Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Analyses
3. Results
3.1. Abundance and Size Structure
3.2. Sex
3.3. Length–Weight Relationships
3.4. Growth, Life Span, and Mortality
3.5. Exploitation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fabricius, J.C. Mantissa Insectorum, Sistens Eorum Species Nuper Detectas, Adjectis Characteribus Genericis, Differentiis Specificis, Emendationibus Observationibus; Impensis C. G. Proft: Copenhagen, Denmark, 1787; Volume 2. [Google Scholar]
- Holthuis, L.B. FAO species catalogue, vol. 13: Marine Lobsters of the World. An Annotated and Illustrated Catalogue of Species of Interest to Fisheries Known to Date. FAO Fish. Synop. 1991, 125, 1–292. [Google Scholar]
- Goñi, R.; Latrouite, D. Review of the biology, ecology and fisheries of Palinurus spp. species of European waters: Palinurus elephas (Fabricius, 1787) and Palinurus mauritanicus (Gruvel, 1911). Cah. Biol. Mar. 2005, 46, 127–142. [Google Scholar]
- Ceccaldi, H.J.; Latrouite, D. The French Fisheries for the European Spiny Lobster Palinurus elephas. In Spiny Lobsters; Phillips, B., Kittaka, J., Eds.; Blackwell Science: Oxford, UK, 2000; pp. 200–209. ISBN 9780470698808. [Google Scholar]
- Hepper, B.T. The fishery for crawfish, Palinurus elephas, off the coast of Cornwall. J. Mar. Biol. Assoc. UK 1977, 57, 925–941. [Google Scholar] [CrossRef]
- Hunter, E.; Shackley, S.E.; Bennett, D.B. Recent studies on the crawfish Palinurus elephas in South Wales and Cornwall. J. Mar. Biol. Assoc. UK 1996, 76, 963–983. [Google Scholar] [CrossRef]
- Gristina, M.; Gagliano, M. Performance of traditional rush and modern plastic traps on the capture of Palinurus elephas (Fabricius, 1787) in laboratory tanks. Fish. Res. 2004, 67, 235–239. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Statistics. Global capture production 1950–2019 (FishstatJ). In FAO Fisheries Division [online]; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 5 February 2022).
- Groeneveld, J.C.; Goñi, R.; Díaz, D. Palinurus Species. In Lobsters: Biology, Management, Aquaculture and Fisheries; Philips, B., Ed.; John Wiley & Sons LTD: Chichester, UK, 2013; pp. 326–356. [Google Scholar]
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Synopsis of biological, ecological and fisheries-related information on priority marine species in the Azores region. Arquipel.-Life Mar. Sci. 2020, 1 (Suppl. 12), 1–138. [Google Scholar]
- Hunter, E. Biology of the European spiny lobster, Palinurus elephas (Fabricius, 1787) (Decapoda, Palinuridea). Crustaceana 1999, 72, 545–565. [Google Scholar] [CrossRef]
- Ruitort, J.J. Peche de la Langouste Rouge Palinurus elephas en Corse. Rapport Final; Universit de Corse Pascal Paoli: Corte, France, 1999. [Google Scholar]
- Goñi, R. Palinurus Elephas. Available online: https://doi.org/10.2305/IUCN.UK.2014-1.RLTS.T169975A1281221.en (accessed on 4 February 2022).
- INE. Annual Nominal Catches by Landed Port and Species. Available online: http://www.ine.pt (accessed on 4 February 2022).
- EC. Council Regulation (EC) No 724/2001 of 4 April 2001 amending Regulation (EC) No 850/98 for the conservation of fishery resources through technical measures for the protection of juveniles of marine organisms. Off. J. Eur. Communities 2001, L102, 16–19. [Google Scholar]
- Portugal. Portaria n° 1102-D/2000, de 22 de novembro. Regulamento da Pesca por Arte de Armadilha. Diário da República I 2000, 270, 10–12.
