Molecular Pathophysiology and Potential Therapeutic Strategies of Ketamine-Related Cystitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Clinical and Pathological Features of Ketamine-Related Cystitis
3. Molecular Evidence of the Pathophysiology and Potential Biomarkers
3.1. Evidence from the Human Bladder Tissue
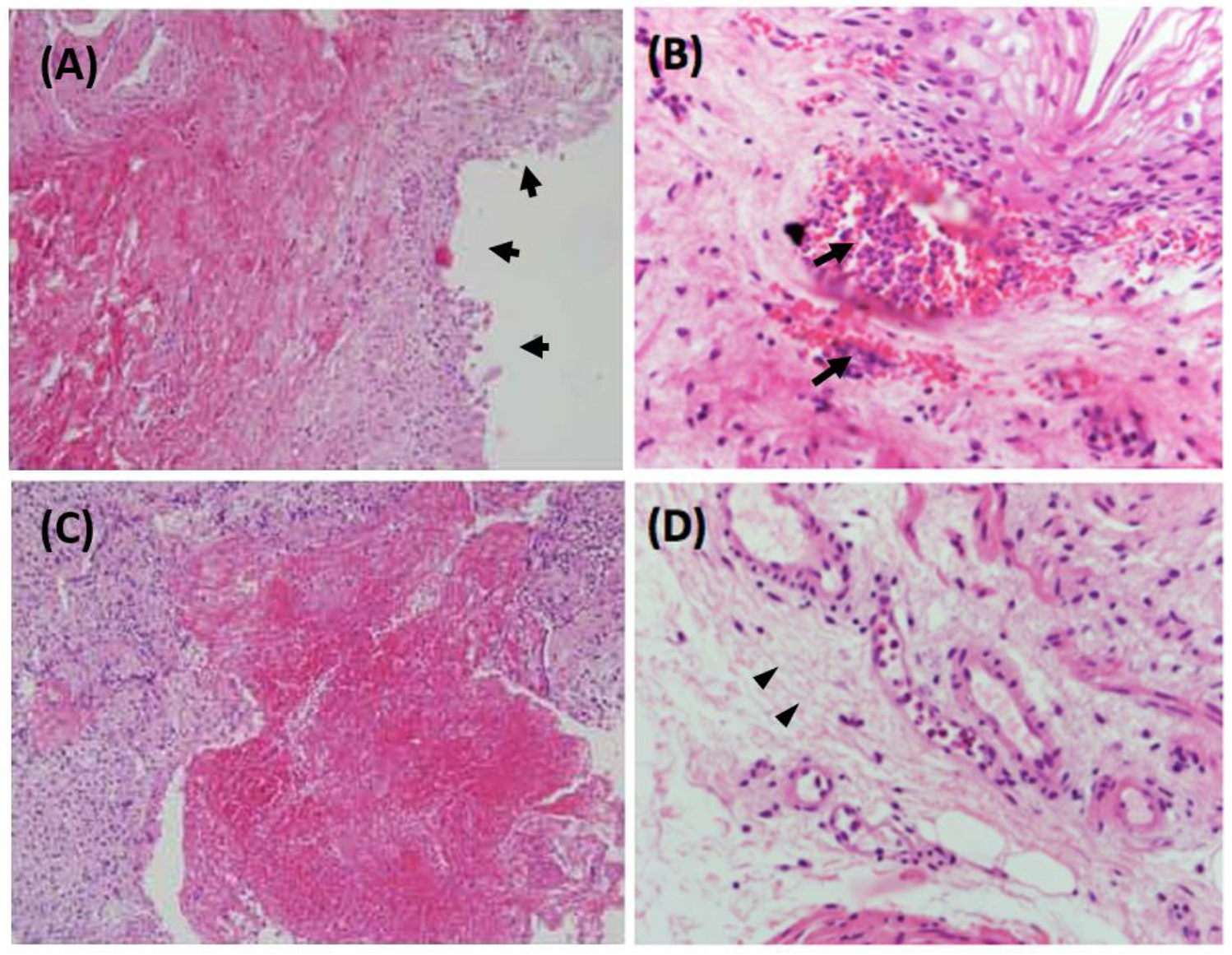
3.1.1. Inflammatory and Immune Reactivity in Human KC Bladder
3.1.2. Ketamine Might Affect Neuronal Growth
3.1.3. Ion Channels Might Be the Mechanosensory in the KC Bladder
3.2. Evidence from the Animal Model
3.2.1. Deficiency of Urothelial Junction-Associated Protein Impaired the Barrier
3.2.2. Oxidative Stress Species Enhanced Bladder Hyperactivity
3.2.3. Ketamine Increased Purinergic Neurotransmission Caused Detrusor Overactivity
3.2.4. Ketamine Moderate Ion Channels in the Bladder Smooth Muscle and Affect the Bladder Function
3.2.5. Change of Extracellular Matrix Gene Expression May Involve Bladder Fibrosis
3.2.6. Immune and Inflammatory Signaling Pathways Altered in KC Bladders
3.2.7. Ketamine Induced Dysregulation of Autophagy and Inhibition of Angiogenesis
3.3. In Vitro Study
3.3.1. Increased Cytosolic Ca2+ Concentration May Be Lethal to the Urothelial Cells
3.3.2. Ketamine Induced Cytotoxicity and Apoptosis of Human Urothelial Cells
4. Current and Potential Treatment for Ketamine-Related Cystitis
4.1. Hyaluronic Acid
4.2. Botulinum Toxin A
4.3. Bay K8644
4.4. Rapamycin
4.5. Wortmannin
4.6. Ba-Wei-Die-Huang-Wan (Hachimi-Jio-Gan)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lankenau, S.E.; Sanders, B. Patterns of ketamine use among young injection drug users. J. Psychoact. Drugs 2007, 39, 21–29. [Google Scholar]
- Wood, D.; Cottrell, A.; Baker, S.C.; Southgate, J.; Harris, M.; Fulford, S.; Woodhouse, C.; Gillatt, D. Recreational ketamine: From pleasure to pain. BJU Int. 2011, 107, 1881–1884. [Google Scholar] [PubMed]
- Pal, R.; Balt, S.; Erowid, E.; Erowid, F.; Baggott, M.J.; Mendelson, J.; Galloway, G.P. Ketamine is associated with lower urinary tract signs and symptoms. Drug Alcohol Depend. 2013, 132, 189–194. [Google Scholar] [PubMed]
- Chu, P.S.; Kwok, S.C.; Lam, K.M.; Chu, T.Y.; Chan, S.W.; Man, C.W.; Ma, W.K.; Chui, K.L.; Yiu, M.K.; Chan, Y.C.; et al. ‘Street ketamine’-associated bladder dysfunction: A report of ten cases. Hong Kong Med. J. 2007, 13, 311–313. [Google Scholar]
- Winstock, A.R.; Mitcheson, L.; Gillatt, D.A.; Cottrell, A.M. The prevalence and natural history of urinary symptoms among recreational ketamine users. BJU Int. 2012, 110, 1762–1766. [Google Scholar]
