Striatal Injury Induces Overall Brain Alteration at the Pallial, Thalamic, and Cerebellar Levels
Abstract
:Simple Summary
Abstract
1. Introduction

2. Materials and Methods
2.1. Animals
2.2. Striatal Lesion
2.3. Experimental Timeline & Brain Scanning
2.4. Behavioral Analysis
2.5. Tissue Processing & Immunohistochemical Staining
2.6. Image & Statistical Analysis
3. Results
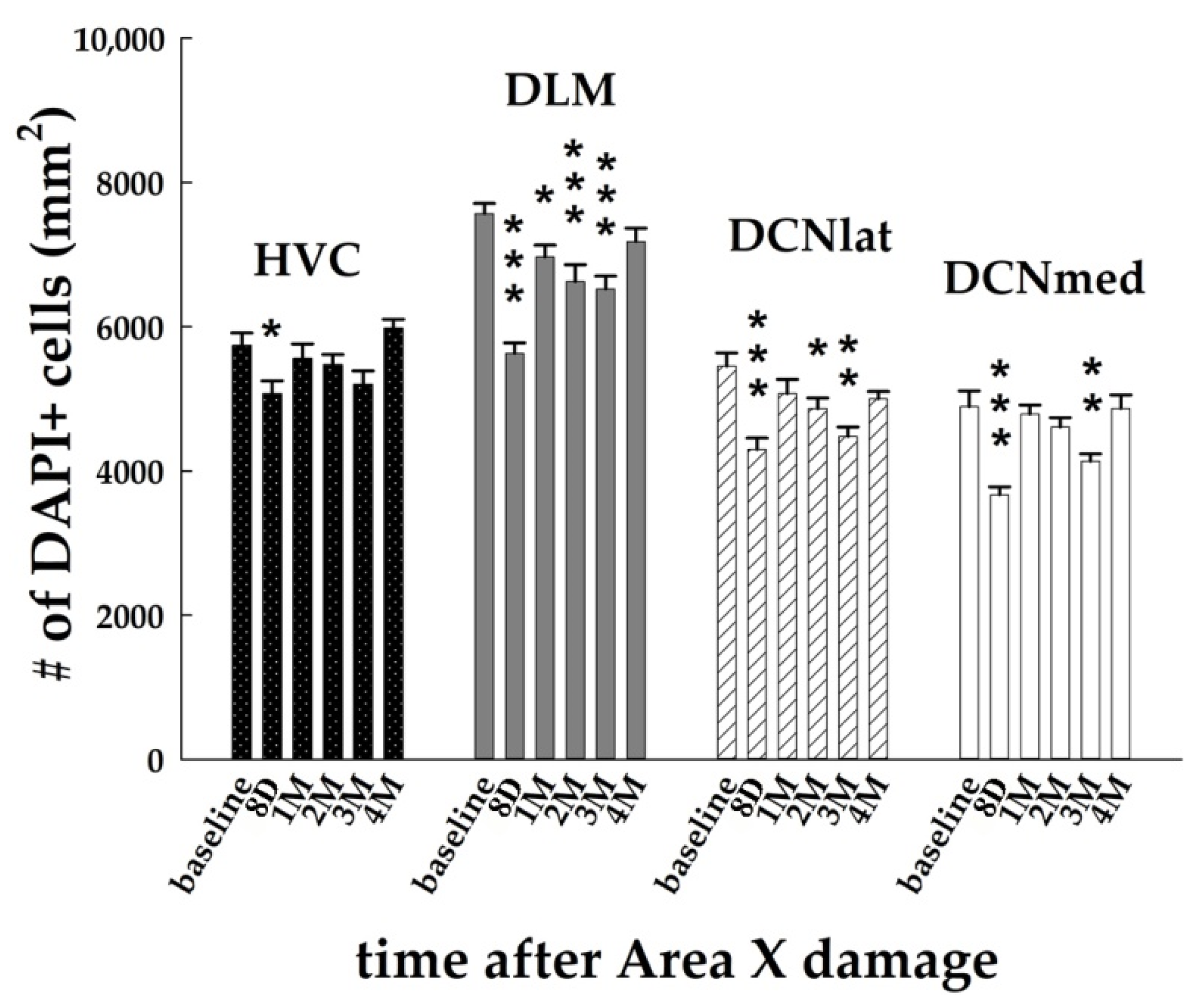
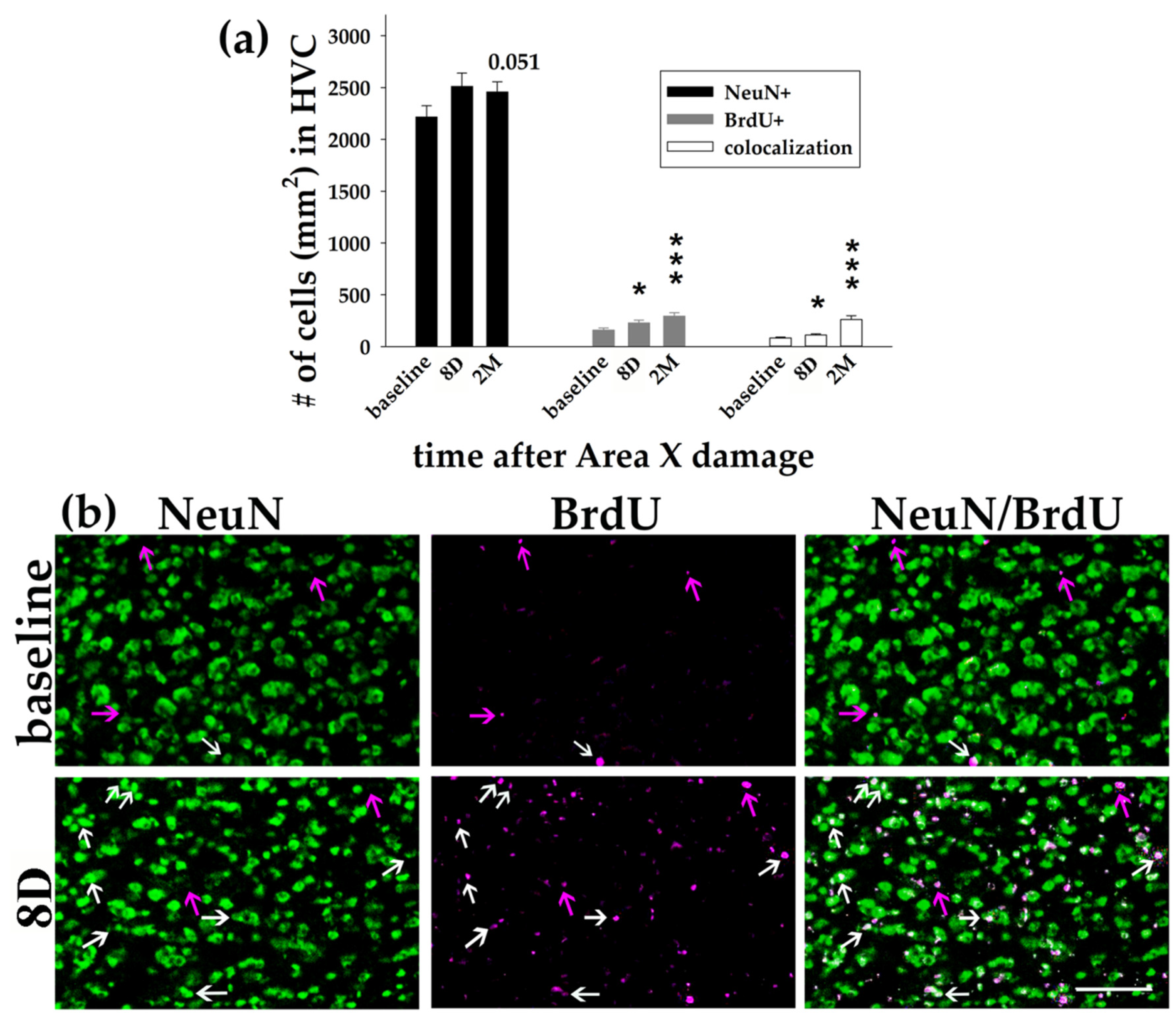
3.1. Area X Lesion Leads to a Temporary Decrease in the Number of Cells in HVC, DLM, and DCN
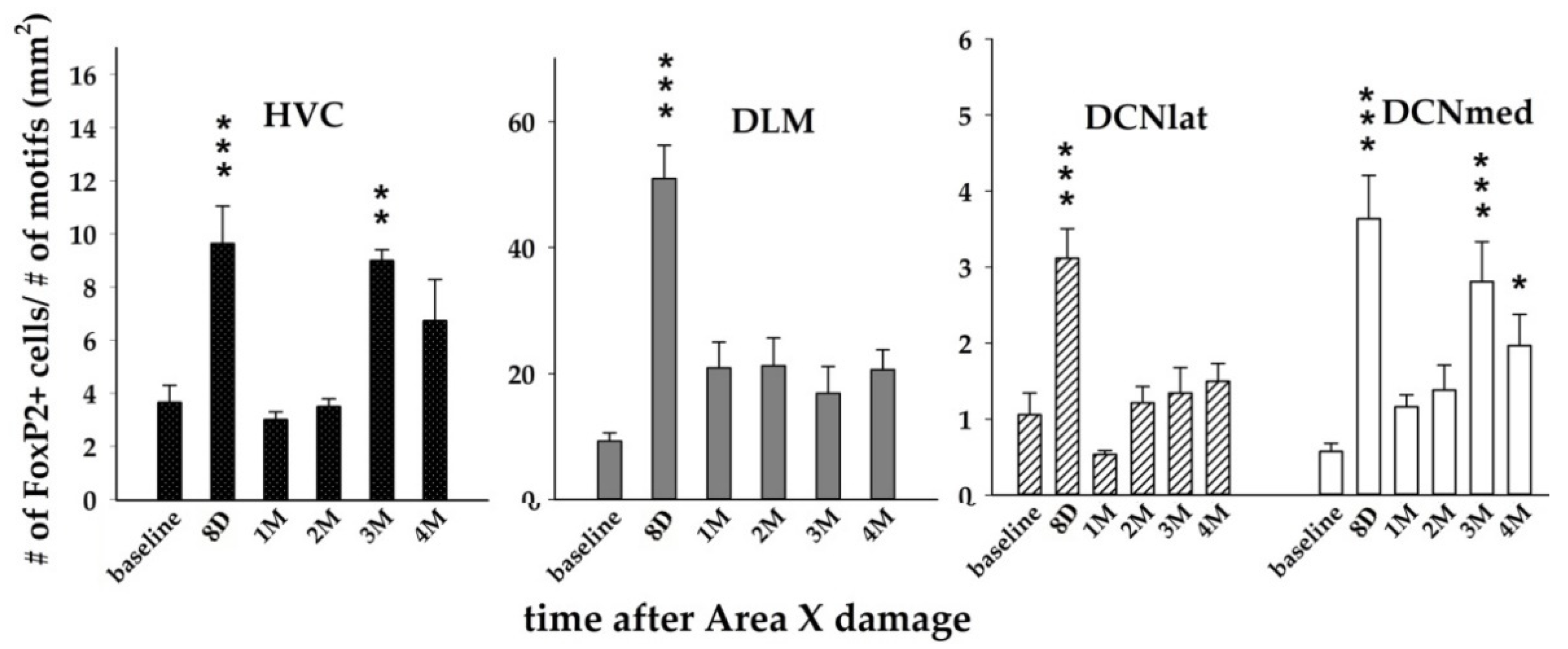
3.2. The Expression of FoxP2 after Area X Lesion Shows a Bi-Phasic Increase
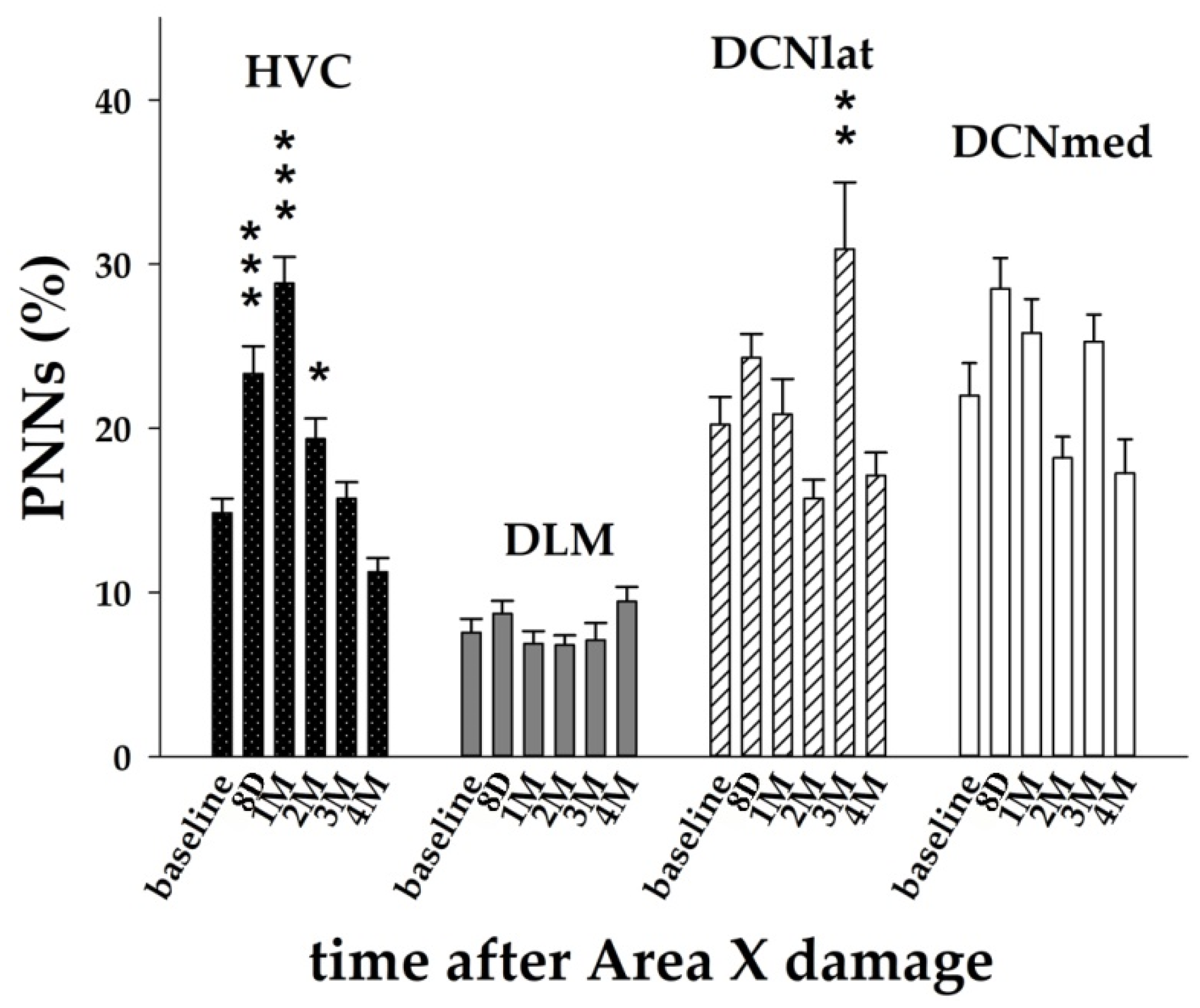
3.3. The Striatal Damage Affects PNNs Expression in Song Control System and Deep Cerebellar Nuclei
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aronov, D.; Andalman, A.S.; Fee, M.S. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 2008, 320, 630–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bottjer, S.W.; Halsema, K.A.; Brown, S.A.; Miesner, E.A. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J. Comp. Neurol. 1989, 279, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Brainard, M.S.; Doupe, A.J. Auditory feedback in learning and maintenance of vocal behaviour. Nat. Rev. Neurosci. 2000, 1, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M. The Role of Auditory Feedback in the Control of Vocalization in the White-Crowned Sparrow 1. Z. Tierpsychol. 1965, 22, 770–783. [Google Scholar] [CrossRef]
- Nottebohm, F.; Arnold, A.P. Sexual dimorphism in vocal control areas of the songbird brain. Science 1976, 194, 211–213. [Google Scholar] [CrossRef]
- Scharff, C.; Nottebohm, F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J. Neurosci. 1991, 11, 2896–2913. [Google Scholar] [CrossRef] [Green Version]
- Sohrabji, F.; Nordeen, E.J.; Nordeen, K.W. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav. Neural Biol. 1990, 53, 51–63. [Google Scholar] [CrossRef]
- Ackermann, H.; Hage, S.R.; Ziegler, W. Brain mechanisms of acoustic communication in humans and nonhuman primates: An evolutionary perspective. Behav. Brain Sci. 2014, 37, 529–546. [Google Scholar] [CrossRef] [Green Version]
- Kubikova, L.; Bosikova, E.; Cvikova, M.; Lukacova, K.; Scharff, C.; Jarvis, E.D. Basal ganglia function, stuttering, sequencing and repair in adult songbirds. Sci. Rep. 2014, 4, 6590. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, D.A.; Roberts, T.F.; Sober, S.J. Thalamostriatal and cerebellothalamic pathways in a songbird, the Bengalese finch. J. Comp. Neurol. 2018, 526, 1550–1570. [Google Scholar] [CrossRef]