- Vasconcellos, G.M. On the size relation and fecundity of the stock of spiny lobster, Palinurus vulgaris Lat., at the coast of Portugal. ICES CM Shellfish Commitee 1960, 8219, 1–6. [Google Scholar]
- Galhardo, A.C.; Serafim, P.; Castro, M. Aspects of the Biology and Fishery of the European Spiny Lobster (Palinurus elephas) from the Southwest Coast of Portugal. J. Crustac. Biol. 2006, 26, 601–609. [Google Scholar] [CrossRef] [Green Version]
- ICES. Azores ecoregion—Ecosystem overview. In Report of the ICES Advisory Committee; ICES: Copenhagen, Denmark, 2020; ICES Advice 2020, Section 3.1. [Google Scholar]
- ICES. Azores ecoregion—Fisheries overview. In Report of the ICES Advisory Committee; ICES: Copenhagen, Denmark, 2020; ICES Advice 2020, Section 3.2. [Google Scholar]
- Santos, R.; Medeiros-Leal, W.; Pinho, M. Stock assessment prioritization in the Azores: Procedures, current challenges and recommendations. Arquipel. Life Mar. Sci. 2020, 37, 20–45. [Google Scholar]
- Lolas, A.; Vafidis, D. Population Dynamics, Fishery, and Exploitation Status of Norway Lobster (Nephrops norvegicus) in Eastern Mediterranean. Water 2021, 13, 289. [Google Scholar] [CrossRef]
- Fariña, A.C.; González Herraiz, I. Trends in catch-per-unit-effort, stock biomass and recruitment in the North and Northwest Iberian Atlantic Nephrops stocks. Fish. Res. 2003, 65, 351–360. [Google Scholar] [CrossRef]
- Dag, O.; Dolgun, A.; Konar, N.M. Onewaytests: An R Package for One-Way Tests in Independent Groups Designs. R J. 2018, 10, 175–199. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Ogle, D.H.; Doll, J.C.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.9.3, 2021. Available online: https://github.com/fishR-Core-Team/FSA (accessed on 5 February 2022).
- von Bertalanffy, L. A quantitative theory of organic growth (inquires on growth laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing sources of uncertainty in length-based estimates of body growth in populations of fishes and macroinvertebrates with bootstrapped ELEFAN. Ecol. Modell. 2019, 393, 37–51. [Google Scholar] [CrossRef] [Green Version]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.H.; Mildenberger, T.K. Extending electronic length frequency analysis in R. Fish. Manag. Ecol. 2017, 24, 230–238. [Google Scholar] [CrossRef]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Fishboot: Bootstrap-Based Methods for the Study of Fish Stocks and Aquatic Populations; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Ricker, W.E. Computation and interpretation of biological statistics of fish populations. Bull. Fish. Res. Bd. Can. 1975, 191, 1–382. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. A review of the lifespans and mortality rates of fish in nature, and their relation to growth and other physiological characteristics. In Ciba Foundation Symposium—The Lifespan of Animals (Colloquia on Ageing); Wolstenholme, G.E.W., O’Conner, M., Eds.; Ciba Foundation: Indianapolis, IN, USA, 1959; Volume 5, pp. 142–180. [Google Scholar]
