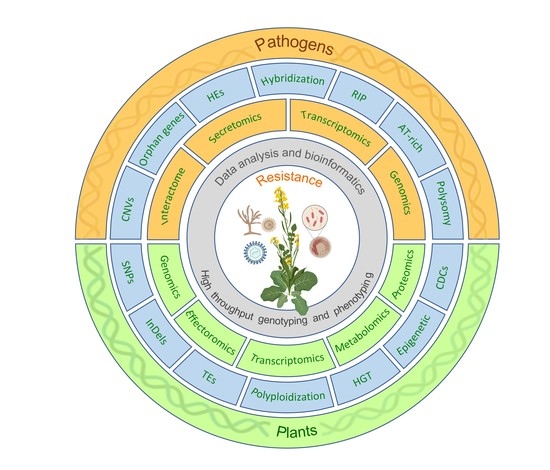
Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions
Abstract
:Simple Summary
Abstract
1. Introduction
2. Plant–Pathogen Interactions
2.1. Gene-for-Gene Relationship
2.2. Zigzag Model of Plant–Pathogen Interactions
2.3. Systemic Acquired Response and Induced Systemic Resistance
2.4. Recognition Models
3. Genomic Variation and Mutational Events in Hosts and Pathogens
3.1. Transposable Elements
3.2. Repeat-Induced Point Mutation
3.3. AT-Rich Isochores
3.4. Chromosomal Rearrangements and Homeologous Exchanges
3.5. Presence/Absence Variation
3.6. Copy Number Variations
3.7. Single Nucleotide Polymorphisms
3.8. Chromosomal Polysomy or Length Polymorphism
3.9. Conditionally Dispensable Chromosomes
3.10. De Novo or Orphan Genes
3.11. Epigenetic Modification of Gene Expression
3.12. Horizontal Gene/Chromosome Transfer
3.13. Hybridization
3.14. Polyploidization
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agrawal, A.A.; Hastings, A.P.; Johnson, M.T.J.; Maron, J.L.; Salminen, J.-P. Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science 2012, 338, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Karasov, T.L.; Horton, M.W.; Bergelson, J. Genomic variability as a driver of plant-pathogen coevolution? Curr. Opin. Plant Biol. 2014, 18, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davison, E.M. Resolving confusions about jarrah dieback—don’t forget the plants. Australas. Plant Pathol. 2014, 43, 691–701. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.; Sakina, A.; Wani, S.H.; Shikari, A.B.; Tripathi, P.; Zaid, A.; Galla, A.; Abdelrahman, M.; Sharma, M.; Singh, A.K.; et al. Harnessing Genome Editing Techniques to Engineer Disease Resistance in Plants. Front. Plant Sci. 2019, 10, 550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Yang, F.; Zhang, J.; Liu, H.; Rahman, S.; Islam, S.; Ma, W.; She, M. Application of CRISPR/Cas9 in Crop quality improvement. Int. J. Mol. Sci. 2021, 22, 4206. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.H. Inheritance of reaction to rust in flax. J. Agric. Res. 1947, 74, 241–262. [Google Scholar]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Bourras, S.; McNally, K.E.; Müller, M.C.; Wicker, T.; Keller, B. Avirulence Genes in Cereal Powdery Mildews: The Gene-for-Gene Hypothesis 2.0. Front. Plant Sci. 2016, 7, 241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neik, T.X.; Ghanbarnia, K.; Ollivier, B.; Scheben, A.; Severn-Ellis, A.; Larkan, N.J.; Haddadi, P.; Fernando, W.G.D.; Rouxel, T.; Batley, J.; et al. Two independent approaches converge to the cloning of a new Leptosphaeria maculans avirulence effector gene, AvrLmS-Lep2. Mol. Plant Pathol. 2022. Early view. [Google Scholar]
- Haddadi, P.; Larkan, N.J.; Van de Wouw, A.; Zhang, Y.; Neik, T.X.; Beynon, E.; Bayer, P.; Edwards, D.; Batley, J.; Borhan, M.H. Brassica napus genes Rlm4 and Rlm7, conferring resistance to Leptosphaeria maculans, are alleles of the Rlm9 wall-associated kinase-like resistance locus. bioRxiv 2021, 12, 471845. [Google Scholar] [CrossRef]
- Balint-Kurti, P. The plant hypersensitive response: Concepts, control and consequences. Mol. Plant Pathol. 2019, 20, 1163–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guidetti-Gonzalez, S.; Freitas-Astúa, J.; Morais do Amaral, A.; Martins, N.F.; Mehta, A.; Silva, M.S.; Carrer, H. Genes associated with hypersensitive response (HR) in the citrus EST database (CitEST). Genet. Mol. Biol. 2007, 30, 943–956. [Google Scholar] [CrossRef] [Green Version]
- Dropkin, V.H. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: Reversal by temperature. Phytopathology 1969, 59, 1632–1637. [Google Scholar]
- Rossi, M.; Goggin, F.L.; Milligan, S.B.; Kaloshian, I.; Ullman, D.E.; Williamson, V.M. The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc. Natl. Acad. Sci. USA 1998, 95, 9750–9754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Münch, S.; Lingner, U.; Floss, D.S.; Ludwig, N.; Sauer, N.; Deising, H.B. The hemibiotrophic lifestyle of Colletotrichum species. J. Plant Physiol. 2008, 165, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Jupe, J.; Stam, R.; Howden, A.J.M.; Morris, J.A.; Zhang, R.; Hedley, P.E.; Huitema, E. Phytophthora capsici-tomato interaction features dramatic shifts in gene expression associated with a hemi-biotrophic lifestyle. Genome Biol. 2013, 14, R63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selin, C.; de Kievit, T.R.; Belmonte, M.F.; Fernando, W.G.D. Elucidating the Role of Effectors in Plant-Fungal Interactions: Progress and Challenges. Front. Microbiol. 2016, 7, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijo, Y.; Loo, E.P.; Yasuda, S. Pattern recognition receptors and signaling in plant–microbe interactions. Plant J. 2018, 93, 592–613. [Google Scholar] [CrossRef] [PubMed]
- Varden, F.A.; De la Concepcion, J.C.; Maidment, J.H.; Banfield, M.J. Taking the stage: Effectors in the spotlight. Curr. Opin. Plant Biol. 2017, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Naveed, Z.A.; Wei, X.; Chen, J.; Mubeen, H.; Ali, G.S. The PTI to ETI Continuum in Phytophthora-Plant Interactions. Front. Plant Sci. 2020, 11, 593905. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Vallet, A.; Saleem-Batcha, R.; Kombrink, A.; Hansen, G.; Valkenburg, D.J.; Thomma, B.P.; Mesters, J.R. Fungal effector Ecp6 outcompetes host immune receptor for chitin binding through intrachain LysM dimerization. Elife 2013, 2, e00790. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Wang, Y.; McDowell, J. Focus on effector-triggered susceptibility. Mol. Plant Microbe Interact. 2018, 31, 5. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunityby cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [PubMed]
- Yuan, Y.; Bayer, P.E.; Batley, J.; Edwards, D. Current status of structural variation studies in plants. Plant Biotechnol. J. 2021, 19, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Tena, G. PTI and ETI are one. Nat. Plants 2021, 7, 1527. [Google Scholar] [CrossRef] [PubMed]
- Sahu, P.P.; Puranik, S.; Khan, M.; Prasad, M. Recent advances in tomato functional genomics: Utilization of VIGS. Protoplasma 2012, 249, 1017–1027. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Brambilla, A.; Sommer, A.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2020, 229, 1234–1250. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kaiyomo, E.; Kumar, D.; Mosher, S.L.; Klessig, D.F. Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 2007, 318, 113–116. [Google Scholar] [CrossRef]