- Yiu-Cheung, C. Acute and chronic toxicity pattern in ketamine abusers in hong kong. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2012, 8, 267–270. [Google Scholar]
- Chu, P.S.; Ma, W.K.; Wong, S.C.; Chu, R.W.; Cheng, C.H.; Wong, S.; Tse, J.M.; Lau, F.L.; Yiu, M.K.; Man, C.W. The destruction of the lower urinary tract by ketamine abuse: A new syndrome? BJU Int. 2008, 102, 1616–1622. [Google Scholar]
- Colebunders, B.; Van Erps, P. Cystitis due to the use of ketamine as a recreational drug: A case report. J. Med. Case Rep. 2008, 2, 219. [Google Scholar]
- Jang, M.Y.; Long, C.Y.; Chuang, S.M.; Huang, C.H.; Lin, H.Y.; Wu, W.J.; Juan, Y.S. Sexual dysfunction in women with ketamine cystitis: A case-control study. BJU Int. 2012, 110, 427–431. [Google Scholar]
- Shahani, R.; Streutker, C.; Dickson, B.; Stewart, R.J. Ketamine-associated ulcerative cystitis: A new clinical entity. Urology 2007, 69, 810–812. [Google Scholar]
- Mak, S.K.; Chan, M.T.; Bower, W.F.; Yip, S.K.; Hou, S.S.; Wu, B.B.; Man, C.Y. Lower urinary tract changes in young adults using ketamine. J. Urol. 2011, 186, 610–614. [Google Scholar]
- Baker, S.C.; Shabir, S.; Georgopoulos, N.T.; Southgate, J. Ketamine-induced apoptosis in normal human urothelial cells: A direct, n-methyl-d-aspartate receptor-independent pathway characterized by mitochondrial stress. Am. J. Pathol. 2016, 186, 1267–1277. [Google Scholar]
- Chen, W.Y.; Huang, M.C.; Lin, S.K. Gender differences in subjective discontinuation symptoms associated with ketamine use. Subst. Abus. Treat. Prev. Policy 2014, 9, 39. [Google Scholar]
- Wei, Y.B.; Yang, J.R.; Yin, Z.; Guo, Q.; Liang, B.L.; Zhou, K.Q. Genitourinary toxicity of ketamine. Hong Kong Med. J. Xianggang Yi Xue Za Zhi 2013, 19, 341–348. [Google Scholar]
- Chen, H.; Vandorpe, D.H.; Xie, X.; Alper, S.L. Disruption of cav1.2-mediated signaling is a pathway for ketamine-induced pathology. Nat. Commun. 2020, 11, 4328. [Google Scholar]
- Mason, K.; Cottrell, A.M.; Corrigan, A.G.; Gillatt, D.A.; Mitchelmore, A.E. Ketamine-associated lower urinary tract destruction: A new radiological challenge. Clin. Radiol. 2010, 65, 795–800. [Google Scholar]
- Lin, H.C.; Lee, H.S.; Chiueh, T.S.; Lin, Y.C.; Lin, H.A.; Lin, Y.C.; Cha, T.L.; Meng, E. Histopathological assessment of inflammation and expression of inflammatory markers in patients with ketamine-induced cystitis. Mol. Med. Rep. 2015, 11, 2421–2428. [Google Scholar]