- Taylor, C.C. Temperature, growth, and mortality—The pacific cockle. ICES J. Mar. Sci. 1960, 26, 117–124. [Google Scholar] [CrossRef]
- Hewitt, D.A.; Hoenig, J.M. Comparison of two approaches for estimating natural mortality based on longevity. Fish. Bull. 2005, 103, 433. [Google Scholar]
- Djabali, F.; Mehailia, A.; Koudil, M.; Brahmi, B. Empirical equations for the estimation of natural mortality in Mediterranean teleosts. NAGA ICLARM Q. 1993, 16, 35–37. [Google Scholar]
- Tanaka, S. Studies on the dynamics and the management of fish populations. Bull. Tokai Fish. Res. Lab. 1960, 28, 1–200. [Google Scholar]
- Alverson, D.L.; Carney, M.J. A graphic review of the growth and decay of population cohorts. ICES J. Mar. Sci. 1975, 36, 133–143. [Google Scholar] [CrossRef]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Hoenig, J.M. Empirical use of longevity data to estimate mortality rates. Fish. Bull. 1983, 82, 898–903. [Google Scholar]
- Alagaraja, K. Simple methods for estimation of parameters for assessing exploited fish stocks. Indian J. Fish. 1984, 31, 177–208. [Google Scholar]
- Pauly, D.; Binohlan, C. FishBase and AUXIM as Tools for Comparing Life-history Patterns, Growth and Natural Mortality of fish: Applications to Snappers and Groupers. In Biology, Fisheries, and Culture of Tropical Groupers and Snappers, Proceedings of an EPOMEX/ICLARM International Workshop on Tropical Snappers and Groupers, Held at the University of Campeche, Campeche, Mexico, 26–29 October 1993; WorldFish: Penang, Malaysia, 1996; pp. 218–243. [Google Scholar]
- Jensen, A.L. Beverton and Holt life history invariants result from optimal trade-off of reproduction and survival. Can. J. Fish. Aquat. Sci. 1996, 53, 820–822. [Google Scholar] [CrossRef]
- Cubillos, L.A.; Alarcón, R.; Brante, A. Empirical estimates of natural mortality for the Chilean hake (Merluccius gayi): Evaluation of precision. Fish. Res. 1999, 42, 147–153. [Google Scholar] [CrossRef]
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News (Books) Ltd.: West Byfleet, UK, 1971. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Marine Fisheries, Great Britain Ministry of Agriculture, Fisheries and Food, Her Majesty’s Stationery Office: London, UK, 1957; Volume 19. [Google Scholar]
