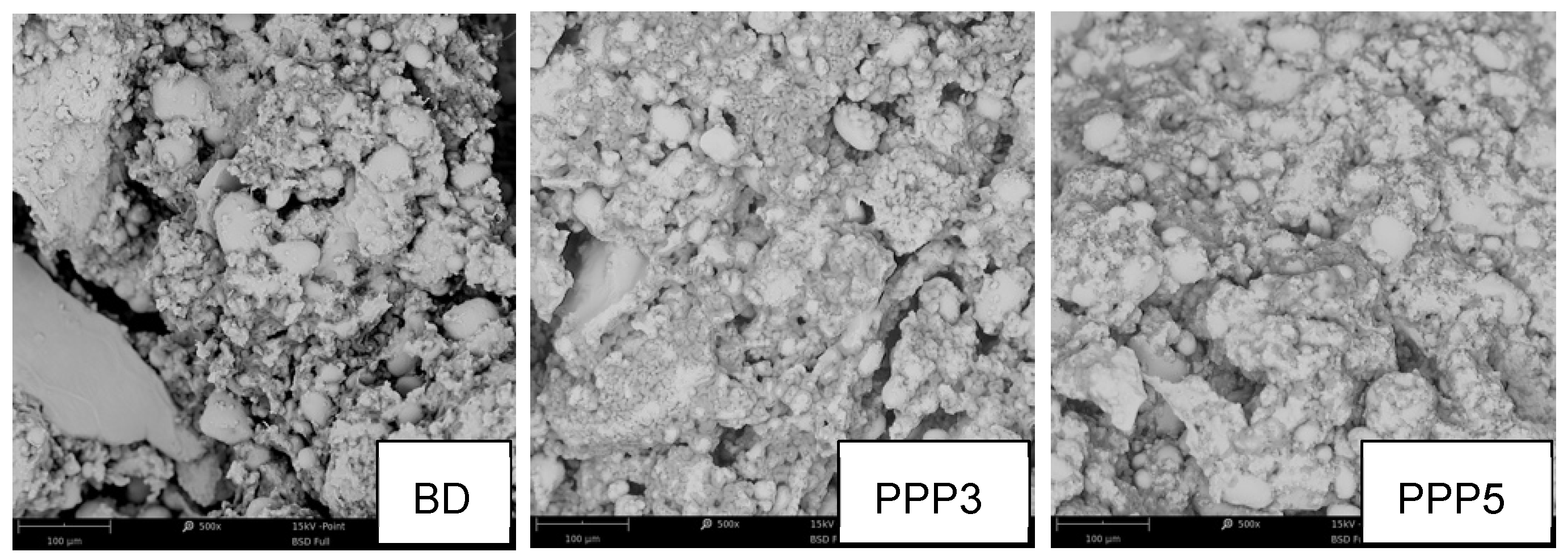
2.3.3. SEM Morphological Analysis
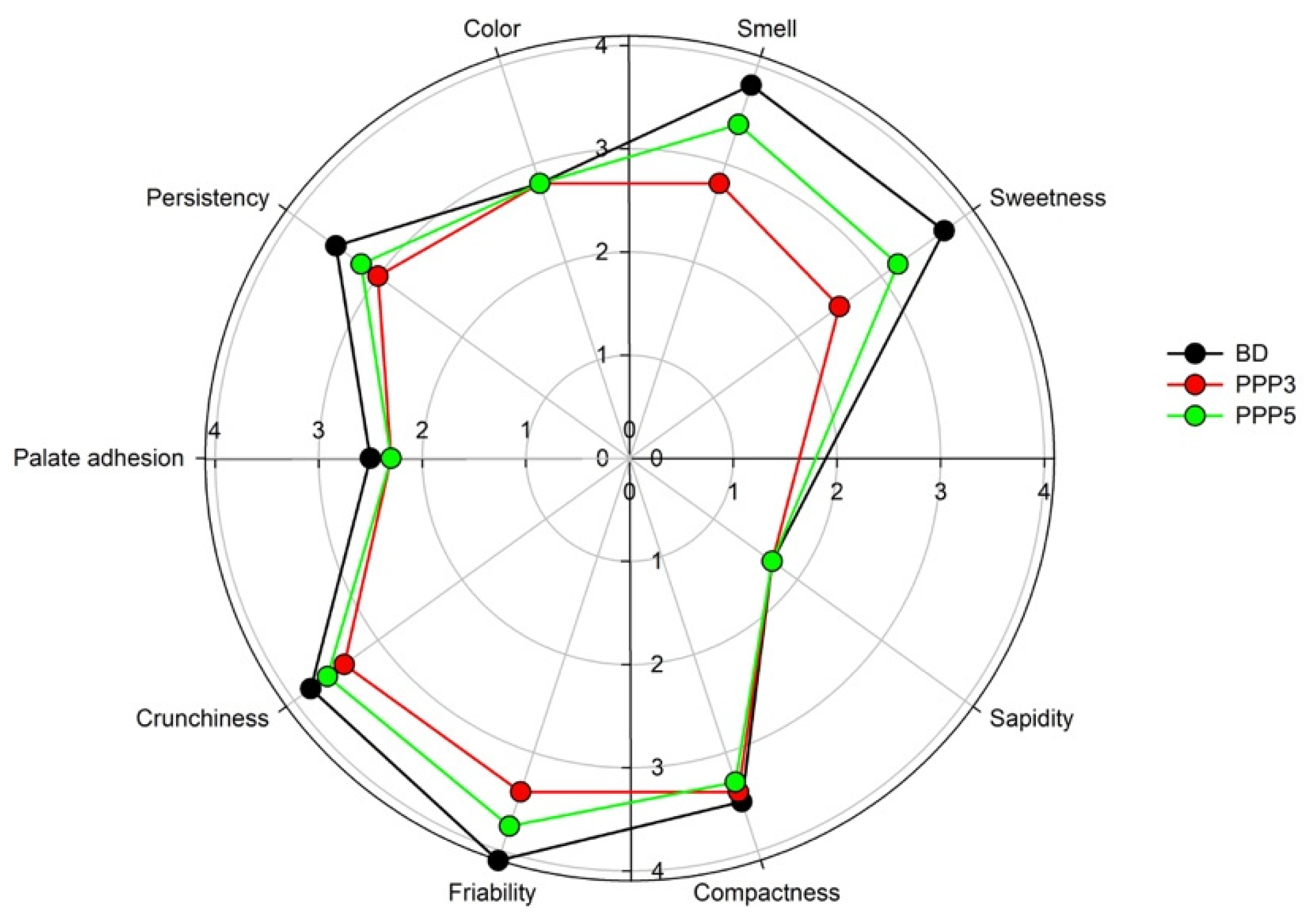
2.3.4. Panel Test
3. Results
3.1. Total Phenolic Content
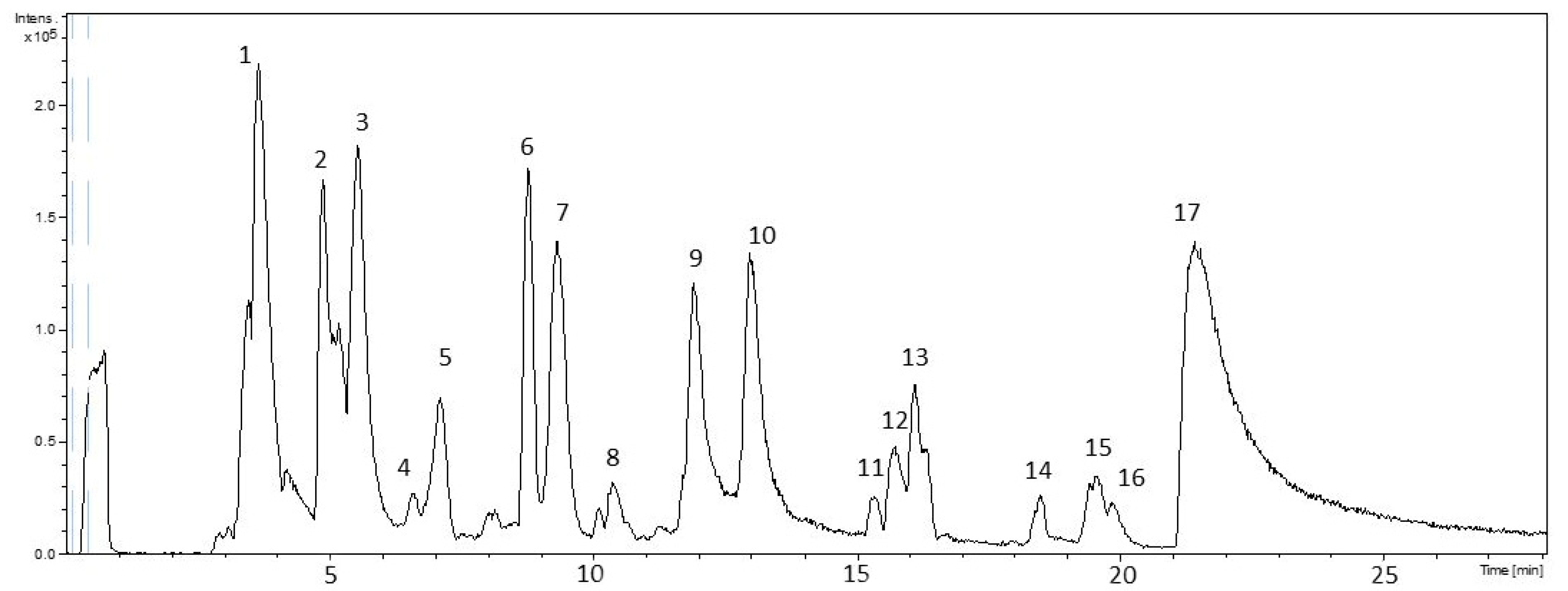
3.2. HPLC-ESI-TOF-MS Analysis
3.3. Biological Effects of the PPPwsf
3.4. Biscuit Dough Preparation and Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Wahaibi, A.; Osman, A.I.; Al-Muhtaseb, A.H.; Alqaisi, O.; Baawain, M.; Fawzy, S.; Rooney, D.W. Techno-economic evaluation of biogas production from food waste via anaerobic digestion. Sci. Rep. 2020, 10, 15719. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I. Catalytic hydrogen production from methane partial oxidation: Mechanism and kinetic study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Ali, H.S.; Mansour, A.F.; Kamil, F.F.; Hussein, M.S. Formulation of nutraceutical biscuits based on died spent coffee grounds. Int. J. Pharmacol. 2018, 14, 584–594. [Google Scholar] [CrossRef]
- Bouazizi, S.; Montevecchi, G.; Antonelli, A.; Hamdi, M. Effects of prickly pear (Opuntia ficus-indica L.) peel flour as an innovative ingredients in biscuit formulation. LWT Food Sci. Technol. 2020, 124, 109155. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Caracciolo, M.; Capocasale, M.; Zappia, C.; Poiana, M. Effects of shortening replacement with extra virgin olive oil on the physical-chemical-sensory properties of Italian Cantuccini biscuits. Foods 2022, 11, 299. [Google Scholar] [CrossRef]
- Hayta, M.; Polat, B. Incorporation of nutraceutical ingredients in baked goods. In Nutraceutical and Functional Food Processing Technology; Boye, J.I., Ed.; Wiley: Chichester, UK, 2015; pp. 211–234. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Presentato, A.; Lino, C.; Piacenza, E.; Albanese, L.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Chillura Martino, D.F.; et al. Volatile compounds of lemon and grapefruit integropectin. Molecules 2020, 26, 51. [Google Scholar] [CrossRef]