- Fan, G.Y.; Cherng, J.H.; Chang, S.J. The immunomodulatory imbalance in patients with ketamine cystitis. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar]
- Kidger, E.; Stahlschmidt, J.; Garthwaite, M.; Fulford, S.; Southgate, J.; Baker, S.C. A rare urachal cyst in a case of ketamine-induced cystitis provides mechanistic insights. Urology 2016, 90, e221–e227. [Google Scholar]
- Castellani, D.; Pirola, G.M. What urologists need to know about ketamine-induced uropathy: A systematic review. Neurourol. Urodyn. 2020, 39, 1049–1062. [Google Scholar]
- Jhang, J.F.; Hsu, Y.H.; Jiang, Y.H.; Kuo, H.C. Elevated serum ige may be associated with development of ketamine cystitis. J. Urol. 2014, 192, 1249–1256. [Google Scholar]
- Gu, D.; Huang, J.; Yin, Y.; Shan, Z.; Zheng, S.; Wu, P. Long-term ketamine abuse induces cystitis in rats by impairing the bladder epithelial barrier. Mol. Biol. Rep. 2014, 41, 7313–7322. [Google Scholar]
- Baker, S.C.; Stahlschmidt, J.; Oxley, J.; Hinley, J.; Eardley, I.; Marsh, F.; Gillatt, D.; Fulford, S.; Southgate, J. Nerve hyperplasia: A unique feature of ketamine cystitis. Acta Neuropathol. Commun. 2013, 1, 64. [Google Scholar]
- Lee, C.L.; Jiang, Y.H.; Kuo, H.C. Increased apoptosis and suburothelial inflammation in patients with ketamine-related cystitis: A comparison with non-ulcerative interstitial cystitis and controls. BJU Int. 2013, 112, 1156–1162. [Google Scholar]
- Shen, C.H.; Wang, S.T.; Lee, Y.R.; Liu, S.Y.; Li, Y.Z.; Wu, J.D.; Chen, Y.J.; Liu, Y.W. Biological effect of ketamine in urothelial cell lines and global gene expression analysis in the bladders of ketamine-injected mice. Mol. Med. Rep. 2015, 11, 887–895. [Google Scholar]
- Liu, K.M.; Chuang, S.M.; Long, C.Y.; Lee, Y.L.; Wang, C.C.; Lu, M.C.; Lin, R.J.; Lu, J.H.; Jang, M.Y.; Wu, W.J.; et al. Ketamine-induced ulcerative cystitis and bladder apoptosis involve oxidative stress mediated by mitochondria and the endoplasmic reticulum. Am. J. Physiol. Ren. Physiol. 2015, 309, F318–F331. [Google Scholar]
- Juan, Y.S.; Lee, Y.L.; Long, C.Y.; Wong, J.H.; Jang, M.Y.; Lu, J.H.; Wu, W.J.; Huang, Y.S.; Chang, W.C.; Chuang, S.M. Translocation of nf-κb and expression of cyclooxygenase-2 are enhanced by ketamine-induced ulcerative cystitis in rat bladder. Am. J Pathol. 2015, 185, 2269–2285. [Google Scholar]
- Adamowicz, P.; Kala, M. Urinary excretion rates of ketamine and norketamine following therapeutic ketamine administration: Method and detection window considerations. J. Anal. Toxicol. 2005, 29, 376–382. [Google Scholar] [PubMed] [Green Version]