- Lafon, V.; Martins, A.; Figueiredo, M.; Rodrigues, M.; Bashmachnikov, I.; Mendonça, A.; Macedo, L.; Goulart, N. Sea surface temperature distribution in the Azores region. Part I: AVHRR imagery and in situ data processing. Arquipel. Life Mar. Sci. 2004, 21A, 1–18. [Google Scholar]
- Martins, A.; Bashmachnikov, I.; Mendonça, A. Multi-sensor (SeaWiFS/MODIS/AVHRR) Surface Signature of the Azores Current. In Proceedings of the Geophysical Research Abstracts; European Geosciences Union General Assembly: Munich, Germany, 2008; Volume 10. [Google Scholar]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Pt. 1: Manual. Pt. 2: Exercises; FAO: Rome, Italy, 1998; ISBN 0429-9345. [Google Scholar]
- Cochrane, K.L. A Fishery Manager’s Guidebook: Management Measures and Their Application; FAO: Rome, Italy, 2002. [Google Scholar]
- Harper, S.; Zeller, D. Fisheries catch reconstructions: Islands, part II. Fish. Cent. Res. Rep. 2011, 19, 143. [Google Scholar]
- Quetglas, A.; Gaamour, A.; Reñones, O.; Missaoui, H.; Zarrouk, T.; Elabed, A.; Goñi, R. Common spiny lobster (Palinurus elephas Fabricius 1787) fisheries in the western Mediterranean: A comparison of Spanish and Tunisian fisheries. Bolletí Soc. d’Història Nat. Balear. 2004, 47, 63–80. [Google Scholar]
- Follesa, M.C.; Cannas, R.; Cau, A.; Cuccu, D.; Mulas, A.; Porcu, C.; Saba, S.; Cau, A. Homing and orientation of Palinurus elephas (Fabricius) in three no-take areas of the central-western Mediterranean: Implications for marine reserve design. Mar. Freshw. Res. 2015, 66, 1–9. [Google Scholar] [CrossRef]
- Marin, J. Exploitation, Biologie et Dynamique du Stock de Langouste Rouge de Corse, Palinurus elephas Fabricius. Ph.D. Thesis, Université d’Aix-Marseille II, Marseille, France, 1987. [Google Scholar]
- Hepper, B.T. Observation on a crawfish (Palinurus vulgaris Latr) tagging experiment off Cornwall in 1966. ICES CM Shellfish Benthos Comm. 1967, 13, 1–4. [Google Scholar]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Sabatini, A.; Cau, A. Emigration and retention of Palinurus elephas (Fabricius, 1787) in a central western Mediterranean marine protected area. Sci. Mar. 2007, 71, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Mulas, A.; Carbonara, P.; Marongiu, M.F.; Sbaraglia, S.; Carugati, L.; Porcu, C.; Bellodi, A.; Cau, A.; Follesa, M.C. Characterizing movements of Palinurus elephas (Fabr. 1787) as a useful tool in Fully Protected Areas design: The case study of the Sardinian FPAs (central-western Mediterranean). In Proceedings of the 2021 International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea), Reggio Calabria, Italy, 4–6 October 2021; pp. 274–278. [Google Scholar]
- Herrnkind, W.F. Movement patterns and orientation. Biol. Crustac. 1983, 7, 41–105. [Google Scholar]