- Backer, R.; Naidoo, S.; van den Berg, N. The nonexpressor of pathogenesis-related genes 1 (NPR1) and related family: Mechanistic insights in plant disease resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slaughter, A.; Daniel, X.; Flors, V.; Luna, E.; Hohn, B.; Mauch-Mani, B. Descendants of primed Arabidopsis plants exhibit resistance to biotic stress. Plant Physiol. 2012, 158, 835–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, E.; Bruce, T.J.; Roberts, M.R.; Flors, V.; Ton, J. Next-generation systemic acquired resistance. Plant Physiol. 2012, 158, 844–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villena, J.; Kitazawa, H.; Van Wees, S.C.M.; Pieterse, C.M.J.; Takahashi, H. Receptors and signaling pathways for recognition of bacteria in livestock and crops: Prospects for beneficial microbes in healthy growth strategies. Front. Immunol. 2018, 9, 2223. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne signals from Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi, R.; Ryu, C.M. Sniffing bacterial volatile compounds for healthier plants. Curr. Opin. Plant Biol. 2018, 44, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Mulla, S.I.; Lee, K.J.; Chae, J.C.; Shukla, P. VOCs-mediated hormonal signaling and crosstalk with plant growth promoting microbes. Crit. Rev. Biotechnol. 2018, 38, 1277–1296. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Rossi, M.J.; Glick, B.R. Ethylene and 1-aminocyclopropane-1-carboxylate (ACC) in plant–bacterial interactions. Front. Plant Sci. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M.J. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J. 2018, 93, 166–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barakat, I.; Chtaina, N.; Grappin, P.; El, G.M.; Ezzahiri, B.; Aligon, A.; Neveu, M.; Marchi, M. Induced Systemic Resistance (ISR) in Arabidopsis thaliana by Bacillus amyloliquefaciens and Trichoderma harzianum Used as Seed Treatments. Agriculture 2019, 9, 166. [Google Scholar]
- Steinbrenner, A.D.; Goritschnig, S.; Staskawicz, B.J. Recognition and activation domains contribute to allele-specific responses of an Arabidopsis NLR receptor to an oomycete effector protein. PLOS Pathog. 2015, 11, e1004665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangl, J.L.; Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Chitrakar, R.; Obulareddy, N.; Panchal, S.; Williams, P.; Melotto, M. Molecular battles between plant and pathogenic bacteria in the phyllosphere. Braz. J. Med. Biol. Res. 2010, 43, 698–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Hoorn, R.A.; Kamoun, S. From guard to decoy: A new model for perception of plant pathogen effectors. Plant Cell 2008, 20, 2009–2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escaramis, G.; Docampo, E.; Rabionet, R. A decade of structural variants: Description, history and methods to detect structural variation. Brief. Funct. Genom. 2015, 14, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Mirlohi, S.; Li, X.; He, Y. Identification of functional single-nucleotide polymorphisms affecting leaf hair number in Brassica Rapa. Plant Physiol. 2018, 177, 490–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levinson, G. Rethinking Evolution: The Revolution That’s Hiding in Plain Sight; World Scientific: London, UK, 2020. ISBN 9781786347268.
- Bennetzen, J.L.; Wang, H. The contributions of transposable elements to the structure, function, and evolution of plant genomes. Annu. Rev. Plant Biol. 2014, 65, 505–530. [Google Scholar] [CrossRef] [PubMed]
- Frantzeskakis, L.; Pietro, A.D.; Rep, M.; Schirawski, J.; Wu, C.H.; Panstruga, R. Rapid evolution in plant-microbe interactions-a molecular genomics perspective. New Phytol. 2020, 225, 1134–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.M.; Stenson, P.D.; Cooper, D.N.; Ferec, C. A systematic analysis of LINE-1 endonuclease-dependent retrotranspositional events causing human genetic disease. Hum. Genet. 2005, 117, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Pritham, E.J.; Putliwala, T.; Feschotte, C. Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene 2007, 390, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Mat Razali, N.; Cheah, B.H.; Nadarajah, K. Transposable elements adaptive role in genome plasticity, pathogenicity and evolution in fungal phytopathogens. Int. J. Mol. Sci. 2019, 20, 3597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, E.; Jia, Y.; Singh, P.; Correll, J.C.; Lee, F.N. Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet. Biol. 2007, 44, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Saunders, D.G.; Mitsuoka, C.; Natsume, S.; Kosugi, S.; Saitoh, H.; Inoue, Y.; Chuma, I.; Tosa, Y.; Cano, L.M. Host specialization of the blast fungus Magnaporthe oryzae is associated with dynamic gain and loss of genes linked to transposable elements. BMC Genom. 2016, 17, 370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandaubert, J.; Lowe, R.G.; Soyer, J.L.; Schoch, C.L.; Van de Wouw, A.P.; Fudal, I.; Robbertse, B.; Lapalu, N.; Links, M.G.; Ollivier, B.; et al. Transposable element-assisted evolution and adaptation to host plant within the Leptosphaeria maculans-Leptosphaeria biglobosa species complex of fungal pathogens. BMC Genom. 2014, 15, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galazka, J.M.; Freitag, M. Variability of chromosome structure in pathogenic fungi of ‘ends and odds’. Curr. Opin. Microbiol. 2014, 20, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faino, L.; Seidl, M.F.; Shi-Kunne, X.; Pauper, M.; Van Den Berg, G.C.M.; Wittenberg, A.H.J.; Thomma, B.P.H.J. Transposons passively and actively contribute to evolution of the two-speed genome of a fungal pathogen. Genome Res. 2016, 26, 1091–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soyer, J.L.; El Ghalid, M.; Glaser, N.; Ollivier, B.; Linglin, J.; Grandaubert, J.; Balesdent, M.-H.; Connolly, L.R.; Freitag, M.; Rouxel, T.; et al. Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus Leptosphaeria maculans. PLoS Genet. 2014, 10, e1004227. [Google Scholar] [CrossRef] [PubMed]
