Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Conventional DPN
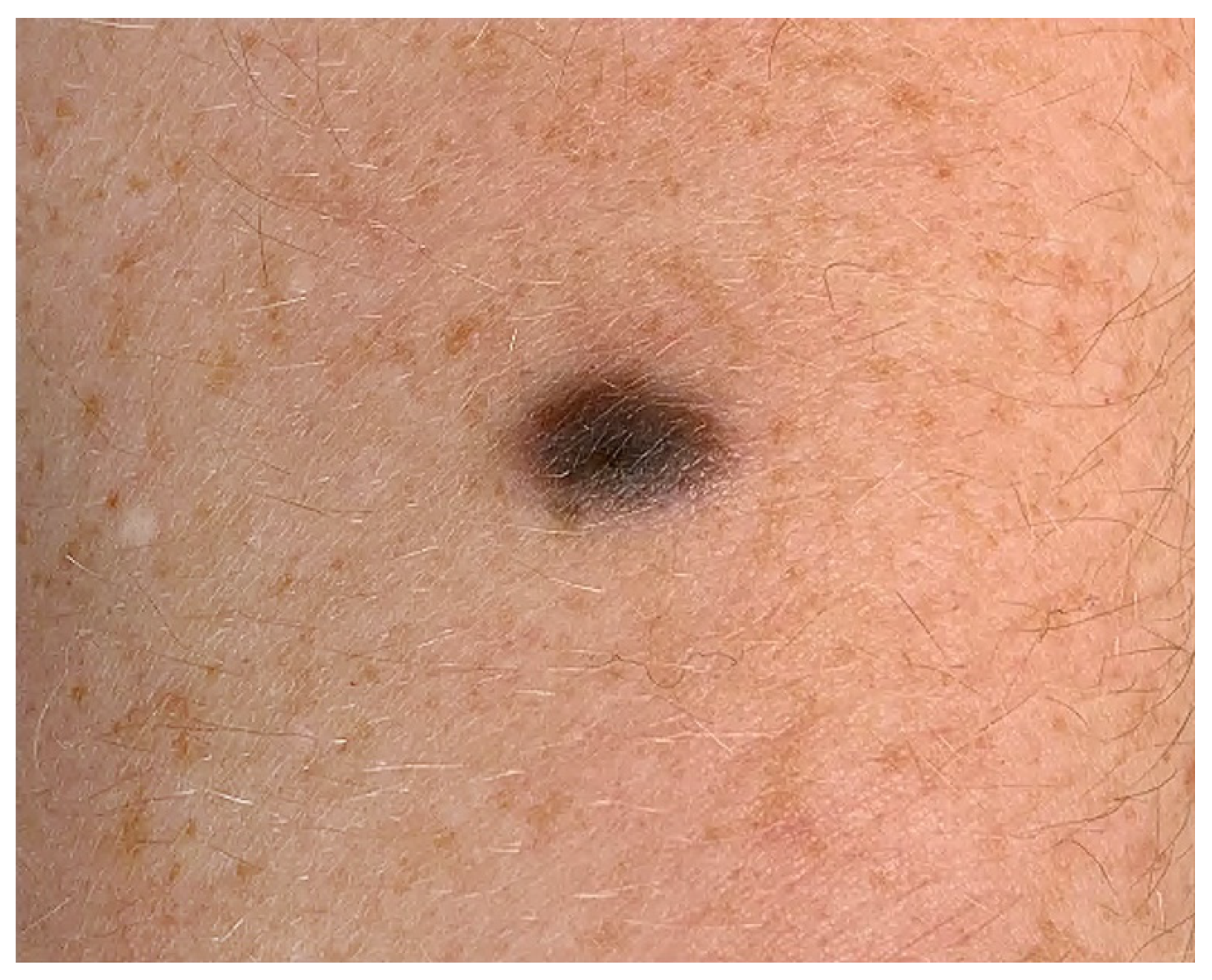
2.1. Clinical Features
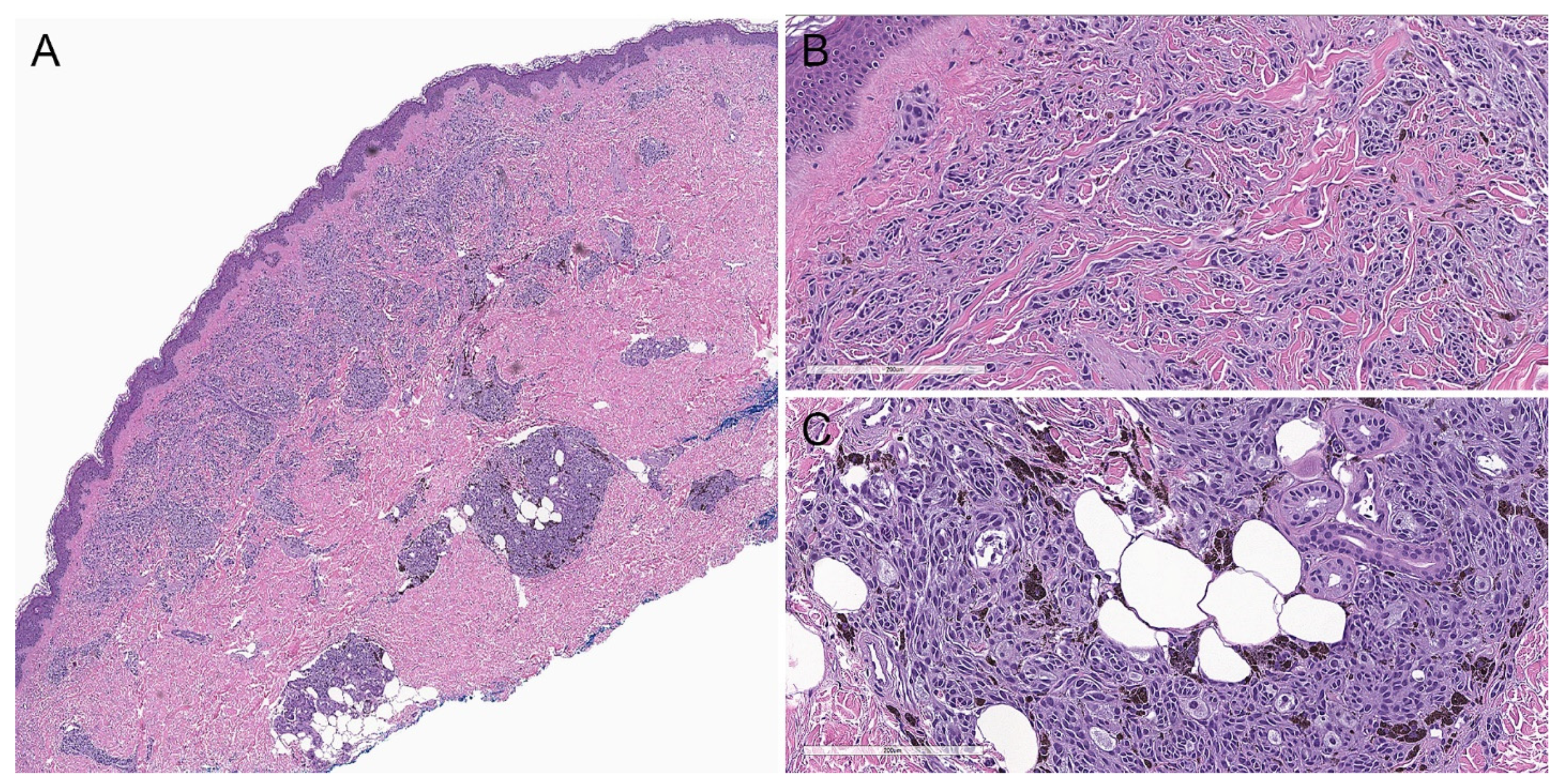
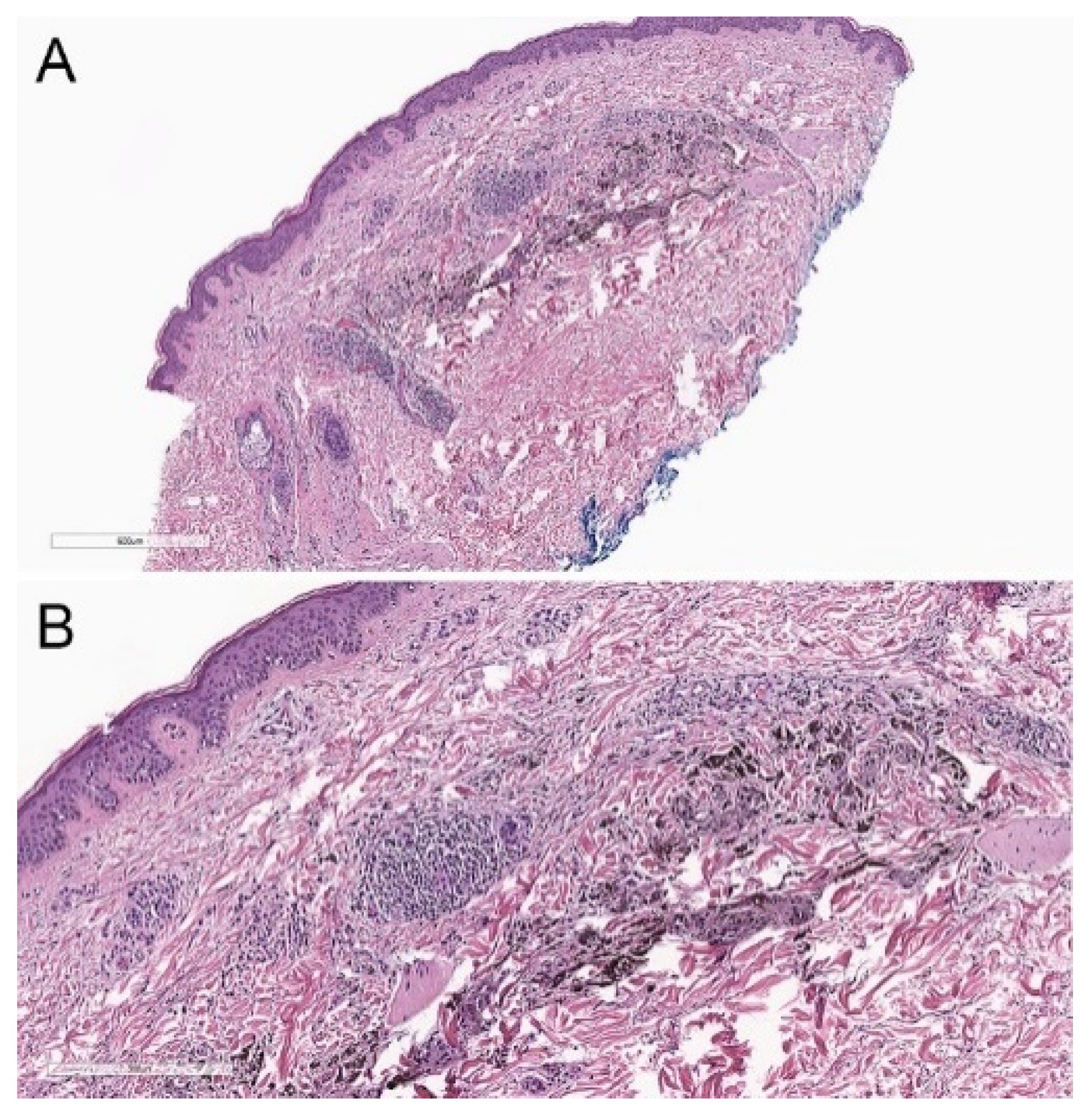
2.2. Histopathological Criteria
2.3. Immunohistochemical Features
2.4. Molecular Biology
2.5. Cytogenetic Findings
2.6. Prognosis and Treatment
3. Atypical DPN
3.1. Clinical Features
3.2. Histopathological Criteria
3.3. Immunohistochemical Features
3.4. Molecular Biology
3.5. Cytogenetic Findings
3.6. Prognosis and Treatment
4. DPN-like Melanoma
4.1. Clinical Features
4.2. Histopathological Criteria
4.3. Molecular Biology
4.4. Cytogenetic Findings
4.5. Prognosis and Treatment
5. DPN Histological Variants
5.1. Plexiform Spindle Cell Nevus (PLEXSCN)
5.2. Clonal/Inverted Type A Nevus
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elder, D.; Massi, D.; Scolier, R.; Willemze, R.W. WHO Classification of Skin Tumors; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Scolyer, R.A.; Murali, R.; McCarthy, S.W.; Thompson, J.F. Histologically ambiguous (“Borderline”) primary cutaneous melanocytic tumors: Approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch. Pathol. Lab. Med. 2010, 134, 1770–1777. [Google Scholar] [CrossRef] [PubMed]
- Muhlbauer, A.; Momtahen, S.; Mihm, M.C.; Wang, J.; Magro, C.M. The correlation of the standard 5 probe FISH assay with melanocytic tumors of uncertain malignant potential. Ann. Diagn. Pathol. 2017, 28, 30–36. [Google Scholar] [CrossRef] [PubMed]
- North, J.P.; Garrido, M.C.; Kolaitis, N.A.; Leboit, P.E.; Mccalmont, T.H.; Bastian, B.C. Fluorescence in situ hybridization as an ancillary tool in the diagnosis of ambiguous melanocytic neoplasms: A review of 804 cases. Am. J. Surg. Pathol. 2014, 38, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Seab, J.A.; Graham, J.H.; Helwig, E.B. Deep Penetrating Nevus. Am. J. Surg. Pathol. 1989, 13, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Barnhill, R.L.; Mihm, M.C.; Magro, C.M. Plexiform spindle cell naevus: A distinctive variant of plexiform melanocytic naevus. Histopathology 1991, 18, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.J.; Golitz, L.E. Melanocytic nevi with focal atypical epithelioid cell components: A review of seventy-three cases. J. Am. Acad. Dermatol. 1994, 30, 724–729. [Google Scholar] [CrossRef]