- Ansell, A.D.; Robb, L. The spiny lobster Palinurus elephas in Scottish waters. Mar. Biol. 1977, 43, 63–70. [Google Scholar] [CrossRef]
- Mercer, J.P. Studies on the Spiny Lobsters (Crustacea: Decapoda: Palinuridae) of the West Coast of Ireland with Particular Reference to Palinurus elephas, Fabricius 1787. Ph.D. Thesis, University College Galway, Galway, Ireland, 1973. [Google Scholar]
- Díaz, D.; Marí, M.; Abelló, P.; Demestre, M. Settlement and juvenile habitat of the European spiny lobster Palinurus elephas (Crustacea: Decapoda: Palinuridae) in the western Mediterranean Sea. Sci. Mar. 2001, 65, 347–356. [Google Scholar] [CrossRef] [Green Version]
- Spanier, E.; McKenzie, T.P.; Cobb, J.S.; Clancy, M. Behavior of juvenile American lobsters, Homarus americanus, under predation risk. Mar. Biol. 1998, 130, 397–406. [Google Scholar] [CrossRef]
- Steneck, R.S. Possible Demographic Consequences of Intraspecific Shelter Competition among American Lobsters. J. Crustac. Biol. 2006, 26, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.; Pinho, M.; Melo, O.; Gonçalves, J.; Leocádio, A.; Aranha, A.; Menezes, G.; Isidro, E. Biological and ecological aspects of the deep-water red crab populations inhabiting isolated seamounts to the west of the Azores (Mid-Atlantic Ridge). Fish. Oceanogr. 2019, 28, 723–734. [Google Scholar] [CrossRef]
- Santos, R.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M.; Isidro, E.; Melo, O.; Santos, R.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M.; et al. Unraveling distributional patterns and life-history traits of a deep-water shrimp Plesionika edwardsii (Decapoda, Pandalidae) under unexploited virgin conditions: A benchmark for fisheries management. Nauplius 2021, 29, 1–13. [Google Scholar] [CrossRef]
- Goñi, R.; Reñones, O.; Quetglas, A. Dynamics of a protected Western Mediterranean population of the European spiny lobster Palinurus elephas (Fabricius, 1787) assessed by trap surveys. Mar. Freshw. Res. 2002, 52, 1577–1587. [Google Scholar] [CrossRef]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Sabatini, A.; Deiana, A.M.; Cau, A. Movement patterns of the spiny lobster Palinurus elephas (Fabricius, 1787) from a central western Mediterranean protected area. Sci. Mar. 2009, 73, 499–506. [Google Scholar] [CrossRef] [Green Version]
- Follesa, M.C.; Cuccu, D.; Cannas, R.; Cau, A. On the growth of the European spiny lobster, Palinurus elephas from Sardinian waters (central western Mediterranean Sea). N. Z. J. Mar. Freshw. Res. 2007, 41, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Wahle, R.A.; Fogarty, M.J. Growth and development: Understanding and modelling growth variability in lobsters. In Lobsters: Biology, Management, Aquaculture and Fisheries; Phillips, B.F., Ed.; Blackwell Publishing: Oxford, UK, 2006; pp. 1–44. [Google Scholar]