- Scurria, A.; Sciortino, M.; Albanese, L.; Nuzzo, D.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Presentato, A.; Pagliaro, M.; Avellone, G.; et al. Flavonoids in lemon and grapefruit integropectin. Chem. Open 2021, 10, 1055–1058. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A.; et al. New neuroprotective effect of lemon integropectin on neuronal cellular model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Noce, A.; Di Laro, M.; Di Daniele, F.; Zaitseva, A.P.; Marrone, G.; Borboni, P.; Di Daniele, N. Natural bioactive compounds useful in clinical management of metabolic syndrome. Nutrients 2021, 16, 630. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Nuzzo, D.; Scordino, M.; Scurria, A.; Giardina, C.; Giordano, F.; Meneguzzo, F.; Mudò, G.; Pagliaro, M.; Picone, P.; Attanzio, A.; et al. Protective, antioxidant and antiproliferative activity of grapefruit integropectin on SH-SY5Y cells. Int. J. Mol. Sci. 2021, 22, 9368. [Google Scholar] [CrossRef] [PubMed]
- Magurano, F.; Sucameli, M.; Picone, P.; Micucci, M.; Baggieri, M.; Marchi, A.; Bucci, P.; Gioacchini, S.; Catinella, G.; Borgonovo, G.; et al. Antioxidant activity of citrus limonoids and investigation of their virucidal potential against SARS-CoV-2 in Cellular Models. Antioxidants 2021, 10, 1794. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A long-neglected broad-spectrum antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef] [PubMed]
- Presentato, A.; Piacenza, E.; Scurria, A.; Albanese, L.; Zabini, F.; Meneguzzo, F.; Nuzzo, D.; Pagliaro, M.; Martino, D.C.; Alduina, R.; et al. A new water-soluble bactericidal agent for the treatment of infections caused by gram-positive and gram-negative bacterial strains. Antibiotics 2020, 9, 586. [Google Scholar] [CrossRef]
- Pineda-Pampliega, J.; Herrera-Duenas, A.; Mulder, E.; Aguirre, J.I.; Hofle, U.; Verhulst, S. Antioxidant supplentation slows telomere shortening in free-living white stork chicks. Proc. Royal Soc. B 2020, 287, 20191917. [Google Scholar] [CrossRef] [Green Version]
- Hassler, E.; Almer, G.; Reishofer, G.; Marsche, G.; Mangge, H.; Deutschmann, H.; Hermann, M.; Leber, M.; Gunzer, F.; Renner, W. Sex-specific association of serum anti-oxidative capacity od leukocyte telomere length. Antioxidants 2021, 10, 1908. [Google Scholar] [CrossRef]