- Vial, C.; Evans, R.J. P2x receptor expression in mouse urinary bladder and the requirement of p2x(1) receptors for functional p2x receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000, 131, 1489–1495. [Google Scholar] [PubMed] [Green Version]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [PubMed] [Green Version]
- Lee, W.C.; Su, C.H.; Tain, Y.L.; Tsai, C.N.; Yu, C.C.; Chuang, Y.C. Potential orphan drug therapy of intravesical liposomal onabotulinumtoxin-a for ketamine-induced cystitis by mucosal protection and anti-inflammation in a rat model. Sci. Rep. 2018, 8, 5795. [Google Scholar]
- Lee, Y.L.; Lin, K.L.; Chuang, S.M.; Lee, Y.C.; Lu, M.C.; Wu, B.N.; Wu, W.J.; Yuan, S.F.; Ho, W.T.; Juan, Y.S. Elucidating mechanisms of bladder repair after hyaluronan instillation in ketamine-induced ulcerative cystitis in animal model. Am. J. Pathol. 2017, 187, 1945–1959. [Google Scholar]
- Yang, H.H.; Jhang, J.F.; Hsu, Y.H.; Jiang, Y.H.; Zhai, W.J.; Kuo, H.C. Smaller bladder capacity and stronger bladder contractility in patients with ketamine cystitis are associated with elevated trpv1 and trpv4. Sci. Rep. 2021, 11, 5200. [Google Scholar]
- Lee, W.C.; Tain, Y.L.; Chuang, Y.C. Ba-wei-die-huang-wan (hachimi-jio-gan) can ameliorate ketamine-induced cystitis by modulating neuroreceptors, inflammatory mediators, and fibrogenesis in a rat model. J. Ethnopharmacol. 2019, 38, 2159–2169. [Google Scholar]
- Lee, W.C.; Wu, C.C.; Chuang, Y.C.; Tain, Y.L.; Chiang, P.H. Ba-wei-die-huang-wan (hachimi-jio-gan) can ameliorate cyclophosphamide-induced ongoing bladder overactivity and acidic adenosine triphosphate solution-induced hyperactivity on rats prestimulated bladder. J. Ethnopharmacol. 2016, 184, 1–9. [Google Scholar]
- Meng, E.; Chang, H.Y.; Chang, S.Y.; Sun, G.H.; Yu, D.S.; Cha, T.L. Involvement of purinergic neurotransmission in ketamine induced bladder dysfunction. J. Urol. 2011, 186, 1134–1141. [Google Scholar]
- Shen, C.H.; Wang, S.T.; Wang, S.C.; Lin, S.M.; Lin, L.C.; Dai, Y.C.; Liu, Y.W. Ketamine-induced bladder dysfunction is associated with extracellular matrix accumulation and impairment of calcium signaling in a mouse model. Mol. Med. Rep. 2019, 19, 2716–2728. [Google Scholar]
- Petkov, G.V. Central role of the bk channel in urinary bladder smooth muscle physiology and pathophysiology. Am. J Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R571–R584. [Google Scholar]