- Rjeibi, O.; Gaamour, A.; Missaoui, H. Etude de la croissance de la langouste rouge Palinurus elephas, dans les eaux Tunisiennes. Bull. l’Institut Natl. Sci. Technol. Mer. 2011, 38, 41–54. [Google Scholar]
- Groeneveld, J.C. Stock assessment, ecology and economics as criteria for choosing between trap and trawl fisheries for spiny lobster Palinurus delagoae. Fish. Res. 2000, 48, 141–155. [Google Scholar] [CrossRef]
- Groeneveld, J.C. Growth of spiny lobster Palinurus gilchristi (Decapoda: Palinuridae) off South Africa. Afr. J. Mar. Sci. 1997, 18, 19–30. [Google Scholar] [CrossRef]
- Pollock, D.E.; Beyers, C.D.B. Environment, distribution and growth rates of West Coast rock-lobster Jasus lalandii (H. Milne Edwards). Trans. R. Soc. S. Afr. 1981, 44, 379–400. [Google Scholar] [CrossRef]
- Pollock, D.E. The Fishery for and Population Dynamics of West Coast Rock Lobster Related to the Environment in the Lambert’s Bay and Port Nolloth Areas; Investigations of the Sea Fisheries Institute of South Africa: Cape Town, South Africa, 1982; ISBN 0621058807. [Google Scholar]
- McKoy, J.L.; Esterman, D.B. Growth of rock lobsters (Jasus edwardsii) in the Gisborne region, New Zealand. N. Z. J. Mar. Freshw. Res. 1981, 15, 121–136. [Google Scholar] [CrossRef]
- Cobb, J.S.; Phillips, B.F. The Biology and Management of Lobsters: Ecology and Management; Academic Press: New York, NY, USA, 1980; Volume II. [Google Scholar]
- Bevacqua, D.; Melià, P.; Follesa, M.C.; De Leo, G.A.; Gatto, M.; Cau, A. Body growth and mortality of the spiny lobster Palinurus elephas within and outside a small marine protected area. Fish. Res. 2010, 106, 543–549. [Google Scholar] [CrossRef]
- Goñi, R.; Hilborn, R.; Díaz, D.; Mallol, S.; Adlerstein, S. Net contribution of spillover from a marine reserve to fishery catches. Mar. Ecol. Prog. Ser. 2010, 400, 233–243. [Google Scholar] [CrossRef] [Green Version]
- Díaz, D.; Mallol, S.; Parma, A.M.; Goñi, R. A 25-year marine reserve as proxy for the unfished condition of an exploited species. Biol. Conserv. 2016, 203, 97–107. [Google Scholar] [CrossRef]
- Latrouite, D.; Noel, P. Pêche de la langouste rouge Palinurus elephas en France. Eléments pour fixer une taille marchande. In Proceedings of the ICES 1997: Theme Session on Biology and Behaviour, Baltimore, MD, USA, 22–24 September 1997; p. 13. [Google Scholar]
- Tidu, C.; Sardá, R.; Pinna, M.; Cannas, A.; Meloni, M.F.; Lecca, E.; Savarino, R. Morphometric relationships of the European spiny lobster Palinurus elephas from northwestern Sardinia. Fish. Res. 2004, 69, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Campillo, A.; Amadei, J. Premieres donnees biologiques sur la langouste de Corse, Palinurus elephas Fabricius. Rev. Trav. l’Institut PÃaches Marit. 1978, 42, 347–373. [Google Scholar]