- El Barnossi, A.; Moussaid, F.; Housseini, A.I. Tangerine, banana and pomegranate peels valorization for sustainable environment: A review. Biotechnol. Rep. 2021, 29, e00574. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Layla, A. Pomegranate bioactive molecules and health benefits. In Bioactive Molecules in Food; Merillon, J.-M., Ramawat, K.G., Eds.; Springer Nature: Cham, Switzerland, 2019; Volume 3, pp. 1253–1279, Chapter 42. [Google Scholar]
- Les, F.; Prieto, J.M.; Arboneés-Mainar, J.M.; Valero, M.S.; Loópez, V. Bioactive properties of commercialised pomegranate (Punica granatum) juice: Antioxidant, antiproliferative and enzyme inhibiting activities. Food Funct. 2015, 6, 2049–2057. [Google Scholar] [CrossRef]
- Naz, S.; Siddiqi, R.; Ahmad, S.; Rasool, S.A.; Sayeed, S.A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007, 72, M341–345. [Google Scholar] [CrossRef]
- Panth, N.; Manandhar, B.; Paudel, K.R. Anticancer activity of Punica granatum (pomegranate): A review. Phytother. Res. 2017, 31, 568–578. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hossin, F.L.A. Effect of pomegranate (Punica granatum) peels and it’s extract on obese hypercholesterolemic rats. Pak. J. Nutr. 2009, 8, 1251–1257. [Google Scholar] [CrossRef] [Green Version]
- Bassiri-Jahromi, S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018, 12, 345. [Google Scholar] [CrossRef]
- Hrncirik, K.; Frische, S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive oil. Eur. J. Lipid Sci. Technol. 2004, 106, 540–549. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Lozano Sanchez, J.; Borras-Linares, I.; Guiducci, A.; Muscolino, E.; San Biagio, P.L.; Dispenza, C.; Bulone, D.; Giacomazza, D.; et al. Moringa oleifera leaf powder as functional additive in cookies to protect SH-SY5Y cells. Appl. Sci. 2021, 11, 9995. [Google Scholar] [CrossRef]
- Rodriguez-Perez, C.; Quintares-Piné, R.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Optimization of the extraction method to obtain a phenolic compounds-rich extract from Moringa oleifera Lam. leaves. Ind. Crop. Prod. 2015, 66, 246–254. [Google Scholar] [CrossRef]
- Garcia, P.; Fredes, C.; Cea, I.; Lozano-Sanchez, J.; Leyva-Jimenez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chrom. Anal. 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Jalal, H.; Pal, M.A.; Hamdani, H.; Rovida, M.; Khan, N.N. Antioxidant activity of pomegranate peel and seed powder extract. J. Pharmacogn. Phytochem. 2018, 7, 992–997. [Google Scholar]