- Wang, Q.; Wu, Q.; Wang, J.; Chen, Y.; Zhang, G.; Chen, J.; Zhao, J.; Wu, P. Ketamine analog methoxetamine induced inflammation and dysfunction of bladder in rats. Int. J. Mol. Sci. 2017, 18, 117. [Google Scholar]
- Lu, J.H.; Wu, Y.H.; Juan, T.J.; Lin, H.Y.; Lin, R.J.; Chueh, K.S.; Lee, Y.C.; Chang, C.Y.; Juan, Y.S. Autophagy alters bladder angiogenesis and improves bladder hyperactivity in the pathogenesis of ketamine-induced cystitis in a rat model. Biology 2021, 10, 488. [Google Scholar]
- Galli, S.J.; Tsai, M. Ige and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A bdnf autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar]
- Bekinschtein, P.; Cammarota, M.; Izquierdo, I.; Medina, J.H. Bdnf and memory formation and storage. Neurosci. A Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2008, 14, 147–156. [Google Scholar]
- Ricci, V.; Martinotti, G.; Gelfo, F.; Tonioni, F.; Caltagirone, C.; Bria, P.; Angelucci, F. Chronic ketamine use increases serum levels of brain-derived neurotrophic factor. Psychopharmacology 2011, 215, 143–148. [Google Scholar]
- Ke, X.; Ding, Y.; Xu, K.; He, H.; Zhang, M.; Wang, D.; Deng, X.; Zhang, X.; Zhou, C.; Liu, Y.; et al. Serum brain-derived neurotrophic factor and nerve growth factor decreased in chronic ketamine abusers. Drug Alcohol Depend. 2014, 142, 290–294. [Google Scholar]
- Sultana, S.; Berger, G.; Cox, A.; Kelly, M.E.M.; Lehmann, C. Rodent models of ketamine-induced cystitis. Neurourol. Urodyn. 2021, 40, 1704–1719. [Google Scholar]
- Yeh, C.H.; Chen, B.H.; Tseng, X.W.; Liao, C.H.; Tsai, W.K. Intravesical instillation of norketamine, a ketamine metabolite, and induced bladder functional changes in rats. Toxics 2021, 9, 154. [Google Scholar]
- Matter, K.; Balda, M.S. Signalling to and from tight junctions. Nat. Reviews. Mol. Cell Biol. 2003, 4, 225–236. [Google Scholar]
- Wu, X.R.; Kong, X.P.; Pellicer, A.; Kreibich, G.; Sun, T.T. Uroplakins in urothelial biology, function, and disease. Kidney Int. 2009, 75, 1153–1165. [Google Scholar]
- Liu, H.T.; Shie, J.H.; Chen, S.H.; Wang, Y.S.; Kuo, H.C. Differences in mast cell infiltration, e-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology 2012, 80, e213–e228. [Google Scholar]
- Shahzad, K. Understanding Mechanisms of Ketamine-Induced Human Urinary Tract Damage. Master’s Thesis, The University of York, York, UK, October 2011. Available online: https://etheses.whiterose.ac.uk/2104/1/Khurram_Shahzad_MSc_Thesis.pdf (accessed on 13 March 2021).