- Fontanillas, E.; Hood, M.E.; Badouin, H.; Petit, E.; Barbe, V.; Gouzy, J.; de Vienne, D.M.; Aguileta, G.; Poulain, J.; Wincker, P.; et al. Degeneration of the non-recombining regions in the mating-type chromosomes of the anther-smut fungi. Mol. Biol. Evol. 2014, 32, 928–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouxel, T.; Grandaubert, J.; Hane, J.K.; Hoede, C.; Van de Wouw, A.P.; Couloux, A.; Dominguez, V.; Anthouard, V.; Bally, P.; Bourras, S.; et al. Effector diversification within compartments of the Leptosphaeria maculans genome affected by Repeat-Induced Point mutations. Nat. Commun. 2011, 2, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Van Der Does, H.C.; Borkovich, K.A.; Coleman, J.J.; Daboussi, M.J.; Di Pietro, A.; Dufresne, M.; Freitag, M.; Grabherr, M.; Henrissat, B.; et al. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 2010, 464, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Chuma, I.; Isobe, C.; Hotta, Y.; Ibaragi, K.; Futamata, N.; Kusaba, M.; Yoshida, K.; Terauchi, R.; Fujita, Y.; Nakayashiki, H.; et al. Multiple translocations of the AVR-Pita effector gene among chromosomes of the rice blast fungus Magnaporthe oryzae and related species. PLOS Pathog. 2011, 7, e1002147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, J.; Chen, M.; Zhong, Z.; Tang, W.; Lin, L.; Zhang, X.; Jiang, H.; Zhang, D.; Miao, C.; Tang, H. Pacbio sequencing reveals transposable elements as a key contributor to genomic plasticity and virulence variation in Magnaporthe oryzae. Molecular. Plant 2017, 10, 1465–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, M.F.; Silva, J.C.; Batista, A.D.; Ribeiro, L.E.; da Silva, G.F.; de Araújo, E.F.; de Queiroz, M.V. Abundance, distribution and potential impact of transposable elements in the genome of Mycosphaerella fijiensis. BMC Genom. 2012, 13, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillon, B.; Gill, N.; Hamelin, R.C.; Goodwin, S.B. The landscape of transposable elements in the finished genome of the fungal wheat pathogen Mycosphaerella graminicola. BMC Genom. 2014, 15, 1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Wyk, S.; Wingfield, B.D.; De Vos, L.; van der Merwe, N.A.; Steenkamp, E.T. Genome-wide analyses of Repeat-Induced Point mutations in the Ascomycota. Front. Microbiol. 2021, 11, 622368. [Google Scholar] [CrossRef] [PubMed]
- Selker, E.U. Premeiotic instability of repeated sequences in Neurospora crassa. Annu. Rev. Genet. 1990, 24, 579–613. [Google Scholar] [CrossRef] [PubMed]
- Cambareri, E.B.; Jensen, B.C.; Schabtach, E.; Selker, E.U. Repeat-induced G-C to A-T mutations in Neurospora. Science 1989, 244, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Neuveglise, C.; Sarfati, J.; Latge, J.P.; Paris, S. Afut1, a retrotransposon-like element from Aspergillus fumigatus. Nucleic Acids Res. 1996, 24, 1428–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.L.; Hermansen, T.D.; Aleksenko, A. A family of DNA repeats in Aspergillus nidulans has assimilated degenerated retrotransposons. Mol. Genet. Genom. 2001, 265, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Hua-van, A.; Héricourt, F.; Capy, P.; Daboussi, M.J.; Langin, T. Three highly divergent subfamilies of the impala transposable element coexist in the genome of the fungus Fusarium oxysporum. Mol. Genet. 1998, 259, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Nakayashiki, H.; Nishimoto, N.; Ikeda, K.; Tosa, Y.; Mayama, S. Degenerate MAGGY elements in a subgroup of Pyricularia grisea: A possible example of successful capture of a genetic invader by a fungal genome. Mol. Genet. 1999, 261, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Rouxel T and Balesdent M H The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Mol. Plant Pathol. 2005, 6, 225–241. [CrossRef] [PubMed]
- Ikeda, K.; Nakayashiki, H.; Kataoka, T.; Tamba, H.; Hashimoto, Y.; Tosa, Y.; Mayama, S. Repeat-induced point mutation (RIP) in Magnaporthe grisea: Implications for its sexual cycle in the natural field context. Mol. Microbiol. 2002, 45, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Williams, A.H.; Taranto, A.P.; Solomon, P.S.; Oliver, R.P. Repeat-Induced Point Mutation: A Fungal-Specific, Endogenous Mutagenesis Process. In Genetic Transformation Systems in Fungi; Van den Berg, M.A., Maruthachalam, K., Eds.; Springer: Cham, Switzerland, 2015; Volume 2. [Google Scholar]
- Testa, A.C.; Oliver, R.P.; Hane, J.K. Occulter Cut: A comprehensive survey of AT-Rich regions in fungal genomes. Genome Biol. Evol. 2016, 8, 2044–2064. [Google Scholar] [CrossRef] [Green Version]
- Rajewska, M.; Wegrzyn, K.; Konieczny, I. AT-rich region and repeated sequences-the essential elements of replication origins of bacterial replicons, FEMS Microbiol. Rev. 2012, 36, 408–434. [Google Scholar]
- De Wit, P.J.; Van Der Burgt, A.; Ökmen, B.; Stergiopoulos, I.; Abd-Elsalam, K.A.; Aerts, A.L.; Bahkali, A.H.; Beenen, H.G.; Chettri, P.; Cox, M.P.; et al. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 2012, 8, e1003088. [Google Scholar] [CrossRef]
- Clutterbuck, A.J. Genomic evidence of repeat-induced point mutation (RIP) in filamentous ascomycetes. Fungal Genet. Biol. 2011, 48, 306–326. [Google Scholar] [CrossRef] [PubMed]
- Schardl, C.L.; Young, C.A.; Hesse, U.; Amyotte, S.G.; Andreeva, K.; Calie, P.J.; Fleetwood, D.J.; Haws, D.C.; Moore, N.; Oeser, B.; et al. Plant-symbiotic fungi as chemical engineers: Multi-genome analysis of the Clavicipitaceae reveals dynamics of alkaloid loci. PLoS Genet. 2013, 9, e1003323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, A.C.; Hane, J.K.; Ellwood, S.R.; Oliver, R.P. Coding Quarry: Highly accurate hidden Markov model gene prediction in fungal genomes using RNA-seq transcripts. BMC Genom. 2015, 16, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fudal, I.; Ross, S.; Brun, H.; Besnard, A.L.; Ermel, M.; Kuhn, M.L.; Balesdent, M.H.; Rouxel, T. Repeat-induced point mutation (RIP) as an alternative mechanism of evolution toward virulence in Leptosphaeria maculans. Mol. Plant Pathol. 2009, 22, 932–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broggini, G.A.L. Identification of Apple Scab Avirulence Gene AvrVg Candidates. Ph.D. Thesis, University of Zurich, Zürich, Switzerland, 2007; 112p. [Google Scholar]