- Cam, M.; Icyer, C.N. Phenolics of pomegranate peels: Extraction optimization by central composite design and alpha glucosidase inhibition potentials. J. Food Sci. Technol. 2015, 52, 1489–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volpe, D.A. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J. Pharm. Sci. 2008, 97, 712–7125. [Google Scholar] [CrossRef] [PubMed]
- Kasten, F.H. Introduction to fluorescent probe: Properties, history and applications. In Fluorescent and Luminescent Probes for Biological Activities, 2nd ed.; Mason, W.T., Ed.; Academic Press: London, UK, 1999; Chapter 2; pp. 19–28. [Google Scholar]
- Teng, Z.; Yuan, C.; Zhang, F.; Huan, M.; Cao, W.; Li, K.; Yang, J.; Cao, D.; Zhou, S.; Mei, Q. Intestinal absorption and first-pass metabolism of polyphenol compounds in rat and their transport dynamics in Caco-2 cells. PLoS ONE 2012, 7, e29647. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bousquieres, J.; Deligny, C.; Riaublanc, A.; Lucas, T. CLSM study of layers in laminated dough: Roll out of layers and elastic recoil. J. Cereal Sci. 2014, 60, 82–91. [Google Scholar] [CrossRef]
- Fisher, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MSn. Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of in vitro gastrointestinal digestion on the antioxidant capacity and anthocyanin content of cornelian cherry fruit extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuno, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Sierra, M.; Lodriguez-Lopez, P.; Leyva-Jimenez, F.J.; Borras-Linares, I.; Giacomazza, D.; Fredes, C.; Robert Canales, P.S.; Segura-Carretero, A.; Lozano Sanchez, J. Encapsulation technologies applied to bioactive phenolic compounds and probiotics with potential application on chronic inflammation. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Hernandez-Ledesma, B., Martinez-Villaluenga, C., Eds.; Elsevier Academic Press: London, UK, 2012; pp. 447–476. [Google Scholar]