- Vicario, D.S. Organization of the zebra finch song control system: Functional organization of outputs from nucleus robustus archistriatalis. J. Comp. Neurol. 1991, 309, 486–494. [Google Scholar] [CrossRef]
- Jarvis, E.D. Learned birdsong and the neurobiology of human language. Ann. N. Y. Acad. Sci. 2004, 1016, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Person, A.L.; Gale, S.D.; Farries, M.A.; Perkel, D.J. Organization of the songbird basal ganglia, including area X. J. Comp. Neurol. 2008, 508, 840–866. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, G.; Zhou, E.P.; Jarvis, E.D. Of mice, birds, and men: The mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS ONE 2012, 7, e46610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petkov, C.I.; Jarvis, E. Birds, primates, and spoken language origins: Behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 2012, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Holmes, G. The symptoms of acute cerebellar injuries due to gunshot injuries. Brain 1917, 40, 461–535. [Google Scholar] [CrossRef]
- Mariën, P.; Ackermann, H.; Adamaszek, M.; Barwood, C.H.; Beaton, A.; Desmond, J.; De Witte, E.; Fawcett, A.J.; Hertrich, I.; Küper, M. Consensus paper: Language and the cerebellum: An ongoing enigma. Cerebellum 2014, 13, 386–410. [Google Scholar] [CrossRef]
- Ziegler, W.; Ackermann, H. Subcortical contributions to motor speech: Phylogenetic, developmental, clinical. Trends Neurosci. 2017, 40, 458–468. [Google Scholar] [CrossRef]
- Pidoux, L.; Le Blanc, P.; Levenes, C.; Leblois, A. A subcortical circuit linking the cerebellum to the basal ganglia engaged in vocal learning. eLife 2018, 7, e32167. [Google Scholar] [CrossRef]
- Hamaide, J.; Lukacova, K.; Van Audekerke, J.; Verhoye, M.; Kubikova, L.; Van der Linden, A. Neuroplasticity in the cerebello-thalamo-basal ganglia pathway: A longitudinal in vivo MRI study in male songbirds. NeuroImage 2018, 181, 190–202. [Google Scholar] [CrossRef]
- Kobayashi, K.; Uno, H.; Okanoya, K. Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport 2001, 12, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Kao, M.H.; Doupe, A.J. Task-related “cortical” bursting depends critically on basal ganglia input and is linked to vocal plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 4756–4761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukacova, K.; Baciak, L.; Pavukova, E.; Pichova, K.; Kasparova, S.; Kubikova, L. Imaging of striatal injury in a songbird brain. Gen. Physiol. Biophys. 2017, 36, 5. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Laverghetta, A.V.; Meade, C.A.; Cuthbertson, S.L.; Bottjer, S.W. An immunohistochemical and pathway tracing study of the striatopallidal organization of area X in the male zebra finch. J. Comp. Neurol. 2004, 469, 239–261. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Theelen, M.; Nottebohm, F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 1990, 5, 101–109. [Google Scholar] [CrossRef]