- Magro, C.M.; Abraham, R.M.; Guo, R.; Li, S.; Wang, X.; Proper, S.; Crowson, A.N.; Mihm, M. Deep penetrating nevus-like borderline tumors: A unique subset of ambiguous melanocytic tumors with malignant potential and normal cytogenetics. Eur. J. Dermatol. 2014, 24, 594–602. [Google Scholar] [CrossRef]
- Magro, C.M.; Crowson, A.N.; Mihm, M.C.; Gupta, K.; Walker, M.J.; Solomon, G. The dermal-based borderline melanocytic tumor: A categorical approach. J. Am. Acad. Dermatol. 2010, 62, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.H. Deep penetrating (plexiform spindle cell) nevus. J. Cutan. Pathol. 1992, 19, 172–180. [Google Scholar] [CrossRef]
- Robson, A.; Morley-Quante, M.; Hempel, H.; McKee, P.H.; Calonje, E. Deep penetrating naevus: Clinicopathological study of 31 cases with further delineation of histological features allowing distinction from other pigmented benign melanocytic lesions and melanoma. Histopathology 2003, 43, 529–537. [Google Scholar] [CrossRef] [PubMed]
- de la Fouchardière, A.; Caillot, C.; Jacquemus, J.; Durieux, E.; Houlier, A.; Haddad, V.; Pissaloux, D. β-Catenin nuclear expression discriminates deep penetrating nevi from other cutaneous melanocytic tumors. Virchows Arch. 2019, 474, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Luzar, B.; Calonje, E. Deep penetrating nevus: A review. Arch. Pathol. Lab. Med. 2011, 135, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Strazzula, L.; Senna, M.M.; Yasuda, M.; Belazarian, L. The deep penetrating nevus. J. Am. Acad. Dermatol. 2014, 71, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.J.; Jen, M.; Chang, M.W.; Grant-Kels, J.M.; Makkar, H. Molecular diagnosis of a benign proliferative nodule developing in a congenital melanocytic nevus in a 3-month-old infant. J. Am. Acad. Dermatol. 