- Mousavi-Derazmahalleh, M.; Chang, S.; Thomas, G.; Derbyshire, M.; Bayer, P.E.; Edwards, D.; Nelson, M.N.; Erskine, W.; Lopez-Ruiz, F.J.; Clements, J.; et al. Prediction of pathogenicity genes involved in adaptation to a lupin host in the fungal pathogens Botrytis cinerea and Sclerotinia sclerotiorum via comparative genomics. BMC Genom. 2019, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- Dal Molin, A.; Minio, A.; Griggio, F.; Delledonne, M.; Infantino, A.; Aragona, M. The genome assembly of the fungal pathogen Pyrenochaeta lycopersici from Single-Molecule Real-Time sequencing sheds new light on its biological complexity. PLoS ONE 2018, 13, e0200217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, A.S.; Wendel, J.F. Homoeologous exchanges, segmental allopolyploidy, and polyploid genome evolution. Front. Genet. 2020, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Coriton, O.; Rousseau-Gueutin, M.; Samans, B.; Schiessl, S.V.; Obermeier, C.; Parkin, I.A.; Chèvre, A.M.; Snowdon, R.J. Mapping of homoeologous chromosome exchanges influencing quantitative trait variation in Brassica napus. Plant Biotechnol. J. 2017, 15, 1478–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurgobin, B.; Golicz, A.A.; Bayer, P.E.; Chan, C.K.K.; Tirnaz, S.; Dolatabadian, A.; Schiessl, S.V.; Samans, B.; Montenegro, J.D.; Parkin, I.A.P.; et al. Homoeologous exchange is a major cause of gene presence/absence variation in the amphidiploid Brassica napus. Plant Biotechnol. J. 2018, 16, 1265–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhanga Goua, X.; Xuna, H.; Biana, Y.; Maa, X.; Lia, J.; Lia, N.; Gonga, L.; Feldmanb, M.; Liua, B.; Levyb, A.A. Homoeologous exchanges occur through intragenic recombination generating novel transcripts and proteins in wheat and other polyploids. Proc. Natl. Acad. Sci. USA 2020, 117, 14561–14571. [Google Scholar] [CrossRef]
- Chalhoub, B.; Denoeud, F.; Liu, S.; Parkin, I.A.; Tang, H.; Wang, X.; Chiquet, J.; Belcram, H.; Tong, C.; Samans, B.; et al. Early allopolyploid evolution in the post-Neolithic Brassica napus oilseed genome. Science 2014, 345, 950–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langner, T.; Harant, A.; Gomez-Luciano, L.B.; Shrestha, R.K.; Malmgren, A.; Latorre, S.M.; Burbano, H.A.; Win, J.; Kamoun, S. Genomic rearrangements generate hypervariable mini-chromosomes in host-specific isolates of the blast fungus. PLoS Genet. 2021, 17, e1009386. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Viegas, W.; Houben, A. A Century of B Chromosomes in Plants: So What? Ann. Bot. 2008, 101, 767–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Möller, M.; Stukenbrock, E.H. Evolution and genome architecture in fungal plant pathogens. Nat. Rev. Microbiol. 2017, 15, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Jin, J.; Nifong, J.M.; Shew, D.; Lewis, R.S. Homoeologous chromosome exchange explains the creation of a QTL affecting soil-borne pathogen resistance in tobacco. Plant Biotechnol. J. 2021, 20, 47–58. [Google Scholar] [CrossRef]
- Zhao, J.; Udall, J.A.; Quijada, P.A.; Grau, C.R.; Meng, J.; Osborn, T.C. Quantitative trait loci for resistance to Sclerotinia sclerotiorum and its association with a homeologous non-reciprocal transposition in Brassica napus L. Theor. Appl. Genet. 2006, 112, 509–516. [Google Scholar] [CrossRef]
- Gabur, I.; Chawla, H.S.; Lopisso, D.T.; von Tiedemann, A.; Snowdon, R.J.; Obermeier, C. Gene presence-absence variation associates with quantitative Verticillium longisporum disease resistance in Brassica napus. Sci. Rep. 2020, 10, 4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.M.; Guan, Z.; Hu, J.; Guo, C.; Yang, Z.; Wang, S.; Liu, D.; Wang, B.; Lu, S.; Zhou, R.; et al. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nat. Plants 2020, 6, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chawla, H.S.; Lee, H.; Gabur, I.; Vollrath, P.; Tamilselvan-Nattar-Amutha, S.; Obermeier, C.; Schiessl, S.V.; Song, J.M.; Liu, K.; Guo, L.; et al. Long-read sequencing reveals widespread intragenic structural variants in a recent allopolyploid crop plant. Plant Biotechnol. J. 2021, 19, 240–250. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.A.; Mundt, C.C. How knowledge of pathogen population biology informs management of Septoria Tritici blotch. Phytopathology 2016, 106, 948–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stukenbrock, E.H. The role of hybridization in the evolution and emergence of new fungal plant pathogens. Phytopathology 2016, 106, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.R.; McDowell, J.M.; Sharpe, A.G.; Zabala, M.D.T.; Lydiate, D.J.; Dangl, J.L. Independent deletions of a pathogen-resistance gene in Brassica and Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 15843–15848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henk, A.D.; Warren, R.F.; Innes, R.W. A new Ac-like transposon of Arabidopsis is associated with a deletion of the RPS5 disease resistance gene. Genetics 1999, 151, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Brunner, S.; Pea, G.; Fengler, K.; Zuccolo, A.; Rafalski, A. Gene duplication and exon shuffling by helitron-like transposons generate intraspecies diversity in maize. Nat. Genet. 2005, 37, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Araki, H.; Chen, L.; Chen, J.Q.; Tian, D. Unique evolutionary mechanism in R-genes under the presence/absence polymorphism in Arabidopsis thaliana. Genetics 2006, 172, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Araki, H.; Wang, Q.; Zhang, P.; Yang, S.; Chen, J.Q.; Tian, D. Highly asymmetric rice genomes. BMC Genom. 2007, 8, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, E.; Borm, T.; Prins, P.; van der Vossen, E.; Uenk, G.; Arens, M.; de Boer, J.; van Eck, H.; Muskens, M.; Vossen, J.; et al. A genome-wide genetic map of NB-LRR disease resistance loci in potato. Theor. Appl. Genet. 2011, 123, 493–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffaele, S.; Farrer, R.A.; Cano, L.M.; Studholme, D.J.; MacLean, D.; Thines, M.; Jiang, R.H.; Zody, M.C.; Kunjeti, S.G.; Donofrio, N.M.; et al. Genome evolution following host jumps in the Irish potato famine pathogen lineage. Science 2010, 330, 1540–1543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Garcia, E.O.; Lin, G.; Hu, Y.; Dalby, M.; Migeon, P.; Tang, H.; Farman, M.; Cook, D.; White, F.F.; et al. Effector gene reshuffling involves dispensable mini chromosomes in the wheat blast fungus. PLoS Genet. 2019, 15, e1008272. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, F.E.; de la Vega, R.C.R.; Brandenburg, J.T.; Carpentier, F.; Giraud, T. Gene Presence–Absence Polymorphism in Castrating Anther-Smut Fungi: Recent Gene Gains and Phylogeographic Structure. Genome Biol. Evol. 2018, 10, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.L.; Burdon, J.J.; Dodds, P.N.; Thrall, P.H. Spatial variation in disease resistance: From molecules to metapopulations. J. Ecol. 2011, 991, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolatabadian, A.; Patel, D.A.; Edwards, D.; Batley, J. Copy number variation and disease resistance in plants. Theor. Appl. Genet. 