- Kosubek-Langer, J.; Schulze, L.; Scharff, C. Maturation, behavioral activation, and connectivity of adult-born medium spiny neurons in a striatal song nucleus. Front. Neurosci. 2017, 11, 323. [Google Scholar] [CrossRef] [Green Version]
- Rochefort, C.; He, X.; Scotto-Lomassese, S.; Scharff, C. Recruitment of FoxP2-expressing neurons to area X varies during song development. Dev. Neurobiol. 2007, 67, 809–817. [Google Scholar] [CrossRef]
- Scott, B.B.; Lois, C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J. Comp. Neurol. 2007, 502, 202–214. [Google Scholar] [CrossRef]
- Thompson, C.K.; Schwabe, F.; Schoof, A.; Mendoza, E.; Gampe, J.; Rochefort, C.; Scharff, C. Young and intense: FoxP2 immunoreactivity in Area X varies with age, song stereotypy, and singing in male zebra finches. Front. Neural Circuits 2013, 7, 24. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.C.; Li, M.Y.; Liu, Y.H.; Ding, J.Y.; Yu, J.Y.; Wang, T.W. Foxp2 regulates neuronal differentiation and neuronal subtype specification. Dev. Neurobiol. 2014, 74, 723–738. [Google Scholar] [CrossRef]
- Schulz, S.; Haesler, S.; Scharff, C.; Rochefort, C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010, 9, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Spiteri, E.; Konopka, G.; Coppola, G.; Bomar, J.; Oldham, M.; Ou, J.; Vernes, S.C.; Fisher, S.E.; Ren, B.; Geschwind, D.H. Identification of the transcriptional targets of FOXP2, a gene linked to speech and language, in developing human brain. Am. J. Hum. Genet. 2007, 81, 1144–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-C.; Kuo, H.-Y.; Bornschein, U.; Takahashi, H.; Chen, S.-Y.; Lu, K.-M.; Yang, H.-Y.; Chen, G.-M.; Lin, J.-R.; Lee, Y.-H. Foxp2 controls synaptic wiring of corticostriatal circuits and vocal communication by opposing Mef2c. Nat. Neurosci. 2016, 19, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Kosubek-Langer, J.; Scharff, C. Dynamic FoxP2 levels in male zebra finches are linked to morphology of adult-born Area X medium spiny neurons. Sci. Rep. 2020, 10, 4787. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, S.; Adam, I.; Scharff, C. FoxP2 in songbirds. Curr. Opin. Neurobiol. 2014, 28, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Haesler, S.; Wada, K.; Nshdejan, A.; Morrisey, E.E.; Lints, T.; Jarvis, E.D.; Scharff, C. FoxP2 expression in avian vocal learners and non-learners. J. Neurosci. 