2008, 59, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Schulte, K.W.; Ruzicka, T.; Megahed, M. Linear arrangement of multiple deep penetrating nevi. Arch. Dermatol. 2003, 139, 1608–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehregan, D.A.; Mehregan, A.H. Deep penetrating nevus. Arch. Dermatol. 1993, 129, 328. [Google Scholar] [CrossRef] [PubMed]
- Scolyer, R.A.; Zhuang, L.; Allan Palmer, A.; Thompson, J.F.; McCarthy, S.W. Combined naevus: A benign lesion frequently misdiagnosed both clinically and pathologically as melanoma. Pathology 2004, 36, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Skelton, H.G.; Smith, K.J.; Barrett, T.L.; Lupton, G.P.; Graham, J.H. HMB-45 staining in benign and malignant melanocytic lesions. Am. J. Dermatopathol. 1991, 13, 543–550. [Google Scholar] [CrossRef]
- Cho, W.C.; Prieto, V.G.; Aung, P.P. Melanocytic lesions with blue naevus-like (dendritic) morphology: An update with an emphasis on histopathological, immunophenotypic, and molecular features. Histopathology 2021, 79, 291–305. [Google Scholar] [CrossRef]
- Erstine, E.M.; Lazar, A.J. Toward an effective use of β-catenin immunohistochemistry in the evaluation of challenging melanocytic lesions. Virchows Arch. 2019, 474, 535–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, I.; Lang, U.E.; Durieux, E.; Tee, M.K.; Jorapur, A.; Shain, A.H.; Haddad, V.; Pissaloux, D.; Chen, X.; Cerroni, L.; et al. Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi. Nat. Commun. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.S.; Saleem, A.; Wang, J.Y.; Rieger, K.E.; Brown, R.A.; Novoa, R.A. Diagnostic utility of LEF1 immunohistochemistry in differentiating deep penetrating nevi from histologic mimics. Am. J. Surg. Pathol. 2020, 44, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.C.; Nájera, L.; Navarro, A.; Huerta, V.; Garrido, E.; Rodriguez-Peralto, J.-L.; Requena, L. Combination of congenital and deep penetrating nevus by acquisition of β-catenin activation. Am. J. Dermatopathol. 2020, 42, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Mehregan, D.R.; Mehregan, D.A.; Mehregan, A.H. Proliferating cell nuclear antigen staining in deep-penetrating nevi. J. Am. Acad. Dermatol. 1995, 33, 685–687. [Google Scholar] [CrossRef]