- Goñi, R.; Quetglas, A.; Reñones, O. Size at maturity, fecundity and reproductive potential of a protected population of the spiny lobster Palinurus elephas (Fabricius, 1787) from the western Mediterranean. Mar. Biol. 2003, 143, 583–592. [Google Scholar] [CrossRef]
- EC. Communication from the Commission on the Precautionary Principle; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Açores. Decreto Legislativo Regional 15/2012/A, de 2 de Abril. Estabelece o regime jurídico da conservação da natureza e da biodiversidade. Diário República I 2012, 66, 1625–1713. [Google Scholar]
- Açores. Portaria n.° 91/2005, de 22 de dezembro. Regulamenta, na Região Autónoma dos Açores, a pesca com redes de emalhar. J. Região Autónoma Açores I 2005, 51, 1141. [Google Scholar]
- Açores. Portaria n.° 30/2004 de 22 de Abril de 2004. Regulamenta o exercício da pesca da Região Autónoma dos Açores, com artes de armadilha. J. Região Autónoma Açores I 2004, 17, 697. [Google Scholar]
- Açores. Portaria n.° 79/2017 de 18 de outubro de 2017. Aprova o Regulamento do Método de Pesca por Armadilha. J. Região Autónoma Açores I 2017, 102, 2221–2228. [Google Scholar]
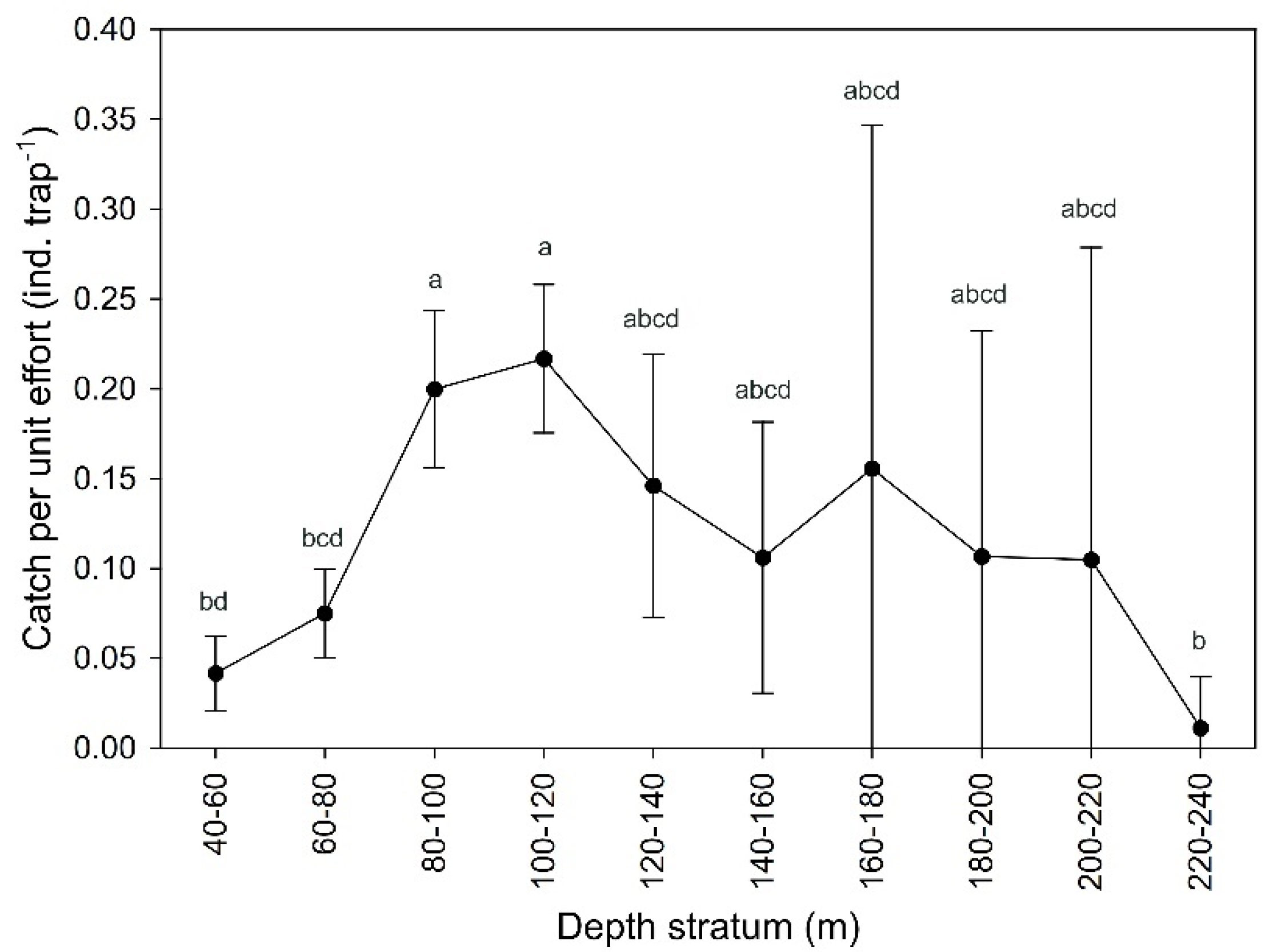



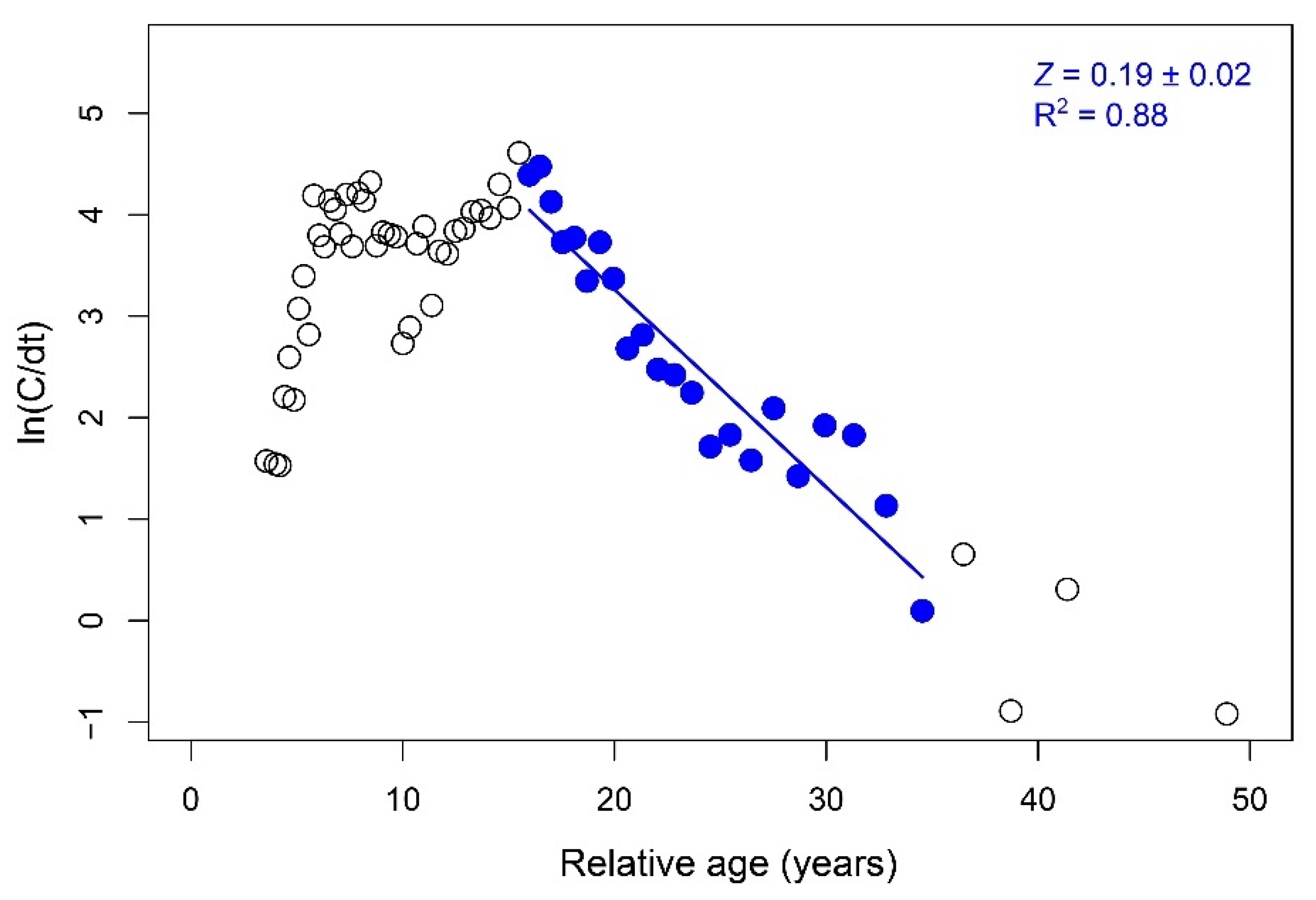

| Sex | n | a | b | SE (a) | SE (b) | R2 | Departure from b = 3 |
|---|---|---|---|---|---|---|---|
| Males | 196 | 0.002 | 2.839 | 0.124 | 0.027 | 0.983 | t = −5.939; p < 0.001 |
| Females | 215 | 0.003 | 2.769 | 0.132 | 0.029 | 0.977 | t = −7.987; p < 0.001 |
| Pooled | 411 | 0.002 | 2.812 | 0.095 | 0.021 | 0.978 | t = −9.018; p < 0.001 |
| Parameter | Estimate | Empirical Formula | Reference |
|---|---|---|---|
| Life span (tmax; years) | 42.86 | tmax = 3/k | [32] |
| Natural mortality (M; year−1) | 0.12 | M = 5/tmax | [34] |
| 0.07 | M = 2.996/tmax | [35] | |
| 0.06 | M = 2.5/tmax | [38] | |
| 0.18 | M = 3 k/(e 0.38 tmax × k − 1) | [39] | |
| 0.45 | M = exp(−0.0066 − 0.279 × log(L∞) + 0.6543 × log(k) + 0.4634 × log(T)) | [40] | |
| 0.07 | M = 3/tmax | [41] | |
| 0.11 | M = 4.6/tmax | [42] | |
| 0.15 | M = 1.0661 × L∞−0.1172 × k 0.5092 | [37] | |
| 0.04 | M = −0.1778 + 3.1687 k | [43] | |
| 0.11 | M = 1.6 k | [44] | |
| 0.11 | M = 1.5 k | [44] | |
| 0.10 | M = 1.4 k | [45] | |
| 0.10 | M = 4.22/tmax | [36] | |
| 0.13 | Average M value | ||
| Fishing mortality (F; year−1) | 0.06 | F = Z − M | |
| Exploitation rate (E; year−1) | 0.33 | E = F/(F + M) | [46] |
| Lc | tc | F10 | Fmax | E50 | E10 | Emax |
|---|---|---|---|---|---|---|