2004, 24, 3164–3175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.E.; Spiteri, E.; Condro, M.C.; Dosumu-Johnson, R.T.; Geschwind, D.H.; White, S.A. Birdsong decreases protein levels of FoxP2, a molecule required for human speech. J. Neurophysiol. 2008, 100, 2015–2025. [Google Scholar] [CrossRef] [Green Version]
- Teramitsu, I.; White, S.A. FoxP2 regulation during undirected singing in adult songbirds. J. Neurosci. 2006, 26, 7390–7394. [Google Scholar] [CrossRef]
- Fisher, S.E.; Scharff, C. FOXP2 as a molecular window into speech and language. Trends Genet. 2009, 25, 166–177. [Google Scholar] [CrossRef]
- Lai, C.S.; Fisher, S.E.; Hurst, J.A.; Vargha-Khadem, F.; Monaco, A.P. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 2001, 413, 519–523. [Google Scholar] [CrossRef]
- Milev, P.; Maurel, P.; Chiba, A.; Mevissen, M.; Popp, S.; Yamaguchi, Y.; Margolis, R.K.; Margolis, R.U. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: Aggrecan, versican, neurocan, and brevican. Biochem. Biophys. Res. Commun. 1998, 247, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Corvetti, L.; Rossi, F. Degradation of chondroitin sulfate proteoglycans induces sprouting of intact purkinje axons in the cerebellum of the adult rat. J. Neurosci. 2005, 25, 7150–7158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Cristo, G.; Chattopadhyaya, B.; Kuhlman, S.J.; Fu, Y.; Bélanger, M.-C.; Wu, C.Z.; Rutishauser, U.; Maffei, L.; Huang, Z.J. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 2007, 10, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The roles of perineuronal nets and the perinodal extracellular matrix in neuronal function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Cornez, G.; Collignon, C.; Müller, W.; Cornil, C.A.; Ball, G.F.; Balthazart, J. Development of perineuronal nets during ontogeny correlates with sensorimotor vocal learning in canaries. eNeuro 2020, 7, e0361-19. [Google Scholar] [CrossRef] [Green Version]
- Cornez, G.; Jonckers, E.; Ter Haar, S.M.; Van der Linden, A.; Cornil, C.A.; Balthazart, J. Timing of perineuronal net development in the zebra finch song control system correlates with developmental song learning. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180849. [Google Scholar] [CrossRef] [Green Version]
- Hensch, T.K. Critical period plasticity in local cortical circuits. Nat. Rev. Neurosci. 2005, 6, 877–888. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Balmer, T.S.; Carels, V.M.; Frisch, J.L.; Nick, T.A. Modulation of perineuronal nets and parvalbumin with developmental song learning. J. Neurosci. 2009, 29, 12878–12885. [Google Scholar] [CrossRef] [Green Version]