2017, 130, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Feuk, L.; Marshall, C.R.; Wintle, R.F.; Scherer, S.W. Structural variants: Changing the landscape of chromosomes and design of disease studies. Hum. Mol. Genet. 2006, 15, 57–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katju, V.; Bergthorsson, U. Copy-number changes in evolution: Rates, fitness effects and adaptive significance. Front. Genet. 2013, 4, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pös, O.; Radvanszky, J.; Buglyó, G.; Pös, Z.; Rusnakova, D.; Nagy, B.; Szemes, T. Copy number variation: Characteristics, evolutionary and pathological aspects. Biomed. J. 2021, 44, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Żmieńko, A.; Samelak, A.; Kozłowski, P.; Figlerowicz, M. Copy number polymorphism in plant genomes. Theor. Appl. Genet. 2014, 127, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, E.G.; Toomajian, C.; Kreitman, M.; Bergelson, J. A genome-wide survey of R gene polymorphisms in Arabidopsis. Plant Cell 2006, 18, 1803–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Liu, X.; Ge, S.; Jensen, J.D.; Hu, F.; Li, X.; Dong, Y.; Gutenkunst, R.N.; Fang, L.; Huang, L.; et al. Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat. Biotechnol. 2012, 30, 105–111. [Google Scholar] [CrossRef]
- Cook, D.E.; Lee, T.G.; Guo, X.; Melito, S.; Wang, K.; Bayless, A.M.; Wang, J.; Hughes, T.J.; Willis, D.K.; Clemente, T.E.; et al. Copy number variation of multiple genes at Rhg1 mediates nematode resistance in soybean. Science 2012, 338, 1206–1209. [Google Scholar] [CrossRef] [Green Version]
- González, V.M.; Aventín, N.; Centeno, E.; Puigdomènech, P. High presence/absence gene variability in defense-related gene clusters of Cucumis melo. BMC Genom. 2013, 14, 782. [Google Scholar] [CrossRef] [Green Version]
- Golicz, A.A.; Batley, J.; Edwards, D. Towards plant pangenomics. Plant Biotechnol. J. 2016, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Qutob, D.; Tedman-Jones, J.; Dong, S.; Kuflu, K.; Pham, H.; Wang, Y.; Do, D.; Kale, S.D.; Arredondo, F.D.; Tyler, B.M.; et al. Copy number variation and transcriptional polymorphisms of Phytophthora sojae RXLR effector genes Avr1a and Avr3a. PLoS ONE 2009, 4, e5066. [Google Scholar] [CrossRef]
- Guo, Y.L.; Fitz, J.; Schneeberger, K.; Ossowski, S.; Cao, J.; Weigel, D. Genome-wide comparison of nucleotide-binding site-leucine-rich repeat-encoding genes in Arabidopsis. Plant Physiol. 2011, 157, 757–769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ding, J.; Zhang, W.; Zhang, Y.; Tang, P.; Chen, J.Q.; Tian, D.; Yang, S. Unique evolutionary pattern of numbers of gramineous NBS-LRR genes. Mol. Genet. Genom. 2010, 283, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Riaz, S.; Morales-Cruz, A.; Amrine, K.C.; McGuire, B.; Gubler, W.D.; Walker, M.A.; Cantu, D. Adaptive genomic structural variation in the grape powdery mildew pathogen, Erysiphe necator. BMC Genom. 2014, 15, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oreiro, E.G.; Grimares, E.K.; Atienza-Grande, G.; Quibod, I.L.; Roman-Reyna, V.; Oliva, R. Genome-wide associations and transcriptional profiling reveal ROS regulation as one underlying mechanism of sheath blight resistance in rice. Mol. Plant Microbe Interact. 2020, 33, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Kankanala, P.; Nandety, R.S.; Mysore, K.S. Genomics of plant disease resistance in legumes. Front. Plant Sci. 2019, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- Glaubitz, J.C.; Casstevens, T.M.; Lu, F.; Harriman, J.; Elshire, R.J.; Sun, Q.; Buckler, E.S. TASSEL-GBS: A high-capacity genotyping by sequencing analysis pipeline. PLoS ONE 2014, 9, e90346. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.W.; Blaxter, M.L. RADSeq: Next-generation population genetics. Brief. Funct. Genom. 2010, 9, 416–423. [Google Scholar] [CrossRef]
- Perseguini, J.M.; Oblessuc, P.R.; Rosa, J.R.; Gomes, K.A.; Chiorato, A.F.; Carbonell, S.A.; Garcia, A.A.; Vianello, R.P.; Benchimol-Reis, L.L. Genome-wide association studies of anthracnose and angular leaf spot resistance in common bean (Phaseolus vulgaris L.). PLoS ONE 2016, 11, e0150506. [Google Scholar] [CrossRef] [Green Version]
- Desgroux, A.; L’anthoëne, V.; Roux-Duparque, M.; Rivière, J.P.; Aubert, G.; Tayeh, N.; Moussart, A.; Mangin, P.; Vetel, P.; Piriou, C.; et al. Genome-wide association mapping of partial resistance to Aphanomyces euteiches in pea. BMC Genom. 2016, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, M.; André, O.; Badis, Y.; Ronfort, J.; Burgarella, C.; Chantret, N.; Prosperi, J.M.; Briskine, R.; Mudge, J.; Debéllé, F.; et al. High-density genome-wide association mapping implicates an F-box encoding gene in Medicago truncatula resistance to Aphanomyces euteiches. New Phytol. 2014, 201, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Barilli, E.; Cobos, M.J.; Carrillo, E.; Kilian, A.; Carling, J.; Rubiales, D. A high-density integrated DArTseq SNP-Based genetic map of Pisum fulvum and identification of QTLs controlling rust resistance. Front. Plant Sci. 2018, 9, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Yu, L.X.; McCord, P.; Miller, D.; Bhamidimarri, S.; Johnson, D.; Monteros, M.J.; Ho, J.; Reisen, P.; Samac, D.A. Identification of molecular markers associated with Verticillium wilt resistance in alfalfa (Medicago sativa L.) using high-resolution melting. PLoS ONE 2014, 9, e115953. [Google Scholar] [CrossRef] [PubMed]
- Dakouri, A.; Lamara, M.; Karim, M.; Wang, J.; Chen, Q.; Gossen, B.D.; Strelkov, S.E.; Hwang, S.F.; Peng, G.; Yu, F. Identification of resistance loci against new pathotypes of Plasmodiophora brassicae in Brassica napus based on genome-wide association mapping. Sci. Rep. 2021, 11, 6599. [Google Scholar] [CrossRef] [PubMed]
- Kifuji, Y.; Hanzawa, H.; Terasawa, Y.; Ashutosh, S.; Nishio, T. QTL analysis of black rot resistance in cabbage using newly developed EST-SNP markers. Euphytica 2013, 190, 289–295. [Google Scholar] [CrossRef]
- Sharma, B.B.; Pritam, K.; Kumar, Y.D.; Dinesh, S.; Raj, S.T. Genetics and molecular mapping of black rot resistance locus Xca1bc on chromosome B–7 in Ethiopian mustard (Brassica carinata Braun). PLoS ONE 2016, 11, e0152290. [Google Scholar] [CrossRef] [PubMed]
- Rey, T.; Bonhomme, M.; Chatterjee, A.; Gavrin, A.; Toulotte, J.; Yang, W.; André, O.; Jacquet, C.; Schornack, S. The Medicago truncatula GRAS protein RAD1 supports arbuscular mycorrhiza symbiosis and Phytophthora palmivora susceptibility. J. Exp. Bot. 2017, 68, 5871–5881. [Google Scholar] [CrossRef] [Green Version]