- Parekh, M.H.; Lobel, R.; O’Connor, L.J.; Leggett, R.E.; Levin, R.M. Protective effect of vitamin e on the response of the rabbit bladder to partial outlet obstruction. J. Urol. 2001, 166, 341–346. [Google Scholar] [PubMed]
- Lin, A.T.; Yang, C.H.; Chen, K.K.; Chang, L.S. Detrusor mitochondrial lipid peroxidation and superoxide dismutase activity in partial bladder outlet obstruction of rabbits. Neurourol. Urodyn. 2005, 24, 282–287. [Google Scholar] [PubMed]
- Yu, H.J.; Chien, C.T.; Lai, Y.J.; Lai, M.K.; Chen, C.F.; Levin, R.M.; Hsu, S.M. Hypoxia preconditioning attenuates bladder overdistension-induced oxidative injury by up-regulation of bcl-2 in the rat. J. Physiol. 2004, 554, 815–828. [Google Scholar] [PubMed]
- Erdem, E.; Leggett, R.; Dicks, B.; Kogan, B.A.; Levin, R.M. Effect of bladder ischaemia/reperfusion on superoxide dismutase activity and contraction. BJU Int. 2005, 96, 169–174. [Google Scholar]
- Chien, C.T.; Yu, H.J.; Lin, T.B.; Lai, M.K.; Hsu, S.M. Substance p via nk1 receptor facilitates hyperactive bladder afferent signaling via action of ros. Am. J. Physiol. Ren. Physiol. 2003, 284, F840–F851. [Google Scholar]
- Chen, W.C.; Hayakawa, S.; Shimizu, K.; Chien, C.T.; Lai, M.K. Catechins prevents substance p-induced hyperactive bladder in rats via the downregulation of icam and ros. Neurosci. Lett. 2004, 367, 213–217. [Google Scholar]
- Aikawa, K.; Leggett, R.; Levin, R.M. Effect of age on hydrogen peroxide mediated contraction damage in the male rat bladder. J. Urol. 2003, 170, 2082–2085. [Google Scholar]
- De Jongh, R.; Haenen, G.R.; van Koeveringe, G.A.; Dambros, M.; De Mey, J.G.; van Kerrebroeck, P.E. Oxidative stress reduces the muscarinic receptor function in the urinary bladder. Neurourol. Urodyn. 2007, 26, 302–308. [Google Scholar]
- Huster, M.; Frei, E.; Hofmann, F.; Wegener, J.W. A complex of ca(v)1.2/pkc is involved in muscarinic signaling in smooth muscle. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 2651–2659. [Google Scholar]
- Petkov, G.V. Role of potassium ion channels in detrusor smooth muscle function and dysfunction. Nat. Reviews. Urol. 2011, 9, 30–40. [Google Scholar]
- Roth, B.L.; Gibbons, S.; Arunotayanun, W.; Huang, X.P.; Setola, V.; Treble, R.; Iversen, L. The ketamine analogue methoxetamine and 3- and 4-methoxy analogues of phencyclidine are high affinity and selective ligands for the glutamate nmda receptor. PLoS ONE 2013, 8, e59334. [Google Scholar]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. Mtor signaling pathway and mtor inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar]
- Li, J.; Kim, S.G.; Blenis, J. Rapamycin: One drug, many effects. Cell Metab. 2014, 19, 373–379. [Google Scholar]
- Fan, N.; Zhang, M.; Xu, K.; Ke, X.; Ding, Y.; Wang, D.; Liu, Y.; Ning, Y.; Deng, X.; He, H. Serum level of vascular endothelial growth factor decreased in chronic ketamine abusers. Drug Alcohol Depend. 2015, 152, 57–61. [Google Scholar]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. Vegf-a/vegfr2 signaling network in endothelial cells relevant to angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar]
- Ow, Y.P.; Green, D.R.; Hao, Z.; Mak, T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008, 9, 532–542. [Google Scholar]