- Cornez, G.; Ter Haar, S.M.; Cornil, C.A.; Balthazart, J. Anatomically discrete sex differences in neuroplasticity in zebra finches as reflected by perineuronal nets. PLoS ONE 2015, 10, e0123199. [Google Scholar] [CrossRef]
- Meyer, C.E.; Boroda, E.; Nick, T.A. Sexually dimorphic perineuronal net expression in the songbird. Basal Ganglia 2014, 3, 229–237. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cornez, G.; Valle, S.; dos Santos, E.B.; Chiver, I.; Müller, W.; Ball, G.F.; Cornil, C.A.; Balthazart, J. Perineuronal nets in HVC and plasticity in male canary song. PLoS ONE 2021, 16, e0252560. [Google Scholar] [CrossRef] [PubMed]
- Tchernichovski, O.; Nottebohm, F.; Ho, C.E.; Pesaran, B.; Mitra, P.P. A procedure for an automated measurement of song similarity. Anim. Behav. 2000, 59, 1167–1176. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Andreotti, J.P.; Prazeres, P.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; Birbrair, A. Neurogenesis in the postnatal cerebellum after injury. Int. J. Dev. Neurosci. 2018, 67, 33–36. [Google Scholar] [CrossRef]
- Takahashi, H.; Takahashi, K.; Liu, F.-C. FOXP genes, neural development, speech and language disorders. In Forkhead Transcription Factors; Springer: Berlin/Heidelberg, Germany, 2009; pp. 117–129. [Google Scholar]
- Tsui, D.; Vessey, J.P.; Tomita, H.; Kaplan, D.R.; Miller, F.D. FoxP2 regulates neurogenesis during embryonic cortical development. J. Neurosci. 2013, 33, 244–258. [Google Scholar] [CrossRef]
- Brown, J.P.; Couillard-Despres, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- Celio, M.R. Parvalbumin in most gamma-aminobutyric acid-containing neurons of the rat cerebral cortex. Science 1986, 231, 995–997. [Google Scholar] [CrossRef]
- Scotto-Lomassese, S.; Rochefort, C.; Nshdejan, A.; Scharff, C. HVC interneurons are not renewed in adult male zebra finches. Eur. J. Neurosci. 2007, 25, 1663–1668. [Google Scholar] [CrossRef]
- Wild, J.M.; Williams, M.N.; Howie, G.J.; Mooney, R. Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). J. Comp. Neurol. 2005, 483, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Bederson, J.B.; Bartkowski, H.M.; Moon, K.; Halks-Miller, M.; Nishimura, M.C.; Brant-Zawadski, M.; Pitts, L.H. Nuclear magnetic resonance imaging and spectroscopy in experimental brain edema in a rat model. J. Neurosurg. 1986, 64, 795–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Horin, N.; Hazvi, S.; Bendel, P.; Schul, R. The ontogeny of a neurotoxic lesion in rat brain revealed by combined MRI and histology. Brain Res. 1996, 718, 97–104. [Google Scholar] [CrossRef]