- Rieger, R.; Michaelis, A.; Green, M.M. A Glossary of Genetics and Cytogenetics: Classical and Molecular; Springer: New York, NY, USA, 1968. [Google Scholar]
- Mun, J.H.; Kwon, S.J.; Seol, Y.J.; Kim, J.A.; Jin, M.; Kim, J.S.; Lim, M.H.; Lee, S.I.; Hong, J.K.; Park, T.H.; et al. Sequence and structure of Brassica rapa chromosome A3. Genome Biol. 2010, 11, R94. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.K.; Tsuchiya, T. Chromosome Engineering in Plants: Genetics, Breeding, Evolution; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1991; pp. 1–630. [Google Scholar]
- Atkinson, N.S.; Hopper, A.K. Chromosome specificity of polysomy promotion by disruptions of the Saccharomyces cerevisiae RNA1 gene. Genetics 1987, 116, 371–375. [Google Scholar] [CrossRef]
- Fierro, F.; Martin, J.F. Molecular mechanisms of chromosomal rearrangement in fungi. Crit. Rev. Microbiol. 1999, 25, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Davière, J.M.; Langin, T.; Daboussi, M.J. Potential role of transposable elements in the rapid reorganization of the Fusarium oxysporum genome. Fungal Genet. Biol. 2001, 34, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Yasunori Akagi, M.T.; Mikihiro, Y.; Takashi, T.; Yukitaka, F.N.; Hiroshi, O.; Motoichiro, K. Chromosome constitution of hybrid strains constructed by protoplast fusion between the tomato and strawberry pathotypes of Alternaria alternata. J Gen. Plant Pathol. 2009, 75, 101–109. [Google Scholar] [CrossRef]
- Hatta, R.; Ito, K.; Hosaki, Y.; Tanaka, T.; Tanaka, A.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. A conditionally dispensable chromosome controls host-specific pathogenicity in the fungal plant pathogen Alternaria alternata. Genetics 2002, 161, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Miao, V.P.; Covert, S.F.; Vanetten, H.D. A fungal gene for antibiotic-resistance on a dispensable (B) chromosome. Science 1991, 254, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Vlaardingerbroek, I.; Beerens, B.; Rose, L.; Fokkens, L.; Cornelissen, B.J.; Rep, M. Exchange of core chromosomes and horizontal transfer of lineage-specific chromosomes in Fusarium oxysporum. Environ. Microbiol. 2016, 18, 3702–3713. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, U.; Alonso-Lifante, M.P.; Barros, K.; Kovarik, A.; Mas de Xaxars, G.; Garcia, S. B-chrom: A database on B-chromosomes of plants, animals and fungi. New Phytol. 2017, 216, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masel, A.M.; He, C.Z.; Poplawski, A.M.; Irwin, J.A.G.; Manners, J.M. Molecular evidence for chromosome transfer between biotypes of Colletotrichum Gloeosporioides. Mol. Plant Microbe Interact. 1996, 9, 339–348. [Google Scholar] [CrossRef]
- Plaumann, P.L.; Schmidpeter, J.; Dahl, M.; Taher, L.; Koch, C. A Dispensable Chromosome Is Required for Virulence in the Hemibiotrophic Plant Pathogen Colletotrichum higginsianum. Front. Microbiol. 2018, 9, 1005. [Google Scholar] [CrossRef] [PubMed]
- Ayukawa, Y.; Asai, S.; Gan, P.; Tsushima, A.; Ichihashi, Y.; Shibata, A.; Komatsu, K.; Houterman, P.M.; Rep, M.; Shirasu, K.; et al. A pair of effectors encoded on a conditionally dispensable chromosome of Fusarium oxysporum suppress host-specific immunity. Commun. Biol. 2021, 4, 707. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, S.; Ueda, K.; Goto, T.; Yamamoto, M.; Nishimura, S.; Kohmoto, K. Structure of AF-toxin II, one of the host-specific toxins produced by Alternaria alternata strawberry pathotype. Tetrahedron Lett. 1986, 27, 2753–2756. [Google Scholar] [CrossRef]
- Nakashima, T.; Ueno, T.; Fukami, H.; Taga, T.; Masuda, H.; Osaki, K. Isolation and structures of AK-Toxin I and II, host-specific phytotoxic metabolites produced by Alternaria alternata Japanese pear pathotype. Agric. Biol. Chem. 1985, 49, 807–815. [Google Scholar] [CrossRef] [Green Version]
- Kohmoto, K.; Itoh, Y.; Shimomura, N.; Kondoh, Y.; Otani, H.; Kodama, M. Isolation and biological activities of 2 host-specific toxins from the tangerine pathotype of Alternaria alternata. Phytopathology 1993, 83, 495–502. [Google Scholar] [CrossRef]
- Johnson, L.J.; Johnson, R.D.; Akamatsu, H.; Salamiah, A.; Otani, H.; Kohmoto, K.; Kodama, M. Spontaneous loss of a conditionally dispensable chromosome from the Alternaria alternata apple pathotype leads to loss of toxin production and pathogenicity. Curr. Genet. 2001, 40, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.F.; Bornberg-Bauer, E. Fact or fiction: Updates on how protein-coding genes might emerge de novo from previously non-coding DNA. F1000Res 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.W.; Chen, X.; Wu, Q.; Hagmann, J.; Han, T.S.; Zou, Y.P.; Ge, S.; Guo, Y.L. On the origin of de novo genes in Arabidopsis thaliana populations. Genome Biol. Evol. 2016, 8, 2190–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLysaght, A.; Guerzoni, D. New genes from non-coding sequence: The role of de novo protein-coding genes in eukaryotic evolutionary innovation. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, J.; Zhao, R.; Jiang, H.; Wang, W. De novo origination of a new protein-coding gene in Saccharomyces cerevisiae. Genetics 2008, 179, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Moghe, G.; Ouyang, S.; Iezzoni, A.; Shiu, S.H.; Gu, X.; Buell, C.R. Comparative analyses reveal distinct sets of lineage-specific genes within Arabidopsis thaliana. BMC Evol. Biol. 2010, 10, 41. [Google Scholar] [CrossRef] [Green Version]
- Donoghue, M.T.; Keshavaiah, C.; Swamidatta, S.H.; Spillane, C. Evolutionary origins of Brassicaceae specific genes in Arabidopsis thaliana. BMC Evol. Biol. 2011, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.L. Gene family evolution in green plants with emphasis on the origination and evolution of Arabidopsis thaliana genes. Plant J. 2013, 73, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Hoen, D.R.; Bureau, T.E. Discovery of novel genes derived from transposable elements using integrative genomic analysis. Mol. Biol. Evol. 2015, 32, 1487–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Trachana, K.; Lercher, M.J.; Bork, P. Younger genes are less likely to be essential than older genes, and duplicates are less likely to be essential than singletons of the same age. Mol. Biol. Evol. 2012, 29, 1703–1706. [Google Scholar] [CrossRef] [Green Version]