- Roesch, A.; Wittschier, S.; Becker, B.; Landthaler, M.; Vogt, T. Loss of dipeptidyl peptidase IV immunostaining discriminates malignant melanomas from deep penetrating nevi. Mod. Pathol. 2006, 19, 1378–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roesch, A.; Becker, B.; Bentink, S.; Spang, R.; Vogl, A.; Hagen, I.; Landthaler, M.; Vogt, T. Ataxia telangiectasia-mutated gene is a possible biomarker for discrimination of infiltrative deep penetrating nevi and metastatic vertical growth phase melanoma. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2486–2490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, D.R.; Forcucci, J.A.; O’Connor, H.; Maize, J.C. Preferentially expressed antigen in MElanoma (PRAME) expression in BRCA1-associated protein (BAP1)-inactivated melanocytic tumors and deep penetrating nevi: A pilot study. J. Cutan. Pathol. 2021, 48, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lezcano, C.; Jungbluth, A.A.; Nehal, K.S.; Hollmann, T.J.; Busam, K.J. PRAME expression in melanocytic tumors. Am. J. Surg. Pathol. 2018, 42, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.P.; McGinniss, M.J.; Esmay, P.; Velazquez, E.F.; Reimann, J.D. Identification of HRAS mutations and absence of GNAQ or GNA11 mutations in deep penetrating nevi. Mod. Pathol. 2013, 26, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Dunn, A.L.J.; Gardner, J.M.; Kaley, J.R.; Bellamy, W.; Shalin, S.C. ALK rearrangements are infrequent in cellular blue nevus and deep penetrating nevus. Am. J. Dermatopathol. 2018, 40, 469–478. [Google Scholar] [CrossRef]