- Málková, L.; Lex, C.K.; Mishkin, M.; Saunders, R.C. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus 2001, 11, 361–370. [Google Scholar] [CrossRef]
- Russo, M.V.; McGavern, D.B. Inflammatory neuroprotection following traumatic brain injury. Science 2016, 353, 783–785. [Google Scholar] [CrossRef] [Green Version]
- Schimmel, S.J.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135–142. [Google Scholar] [CrossRef]
- Bayly, P.V.; Dikranian, K.T.; Black, E.E.; Young, C.; Qin, Y.-Q.; Labruyere, J.; Olney, J.W. Spatiotemporal evolution of apoptotic neurodegeneration following traumatic injury to the developing rat brain. Brain Res. 2006, 1107, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Ren, Z.; Iliff, J.J.; Yang, L.; Yang, J.; Chen, X.; Chen, M.J.; Giese, R.N.; Wang, B.; Shi, X.; Nedergaard, M. ‘Hit & Run’model of closed-skull traumatic brain injury (TBI) reveals complex patterns of post-traumatic AQP4 dysregulation. J. Cereb. Blood Flow Metab. 2013, 33, 834–845. [Google Scholar]
- Susarla, B.T.; Villapol, S.; Yi, J.-H.; Geller, H.M.; Symes, A.J. Temporal patterns of cortical proliferation of glial cell populations after traumatic brain injury in mice. ASN Neuro 2014, 6, e00143. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, M.K.; Riederer, P.; Brückner, G.; Arendt, T. Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef]
- Cabungcal, J.-H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Fawcett, J. The perineuronal net and the control of CNS plasticity. Cell Tissue Res. 2012, 349, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Takesian, A.E.; Hensch, T.K. Balancing plasticity/stability across brain development. Prog. Brain Res. 2013, 207, 3–34. [Google Scholar] [PubMed]
- Farries, M.A.; Perkel, D.J. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J. Neurosci. 2002, 22, 3776–3787. [Google Scholar] [CrossRef]
- Chen, C.H.; Fremont, R.; Arteaga-Bracho, E.E.; Khodakhah, K. Short latency cerebellar modulation of the basal ganglia. Nat. Neurosci. 2014, 17, 1767–1775. [Google Scholar] [CrossRef]
- Timmann, D.; Drepper, J.; Frings, M.; Maschke, M.; Richter, S.; Gerwig, M.; Kolb, F.P. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex 2010, 46, 845–857. [Google Scholar] [CrossRef]
- Carulli, D.; Broersen, R.; de Winter, F.; Muir, E.M.; Mešković, M.; de Waal, M.; de Vries, S.; Boele, H.-J.; Canto, C.B.; De Zeeuw, C.I. Cerebellar plasticity and associative memories are controlled by perineuronal nets. Proc. Natl. Acad. Sci. USA 2020, 117, 6855–6865. [Google Scholar] [CrossRef] [Green Version]