- Luhua, S.; Hegie, A.; Suzuki, N.; Shulaev, E.; Luo, X.; Cenariu, D.; Ma, V.; Kao, S.; Lim, J.; Gunay, M.B.; et al. Linking genes of unknown function with abiotic stress responses by high-throughput phenotype screening. Physiol. Plant. 2013, 148, 322–333. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Güldener, U.; Xu, J.R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.J.; Baker, S.E.; Rep, M.; et al. The Fusarium graminearum genome reveals a link between localized polymorphism and pathogen specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Rajasingh, S.; Barani, B.; Samanta, S.; Dawn, B.; Wang, R.; Rajasingh, J. Therapy of Infectious Diseases Using Epigenetic Approaches. Epigenetics in Human Disease, 2nd ed.; Academic Press: London, UK, 2018; Chapter 22; Volume 6, pp. 689–715. [Google Scholar]
- Chang, Y.N.; Zhu, C.; Jiang, J.; Zhang, H.; Zhu, J.K.; Duan, C.G. Epigenetic regulation in plant abiotic stress responses. J. Integr. Plant Biol. 2020, 62, 563–580. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic mechanisms of plant adaptation to biotic and abiotic stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef]
- Dowen, R.H.; Pelizzola, M.; Schmitz, R.J.; Lister, R.; Dowen, J.M.; Nery, J.R.; Dixon, J.E.; Ecker, J.R. Widespread dynamic DNA methylation in response to biotic stress. Proc. Natl. Acad. Sci. USA 2012, 109, 2183–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, A.; Lepère, G.; Jay, F.; Wang, J.; Bapaume, L.; Wang, Y.; Abraham, A.L.; Penterman, J.; Fischer, R.L.; Voinnet, O.; et al. Dynamics and biological relevance of DNA demethylation in Arabidopsis antibacterial defense. Proc. Natl. Acad. Sci. USA 2013, 110, 2389–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambani, A.; Rice, J.H.; Liu, J.; Lane, T.; Ranjan, P.; Mazarei, M.; Pantalone, V.; Stewart, C.N., Jr.; Staton, M.; Hewezi, T. The methylome of soybean roots during the compatible interaction with the soybean cyst nematode. Plant Physiol. 2015, 168, 1364–1377. [Google Scholar] [CrossRef]
- Kellenberger, R.T.; Schlüter, P.M.; Schiestl, F.P. Herbivore-induced DNA demethylation changes floral signalling and attractiveness to pollinators in Brassica rapa. PLoS ONE 2016, 11, e0166646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López Sánchez, A.; Stassen, J.H.; Furci, L.; Smith, L.M.; Ton, J. The role of DNA (de)methylation in immune responsiveness of Arabidopsis. Plant J. 2016, 88, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Xu, W.; Zou, J.; Qiu, Y.; Kong, J.; Yang, Y.; Zhang, B.; Zhu, S. Epigenetic changes in the regulation of Nicotiana tabacum response to cucumber mosaic virus infection and symptom recovery through single-base resolution methylomes. Viruses 2018, 10, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, S.; Kong, X.; Song, G.; Jia, M.; Guan, J.; Wang, F.; Qin, Z.; Wu, L.; Lan, X.; Li, A.; et al. DNA methylation dynamics during the interaction of wheat progenitor Aegilops tauschii with the obligate biotrophic fungus Blumeria graminis f. sp. tritici. New Phytol. 2019, 221, 1023–1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Fan, M.; He, Y. DNA methylation analysis of the Citrullus lanatus response to cucumber green mottle mosaic virus infection by whole-genome bisulfite sequencing. Genes 2019, 10, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atighi, M.R.; Verstraeten, B.; De Meyer, T.; Kyndt, T. Genome-wide DNA hypomethylation shapes nematode pattern-triggered immunity in plants. New Phytol. 2020, 227, 545–558. [Google Scholar] [CrossRef]
- Annacondia, M.L.; Markovic, D.; Reig Valiente, J.L.; Scaltsoyiannes, V.; Pieterse, C.M.; Ninkovic, V.; Slotkin, R.K.; Martinez Arias, G. Aphid feeding induces the relaxation of epigenetic control and the associated regulation of the defense response in Arabidopsis. New Phytol. 2021, 230, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Pavet, V.; Quintero, C.; Cecchini, N.M.; Rosa, A.L.; Alvarez, M.E. Arabidopsis displays centromeric DNA hypomethylation and cytological alterations of heterochromatin upon attack by Pseudomonas syringae. Mol. Plant Microbe Interact. 2006, 19, 577–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agorio, A.; Vera, P. ARGONAUTE4 is required for resistance to Pseudomonas syringae in Arabidopsis. Plant Cell 2007, 19, 3778–3790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imhof, A.; Wolffe, A.P. Transcription: Gene control by targeted histone acetylation. Curr. Biol. 1998, 8, 422–424. [Google Scholar] [CrossRef] [Green Version]
- Zhi, P.; Chang, C. Exploiting Epigenetic Variations for Crop Disease Resistance Improvement. Front. Plant Sci. 2021, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- De-La-Peña, C.; Rangel-Cano, A.; Alvarez-Venegas, R. Regulation of disease-responsive genes mediated by epigenetic factors: Interaction of Arabidopsis-Pseudomonas. Mol. Plant Pathol. 2012, 13, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Fu, F.; Xu, S.; Lee, S.Y.; Yun, D.J.; Mengiste, T. Global regulation of plant immunity by histone lysine methyl transferases. Plant Cell 2016, 28, 1640–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.M.; Song, H.R.; Han, S.K.; Han, M.; Kim, C.Y.; Park, J.; Lee, Y.H.; Jeon, J.S.; Noh, Y.S.; Noh, B. HDA19 is required for the repression of salicylic acid biosynthesis and salicylic acid-mediated defense responses in Arabidopsis. Plant J. 2012, 71, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Latrasse, D.; Jégu, T.; Li, H.; de Zelicourt, A.; Raynaud, C.; Legras, S.; Gust, A.; Samajova, O.; Veluchamy, A.; Rayapuram, N.; et al. MAPK-triggered chromatin reprogramming by histone deacetylase in plant innate immunity. Genome Biol. 2017, 18, 131. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M.; Hirt, H. Plant immunity: From signaling to epigenetic control of defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhi, P.; Liu, J.; Li, H.; Zhang, X.; Xu, J.; Zhou, J.; Wang, X.; Chang, C. Epigenetic activation of Enoyl-CoA Reductase by an acetyltransferase complex triggers wheat wax biosynthesis. Plant Physiol. 2020, 183, 1250–1267. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiong, Y.; Lai, L.B.; Zhang, K.; Li, Z.; Kang, H.; Dai, L.; Gopalan, V.; Wang, G.L.; Liu, W. The rice RNase P protein subunit Rpp30 confers broad-spectrum resistance to fungal and bacterial pathogens. Plant Biotechnol. J. 2021, 19, 1988. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dai, Y.; Cui, S.; Ma, L. Histone H2B monoubiquitination in the chromatin of FLOWERING LOCUS C regulates flowering time in Arabidopsis. Plant Cell 2008, 20, 2586–2602. [Google Scholar] [CrossRef] [Green Version]