- Robson, A.; Assaf, C.; Bagot, M.; Burg, G.; Calonje, E.; Castillo, C.; Cerroni, L.; Chimenti, N.; Dechelotte, P.; Franck, F.; et al. Aggressive epidermotropic cutaneous CD8+ lymphoma: A cutaneous lymphoma with distinct clinical and pathological features. Report of an EORTC Cutaneous Lymphoma Task Force Workshop. Histopathology 2015, 67, 425–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, A.; Sini, M.C.; Cesinaro, A.M.; Portelli, F.; Urso, C.; Lentini, M.; Cardia, R.; Alos, L.; Cook, M.; Simi, S.; et al. NGS-based analysis of atypical deep penetrating nevi. Cancers 2021, 13, 3066. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.M.; Ming, M.E.; Elder, D.E.; Xu, X. An atypical melanocytic lesion without genomic abnormalities shows locoregional metastasis. J. Cutan. Pathol. 2012, 39, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Isales, M.C.; Khan, A.U.; Zhang, B.; Compres, E.V.; Kim, D.; Tan, T.L.; Beaubier, N.; Gerami, P. Molecular analysis of atypical deep penetrating nevus progressing to melanoma. J. Cutan. Pathol. 2020, 47, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Vilain, R.E.; Granter, S.R.; Hu, N.R.; Bresler, S.C.; Xu, S.; Frank, A.H.; Mihm, M.C.; Saw, R.P.; Fletcher, C.D.; et al. 5-Hydroxymethylcytosine is a nuclear biomarker to assess biological potential in histologically ambiguous heavily pigmented melanocytic neoplasms. J. Cutan. Pathol. 2017, 44, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Cerroni, L.; Barnhill, R.; Elder, D.; Gottlieb, G.; Heenan, P.; Kutzner, H.; LeBoit, P.E.; Mihm, M.; Rosai, J.; Kerl, H. Melanocytic tumors of uncertain malignant potential: Results of a tutorial held at the XXIX symposium of the international society of dermatopathology in Graz, October 2008. Am. J. Surg. Pathol. 2010, 34, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, I.; Griewank, K.G.; Ungureanu, L.; Tamayo, A.; Siepmann, T. Deep penetrating nevus and borderline-deep penetrating nevus: A literature review. Front. Oncol. 2020, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- McCalmont, T.H.; Bastian, B.C. An unconventional deep penetrating melanocytic nevus with microscopic involvement of regional lymph nodes. J. Cutan. Pathol. 2012, 39, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Massi, G.; LeBoit, P.E. Melanoma with a plexiform pattern. In Histological Diagnosis of Nevi and Melanoma; Springer: Berlin/Heidelberg, Germany, 2014; pp. 581–588. [Google Scholar] [CrossRef]