- Carulli, D.; Rhodes, K.E.; Brown, D.J.; Bonnert, T.P.; Pollack, S.J.; Oliver, K.; Strata, P.; Fawcett, J.W. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 2006, 494, 559–577. [Google Scholar] [CrossRef]
- Carmichael, S.T.; Archibeque, I.; Luke, L.; Nolan, T.; Momiy, J.; Li, S. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp. Neurol. 2005, 193, 291–311. [Google Scholar] [CrossRef]
- Carbo-Gas, M.; Moreno-Rius, J.; Guarque-Chabrera, J.; Vazquez-Sanroman, D.; Gil-Miravet, I.; Carulli, D.; Hoebeek, F.; De Zeeuw, C.; Sanchis-Segura, C.; Miquel, M. Cerebellar perineuronal nets in cocaine-induced pavlovian memory: Site matters. Neuropharmacology 2017, 125, 166–180. [Google Scholar] [CrossRef]
- Smith, C.C.; Mauricio, R.; Nobre, L.; Marsh, B.; Wüst, R.C.; Rossiter, H.B.; Ichiyama, R.M. Differential regulation of perineuronal nets in the brain and spinal cord with exercise training. Brain Res. Bull. 2015, 111, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Scharff, C.; Kirn, J.R.; Grossman, M.; Macklis, J.D.; Nottebohm, F. Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron 2000, 25, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Enard, W.; Gehre, S.; Hammerschmidt, K.; Hölter, S.M.; Blass, T.; Somel, M.; Brückner, M.K.; Schreiweis, C.; Winter, C.; Sohr, R. A humanized version of Foxp2 affects cortico-basal ganglia circuits in mice. Cell 2009, 137, 961–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- French, C.; Jin, X.; Campbell, T.; Gerfen, E.; Groszer, M.; Fisher, S.E.; Costa, R. An aetiological Foxp2 mutation causes aberrant striatal activity and alters plasticity during skill learning. Mol. Psychiatry 2012, 17, 1077–1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heston, J.B.; White, S.A. Behavior-linked FoxP2 regulation enables zebra finch vocal learning. J. Neurosci. 2015, 35, 2885–2894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norton, P.; Barschke, P.; Scharff, C.; Mendoza, E. Differential song deficits after lentivirus-mediated knockdown of FoxP1, FoxP2, or FoxP4 in area X of juvenile zebra finches. J. Neurosci. 2019, 39, 9782–9796. [Google Scholar] [CrossRef] [PubMed]
- Day, N.F.; Hobbs, T.G.; Heston, J.B.; White, S.A. Beyond critical period learning: Striatal FoxP2 affects the active maintenance of learned vocalizations in adulthood. eNeuro 2019, 6, e0071-19. [Google Scholar] [CrossRef]
- Murugan, M.; Harward, S.; Scharff, C.; Mooney, R. Diminished FoxP2 levels affect dopaminergic modulation of corticostriatal signaling important to song variability. Neuron 2013, 80, 1464–1476. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Merullo, D.P.; Koch, T.M.I.; Cao, M.; Co, M.; Kulkarni, A.; Konopka, G.; Roberts, T.F. Expression of FoxP2 in the basal ganglia regulates vocal motor sequences in the adult songbird. Nat Commun 2021, 12, 2617. [Google Scholar] [CrossRef]