- Hervert-Hernandez, D.; Pintado, C.; Rotger, R.; Goni, I. Stimulatory role of grape pomace polyphenols an lactobacillus acidophilus growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Sorrenti, V.; Randazzo, C.L.; Caggia, C.; Ballistreri, G.; Romeo, F.V.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial effects of pomegranate peel extract and probiotics on pre-adipocyte differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant acivity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food. Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Topkaya, C.; Isik, F. Effects of pomegranate peel supplementation on chemical, physical and nutritional properties of muffin cakes. J. Food Process. Preserv. 2019, 43, e13868. [Google Scholar] [CrossRef]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental conditions affect color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E.; Pan, Z.; The, H.E.; Menon, V.; Modereger, B.; Pesek, J.J.; Matyska, M.T.; Dao, L.; Takeoka, G. Phenolic composition of pomegranate peel extracts using a liquid chromatography-mass spectrometry approach with silica hydride columns. J. Sep. Sci. 2017, 40, 1449–1456. [Google Scholar] [CrossRef]
- Hering, N.A.; Luetting, J.; Jebautzke, B.; Schulzke, J.D.; Rosenthal, R. The punicalagin metabolites ellagic acid and urolithin A exert different strengthening and anti-iflammatory effects on tight junction-mediated intestinal barrier function in vitro. Front. Pharmacol. 2021, 12, 610164. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite throgh the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.; Park, J.-S.; Lee, E.-J.; Ahn, J.-H.; Kim, H.S. Anti-inflammatory and antioxidant mechanisms of urolithin B in activated microglia. Phytomedicine 2019, 55, 50–57. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Hou, C.; Li, J.; Luo, Y.; Li, B. Punicalagin and ellagic acid from pomegranate peel induce apoptosis and inhibit proliferation in human HepG2 hepatoma cells throgh targeting mithocondria. Food Agric. Immunol. 2019, 30, 897–912. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.-Y.; Lin, L.C.; Chen, S.-R.; Farooqi, A.A.; Cheng, Y.B.; Tang, J.Y.; Chang, H.-W. Pomegranate extract (POMx) induces mitochondrial dysfunction and apoptosis of oral cancer cells. Antioxidants 2021, 10, 1117. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Suzuki, A.; Fujii, A.; Yamamoto, N.; Yamamoto, M.; Ohminami, H.; Kameyama, A.; Shibuya, Y.; Nishizawa, Y.; Tokimitsu, I.; Saito, I. Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett. 2006, 580, 2317–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, R.; Gavin-Smith, B.; Ferraboschi, C.; Kraemer, K. Food fortification: The advantages, disadvantages and lessons from Sight and Life Programs. Nutrients 2021, 13, 1118. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, A.; Elmadfa, I.; Djazayery, A.; Golalipour, M.H.; Sadighi, J.; Sadeghian Sharif, S. Efficacy of flour fortification with folic acid in women of childbearing age in Iran. Ann. Nutr. Metab. 2011, 58, 188–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gera, T.; Sachdev, H.S.; Boy, E. Effect of iron-fortified foods on hematologic and biological outcomes: Systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2012, 96, 309–324. [Google Scholar] [CrossRef] [Green Version]
- Solon, F.S.; Solon, M.S.; Mehansho, H. Evaluation of the effect of vitamin A-fortified margarine on the vitamin A status of preschool Filipino children. Eur. J. Clin. Nutr. 1996, 50, 720–723, PMID 8933117. [Google Scholar]
- Singh, M.; Thrimawithana, T.; Shula, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Future Foods 2020, 1, 100002. [Google Scholar] [CrossRef]

| Items | Forward | Reverse |
|---|---|---|
| TNFα | 5′-CCTCTCTCTAATCAGCCCTCTG-3′ | 5′-GAGGACCTGGGAGTAGATGAG-3′ |
| L-1β | 5′-CCTGTCCTGCGTGTTGAAAGA-3′ | 5′-GGGAACTGGGCAGACTCAAA-3′ |
| L-8 | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ |
| β-actin | 5′-CATGTACGTTGCTATCCAGGC-3′ | 5′-CTCCTTAATGCTACGCACGAT-3′ |
| Molecules | Concentration (mg/kg) |
|---|---|
| Polyphenols * | 16,220 ± 1206 |
| Flavonoids | 3300 ± 96 |
| Antocyanins | 363 ± 24 |
| Peak | RT | Meas. m/z | Calc. m/z | Err. [ppm] | Formula | Proposed Compound | Chemical Class |
|---|---|---|---|---|---|---|---|
| 1 | 3.6 | 353.072 | 353.0725 | 1.6 | C12H17O12 | Sugar | Sugar |
| 2 | 4.9 | 353.0716 | 353.0878 | 46 | C16H17O9 | Chlorogenic acid | Phenolic acids |
| 3 | 5.5 | 191.0195 | 191.0197 | 0.9 | C6H7O7 | Citric acid | Organic acids |
| 6 | 8.8 | 169.0127 | 169.0142 | 9.4 | C7H5O5 | Gallic acid | Phenolic acids |