| 90.00 | 10.16 | 0.12 | 0.16 | 0.27 | 0.48 | 0.55 |
| 101.65 | 12.22 | 0.08 | 0.17 | 0.27 | 0.38 | 0.56 |
| 113.30 | 14.63 | 0.05 | 0.18 | 0.27 | 0.27 | 0.58 |
| Source | Area | Method | n | Size | Sex | Growth Parameter | Mortality Rate | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ | k | t0 | ϕ′ | Z | M | F | ||||||
| Mediterranean Sea | ||||||||||||
| [55] | Corsica | MR, TA | 61 | CL | M | 166.0 | 0.15 | −0.35 | 3.62 | 0.30–0.52 | 0.15–0.30 | 0.15–0.22 |
| MR, TA | 50 | CL | F | 135.9 | 0.19 | −0.34 | 3.54 | 0.23–0.42 | 0.15–0.30 | 0.08–0.12 | ||
| [69] | CW Mediterranean Sea | MR | 146 | CL | M | 167.9 | 0.13 | −0.40 | 3.56 | |||
| MR | 146 | CL | M | 167.0 | 0.13 | −0.40 | 3.56 | |||||
| MR | 102 | CL | F | 120.2 | 0.21 | −0.35 | 3.48 | |||||
| MR | 102 | CL | F | 125.0 | 0.19 | −0.37 | 3.47 | |||||
| [78] | CW Mediterranean Sea | MR | 147 | CL | M | 189.0 | 0.10 | 3.50 | ||||
| MR | 107 | CL | F | 117.0 | 0.16 | 3.30 | ||||||
| MR | 254 | CL | P | 0.70 | 0.27 | 0.43 | ||||||
| [79] | W Mediterranean Sea | MR | 270 | CL | M | 0.20 | ||||||
| MR | 296 | CL | F | 0.16 | ||||||||
| [71] | Tunisia | LFD | - | CL | M | 201.6 | 0.16 | −0.27 | 3.81 | |||
| LFD | - | CL | F | 155.4 | 0.22 | −0.25 | 3.73 | |||||
| [80] | W Mediterranean Sea | LCC | - | CL | M | 0.19–0.80 | ||||||
| LCC | - | CL | F | 0.17–0.57 | ||||||||
| Atlantic Ocean | ||||||||||||
| [5] | Cornwall | MR | 286 | CL | P | 0.23 | 0.11 | 0.12 | ||||
| This study | Azores | LFD | 325 | CL | M | 176.9 | 0.05 | 3.32 | ||||
| LFD | 559 | CL | F | 157.2 | 0.07 | 3.26 | ||||||
| LFD, LCC | 884 | CL | P | 176.8 | 0.07 | 3.32 | 0.19 | 0.13 | 0.06 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, R.; Peixoto, U.I.; Medeiros-Leal, W.; Sequeira, R.M.; Novoa-Pabon, A.; Pinho, M. Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management. Biology 2022, 11, 474. https://doi.org/10.3390/biology11030474
Santos R, Peixoto UI, Medeiros-Leal W, Sequeira RM, Novoa-Pabon A, Pinho M. Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management. Biology. 2022; 11(3):474. https://doi.org/10.3390/biology11030474
Chicago/Turabian StyleSantos, Régis, Ualerson Iran Peixoto, Wendell Medeiros-Leal, Rui M. Sequeira, Ana Novoa-Pabon, and Mário Pinho. 2022. "Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management" Biology 11, no. 3: 474. https://doi.org/10.3390/biology11030474
APA StyleSantos, R., Peixoto, U. I., Medeiros-Leal, W., Sequeira, R. M., Novoa-Pabon, A., & Pinho, M. (2022). Demographics and Yield–Per–Recruit Assessment of the Vulnerable Spiny Lobster Palinurus elephas in the Azores—Implications for Conservation and Fisheries Management. Biology, 11(3), 474. https://doi.org/10.3390/biology11030474