- Jarvis, E.D.; Yu, J.; Rivas, M.V.; Horita, H.; Feenders, G.; Whitney, O.; Jarvis, S.C.; Jarvis, E.R.; Kubikova, L.; Puck, A.E.; et al. Global view of the functional molecular organization of the avian cerebrum: Mirror images and functional columns. J. Comp. Neurol. 2013, 521, 3614–3665. [Google Scholar] [CrossRef] [Green Version]
- Reiner, A.; Perkel, D.J.; Bruce, L.L.; Butler, A.B.; Csillag, A.; Kuenzel, W.; Medina, L.; Paxinos, G.; Shimizu, T.; Striedter, G.; et al. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004, 473, 377–414. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Masterson, S.P.; Damron, J.K.; Guido, W.; Bickford, M.E. The Mouse Pulvinar Nucleus Links the Lateral Extrastriate Cortex, Striatum, and Amygdala. J. Neurosci. 2018, 38, 347–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, X.L.; Fang, Q.; Yan, L.; Zhong, W.; Peng, B.; Li, H.; Wei, J.; Tao, H.W.; Zhang, L.I. Contextual and cross-modality modulation of auditory cortical processing through pulvinar mediated suppression. eLife 2020, 9, e54157. [Google Scholar] [CrossRef] [PubMed]
- Overton, P.G.; Coizet, V. The neuropathological basis of anxiety in Parkinson’s disease. Med. Hypotheses 2020, 144, 110048. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Yamamoto, K.; Karten, H.J. Organization and evolution of the avian forebrain. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005, 287, 1080–1102. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukacova, K.; Hamaide, J.; Baciak, L.; Van der Linden, A.; Kubikova, L. Striatal Injury Induces Overall Brain Alteration at the Pallial, Thalamic, and Cerebellar Levels. Biology 2022, 11, 425. https://doi.org/10.3390/biology11030425
Lukacova K, Hamaide J, Baciak L, Van der Linden A, Kubikova L. Striatal Injury Induces Overall Brain Alteration at the Pallial, Thalamic, and Cerebellar Levels. Biology. 2022; 11(3):425. https://doi.org/10.3390/biology11030425
Chicago/Turabian StyleLukacova, Kristina, Julie Hamaide, Ladislav Baciak, Annemie Van der Linden, and Lubica Kubikova. 2022. "Striatal Injury Induces Overall Brain Alteration at the Pallial, Thalamic, and Cerebellar Levels" Biology 11, no. 3: 425. https://doi.org/10.3390/biology11030425
APA StyleLukacova, K., Hamaide, J., Baciak, L., Van der Linden, A., & Kubikova, L. (2022). Striatal Injury Induces Overall Brain Alteration at the Pallial, Thalamic, and Cerebellar Levels. Biology, 11(3), 425. https://doi.org/10.3390/biology11030425