- Zou, B.; Yang, D.L.; Shi, Z.; Dong, H.; Hua, J. Monoubiquitination of histone 2B at the disease resistance gene locus regulates its expression and impacts immune responses in Arabidopsis. Plant Physiol. 2014, 165, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Quispe-Huamanquispe, D.G.; Gheysen, G.; Kreuze, J.F. Horizontal Gene Transfer Contributes to Plant Evolution: The Case of Agrobacterium T-DNAs. Front. Plant Sci. 2017, 8, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.; Rivera, M.C.; Moore, J.E.; Lake, J.A. Horizontal gene transfer accelerates genome innovation and evolution. Mol. Biol. Evol. 2003, 20, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.A.; Leonard, G.; Soanes, D.M.; Talbot, N.J. Gene transfer into the fungi. Fungal Biol. Rev. 2011, 25, 98–110. [Google Scholar] [CrossRef]
- Soanes, D.; Richards, T.A. Horizontal gene transfer in eukaryotic plant pathogens. Annu. Rev. Phytopathol. 2014, 52, 583–614. [Google Scholar] [CrossRef] [PubMed]
- Van der Does, H.C.; Rep, M. Virulence genes and the evolution of host specificity in plant-pathogenic fungi. Mol. Plant-Microbe Interact. 2007, 20, 1175–1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Cai, G.; Luo, J.; Bhattacharya, D.; Zhang, N. Extensive horizontal gene transfers between plant pathogenic fungi. BMC Biol. 2016, 14, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, Z.; Zhu, B.; Feng, H.; Huang, L. Horizontal gene transfer drives adaptive colonization of apple trees by the fungal pathogen Valsa mali. Sci. Rep. 2016, 6, 33129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Rusu, A.G.; Poplawski, A.M.; Irwin, J.A.G.; Manners, J.M. Transfer of a supernumerary chromosome between vegetatively incompatible biotypes of the fungus Colletotrichum gloeosporioides. Genetics 1998, 150, 1459–1466. [Google Scholar] [CrossRef]
- Akagi, Y.; Akamatsu, H.; Otani, H.; Kodama, M. Horizontal chromosome transfer, a mechanism for the evolution and differentiation of a plant-pathogenic fungus. Eukaryot Cell 2009, 8, 1732–1738. [Google Scholar] [CrossRef] [Green Version]
- Chèvre, A.M.; Eber, F.; This, P.; Barret, P.; Tanguy, X.; Brun, H.; Delseny, M.; Renard, M. Characterization of Brassica nigra chromosomes and of blackleg resistance in B. napus-B. nigra addition lines. Plant Breed. 1996, 115, 113–118. [Google Scholar] [CrossRef]
- Navabi, Z.K.; Parkin, I.A.; Pires, J.C.; Xiong, Z.; Thiagarajah, M.R.; Good, A.G.; Rahman, M.H. Introgression of B-genome chromosomes in a doubled haploid population of Brassica napus × B. carinata. Genome 2010, 53, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Tonguç, M.; Griffiths, P.D. Transfer of powdery mildew resistance from Brassica carinata to Brassica oleracea through embryo rescue. Plant Breed. 2004, 123, 587–589. [Google Scholar] [CrossRef]
- Sharma, B.B.; Kalia, P.; Singh, D.; Sharma, T.R. Introgression of black rot resistance from Brassica carinata to cauliflower (Brassica oleracea botrytis group) through embryo rescue. Front. Plant Sci. 2017, 8, 1255. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Bajaj, Y.P.S. Interspecific hybridization in Brassica juncea-Brassica hirta using embryo rescue. Euphytica 1987, 36, 321–326. [Google Scholar] [CrossRef]
- Bertier, L.; Leus, L.; D’hondt, L.; De Cock, A.W.; Höfte, M. Host adaptation and speciation through hybridization and polyploidy in Phytophthora. PLoS ONE 2013, 8, e85385. [Google Scholar]
- Beest, M.T.; Roux, J.J.L.; Richardson, D.M.; Brysting, A.K.; Suda, J.; Kubešová, M.; Pyšek, P. The more the better? The role of polyploidy in facilitating plant invasions. Ann. Bot. 2012, 109, 19–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Innes, R.W.; Ameline-Torregrosa, C.; Ashfield, T.; Cannon, E.; Cannon, S.B.; Chacko, B.; Chen, N.W.; Couloux, A.; Dalwani, A.; Denny, R.; et al. Differential accumulation of retroelements and diversification of NB-LRR disease resistance genes in duplicated regions following polyploidy in the ancestor of soybean. Plant Physiol. 2008, 148, 1740–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oswald, B.P.; Nuismer, S.L. Neopolyploidy and pathogen resistance. Proc. R. Soc. 2007, 274, 2393–2397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santa, J.; Berdugo, J.; Cely-Pardo, L.; Soto-Suarez, M.; Mosquera, T.; Galeano, C. QTL analysis reveals quantitative resistant loci for Phytophthora infestans and Tecia solanivora in tetraploid potato (Solanum tuberosum L.). PLoS ONE 2018, 13, e0199716. [Google Scholar] [CrossRef] [Green Version]
- Hias, N.; Svara, A.; Keulemans, J.H. Effect of polyploidization on the response of apple (Malus × domestica Borkh.) to Venturia inaequalis infection. Eur. J. Plant Pathol. 2018, 151, 515–526. [Google Scholar] [CrossRef]
- Hannweg, K.; Steyn, W.; Bertling, I. In vitro-induced tetraploids of Plectranthus esculentus are nematode-tolerant and have enhanced nutritional value. Euphytica 2016, 207, 343–351. [Google Scholar] [CrossRef]
- Li, Y.; Shen, H.; Zhou, Q.; Qian, K.; van der Lee, T.; Huang, S. Changing ploidy as a strategy: The Irish potato famine pathogen shifts ploidy in relation to its sexuality. Mol. Plant Microbe Interact. 2017, 30, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Oustric, J.; Santini, J.; Morillon, R. Synthetic Polyploidy in Grafted Crops. Front. Plant Sci. 2020, 11, 540894. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, K.; Gil, J.; Bertier, L.D.; Kenefick, A.; Wood, K.J.; Zhang, L.; Reyes-Chin-Wo, S.; Cavanaugh, K.; Tsuchida, C.; Wong, J.; et al. Genomic signatures of heterokaryosis in the oomycete pathogen Bremia lactucae. Nat. Commun. 2019, 10, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imam, J.; Singh, P.K.; Shukla, P. Plant Microbe Interactions in Post Genomic Era: Perspectives and Applications. Front. Microbiol. 2016, 7, 1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolatabadian, A.; Fernando, W.G.D. Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions. Biology 2022, 11, 421. https://doi.org/10.3390/biology11030421
Dolatabadian A, Fernando WGD. Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions. Biology. 2022; 11(3):421. https://doi.org/10.3390/biology11030421
Chicago/Turabian StyleDolatabadian, Aria, and Wannakuwattewaduge Gerard Dilantha Fernando. 2022. "Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions" Biology 11, no. 3: 421. https://doi.org/10.3390/biology11030421
APA StyleDolatabadian, A., & Fernando, W. G. D. (2022). Genomic Variations and Mutational Events Associated with Plant–Pathogen Interactions. Biology, 11(3), 421. https://doi.org/10.3390/biology11030421