- Giubellino, A.; Nelson, A.C.; He, Y.; Munro, S.A.; Song, K.Y.; Oliva, I.C.G.; Torres-Cabala, C. Molecular characterization of biphenotypic epithelioid and plexiform melanoma with deep penetrating nevus-like features. Pigment Cell Melanoma Res. 2022, 35, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.; Yang, A.; Mihm, M.C.; Barnhill, R.L. The plexiform spindle cell nevus nevi and atypical variants: Report of 128 cases. Hum. Pathol. 2014, 45, 2369–2378. [Google Scholar] [CrossRef]
- Barnhill, R.L.; Barnhill, M.A.; Berwick, M.; Mihm, M.C. The histologic spectrum of pigmented spindle cell nevus: A review of 120 cases with emphasis on atypical variants. Hum. Pathol. 1991, 22, 52–58. [Google Scholar] [CrossRef]
- Dadras, S.S.; Lu, J.; Zembowicz, A.; Flotte, T.J.; Mihm, M.C. Histological features and outcome of inverted type-A melanocytic nevi. J. Cutan. Pathol. 2018, 45, 254–262. [Google Scholar] [CrossRef] [PubMed]
- High, W.A.; Alanen, K.W.; Golitz, L.E. Is melanocytic nevus with focal atypical epithelioid components (clonal nevus) a superficial variant of deep penetrating nevus? J. Am. Acad. Dermatol. 2006, 55, 460–466. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, P.; Aung, P.P. Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants. Biology 2022, 11, 460. https://doi.org/10.3390/biology11030460
Gill P, Aung PP. Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants. Biology. 2022; 11(3):460. https://doi.org/10.3390/biology11030460
Chicago/Turabian StyleGill, Pavandeep, and Phyu P. Aung. 2022. "Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants" Biology 11, no. 3: 460. https://doi.org/10.3390/biology11030460
APA StyleGill, P., & Aung, P. P. (2022). Conventional and Atypical Deep Penetrating Nevus, Deep Penetrating Nevus-like Melanoma, and Related Variants. Biology, 11(3), 460. https://doi.org/10.3390/biology11030460