| 8 | 10.4 | 541.0266 | 541.026 | −1 | C24H13O15 | Punicalagin α | Hydrolysable tannins |
| 9 | 11.9 | 541.0277 | 541.026 | −3.1 | C24H13O15 | Punicalagin β | Hydrolysable tannins |
| 10 | 13 | 541.0263 | 541.026 | −0.5 | C24H13O15 | Punicalagin γ | Hydrolysable tannins |
| 11 | 15.4 | 633.0712 | 633.0733 | 3.4 | C27H21O18 | Corilagin | Hydrolysable tannins |
| 12 | 15.6 | 463.0503 | 463.0518 | 3.3 | C20H15O13 | Ellagic acid hexoside | Hydrolysable tannins |
| 15 | 19.5 | 447.0552 | 447.0569 | 3.9 | C20H15O12 | Ellagic acid-deoxyhexoside | Hydrolysable tannins |
| 16 | 19.8 | 433.0387 | 433.0412 | 5.9 | C19H13O12 | Ellagic acid pentoside | Hydrolysable tannins |
| 17 | 21.3 | 300.9973 | 300.999 | 5.6 | C14H5O8 | Ellagic acid | Hydrolysable tannins |
| Molecules | Concentration (mg/kg) |
|---|---|
| Polyphenols | 6087 ± 525 |
| Flavonoids | 294 ± 36 |
| Antocyanins | 137 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzzo, D.; Picone, P.; Lozano Sanchez, J.; Borras-Linares, I.; Guiducci, A.; Muscolino, E.; Giacomazza, D.; Sanfilippo, T.; Guggino, R.; Bulone, D.; et al. Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment. Biology 2022, 11, 416. https://doi.org/10.3390/biology11030416
Nuzzo D, Picone P, Lozano Sanchez J, Borras-Linares I, Guiducci A, Muscolino E, Giacomazza D, Sanfilippo T, Guggino R, Bulone D, et al. Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment. Biology. 2022; 11(3):416. https://doi.org/10.3390/biology11030416
Chicago/Turabian StyleNuzzo, Domenico, Pasquale Picone, Jesus Lozano Sanchez, Isabel Borras-Linares, Alessandro Guiducci, Emanuela Muscolino, Daniela Giacomazza, Tiziana Sanfilippo, Rossella Guggino, Donatella Bulone, and et al. 2022. "Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment" Biology 11, no. 3: 416. https://doi.org/10.3390/biology11030416
APA StyleNuzzo, D., Picone, P., Lozano Sanchez, J., Borras-Linares, I., Guiducci, A., Muscolino, E., Giacomazza, D., Sanfilippo, T., Guggino, R., Bulone, D., Dispenza, C., San Biagio, P. L., & Lapasin, R. (2022). Recovery from Food Waste—Biscuit Doughs Enriched with Pomegranate Peel Powder as a Model of Fortified Aliment. Biology, 11(3), 416. https://doi.org/10.3390/biology11030416