- Santucci, R.; Sinibaldi, F.; Cozza, P.; Polticelli, F.; Fiorucci, L. Cytochrome c: An extreme multifunctional protein with a key role in cell fate. Int. J. Biol. Macromol. 2019, 136, 1237–1246. [Google Scholar]
- Otori, K.; Yano, Y.; Takada, N.; Lee, C.C.; Hayashi, S.; Otani, S.; Fukushima, S. Reversibility and apoptosis in rat urinary bladder papillomatosis induced by uracil. Carcinogenesis 1997, 18, 1485–1489. [Google Scholar]
- Jock, M.; Leggett, R.E.; Schuler, C.; Callaghan, C.; Levin, R.M. Effect of partial bladder outlet obstruction and reversal on rabbit bladder physiology and biochemistry: Duration of recovery period and severity of function. BJU Int. 2014, 114, 946–954. [Google Scholar]
- Lai, Y.; Wu, S.; Ni, L.; Chen, Z.; Li, X.; Yang, S.; Gui, Y.; Guan, Z.; Cai, Z.; Ye, J. Ketamine-associated urinary tract dysfunction: An underrecognized clinical entity. Urol. Int. 2012, 89, 93–96. [Google Scholar]
- Meng, E.; Tsao, C.W.; Tang, S.H.; Wu, S.T.; Cha, T.L.; Sun, G.H.; Yu, D.S.; Chang, S.Y. Intravesical hyaluronic acid treatment for ketamine-associated cystitis: Preliminary results. Urol. Sci. 2015, 26, 176–179. [Google Scholar]
- Zeng, J.; Lai, H.; Zheng, D.; Zhong, L.; Huang, Z.; Wang, S.; Zou, W.; Wei, L. Effective treatment of ketamine-associated cystitis with botulinum toxin type a injection combined with bladder hydrodistention. J. Int. Med. Res. 2017, 45, 792–797. [Google Scholar]
- Parsons, C.L.; Boychuk, D.; Jones, S.; Hurst, R.; Callahan, H. Bladder surface glycosaminoglycans: An epithelial permeability barrier. J. Urol. 1990, 143, 139–142. [Google Scholar]
- Reitinger, S.; Lepperdinger, G. Hyaluronan, a ready choice to fuel regeneration: A mini-review. Gerontology 2013, 59, 71–76. [Google Scholar]
- Chuang, Y.C.; Yoshimura, N.; Huang, C.C.; Chiang, P.H.; Chancellor, M.B. Intravesical botulinum toxin a administration produces analgesia against acetic acid induced bladder pain responses in rats. J. Urol. 2004, 172, 1529–1532. [Google Scholar]
- Jhang, J.F.; Kuo, H.C. Botulinum toxin a and lower urinary tract dysfunction: Pathophysiology and mechanisms of action. Toxins 2016, 8, 120. [Google Scholar]
- Kuo, H.C. Repeated intravesical onabotulinumtoxina injections are effective in treatment of refractory interstitial cystitis/bladder pain syndrome. Int. J. Clin. Pract. 2013, 67, 427–434. [Google Scholar]
- Liu, H.T.; Kuo, H.C. Intravesical botulinum toxin a injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 2007, 70, 463–468. [Google Scholar]
- Imamura, T.; Ishizuka, O.; Zhong, C.; Ogawa, T.; Nakayama, T.; Kurizaki, Y.; Tanabe, T.; Nishizawa, O.; Andersson, K.E. An extract (thc-002) of ba-wei-die-huang-wan inhibits expression of tachykinins, and p2x3 and trpv1 receptors, and inhibits atp-induced detrusor overactivity in spontaneously hypertensive rats. Neurourol. Urodyn. 2009, 28, 529–534. [Google Scholar]
- Tong, Y.C.; Cheng, J.T.; Wan, W.C. Effects of ba-wei-die-huang-wan on the cholinergic function and protein expression of m2 muscarinic receptor of the urinary bladder in diabetic rats. Neurosci. Lett. 2002, 330, 21–24. [Google Scholar] [PubMed]
- Tsai, W.H.; Wu, C.H.; Cheng, C.H.; Chien, C.T. Ba-wei-di-huang-wan through its active ingredient loganin counteracts substance p-enhanced nf-κb/icam-1 signaling in rats with bladder hyperactivity. Neurourol. Urodyn. 2016, 35, 771–779. [Google Scholar] [PubMed]
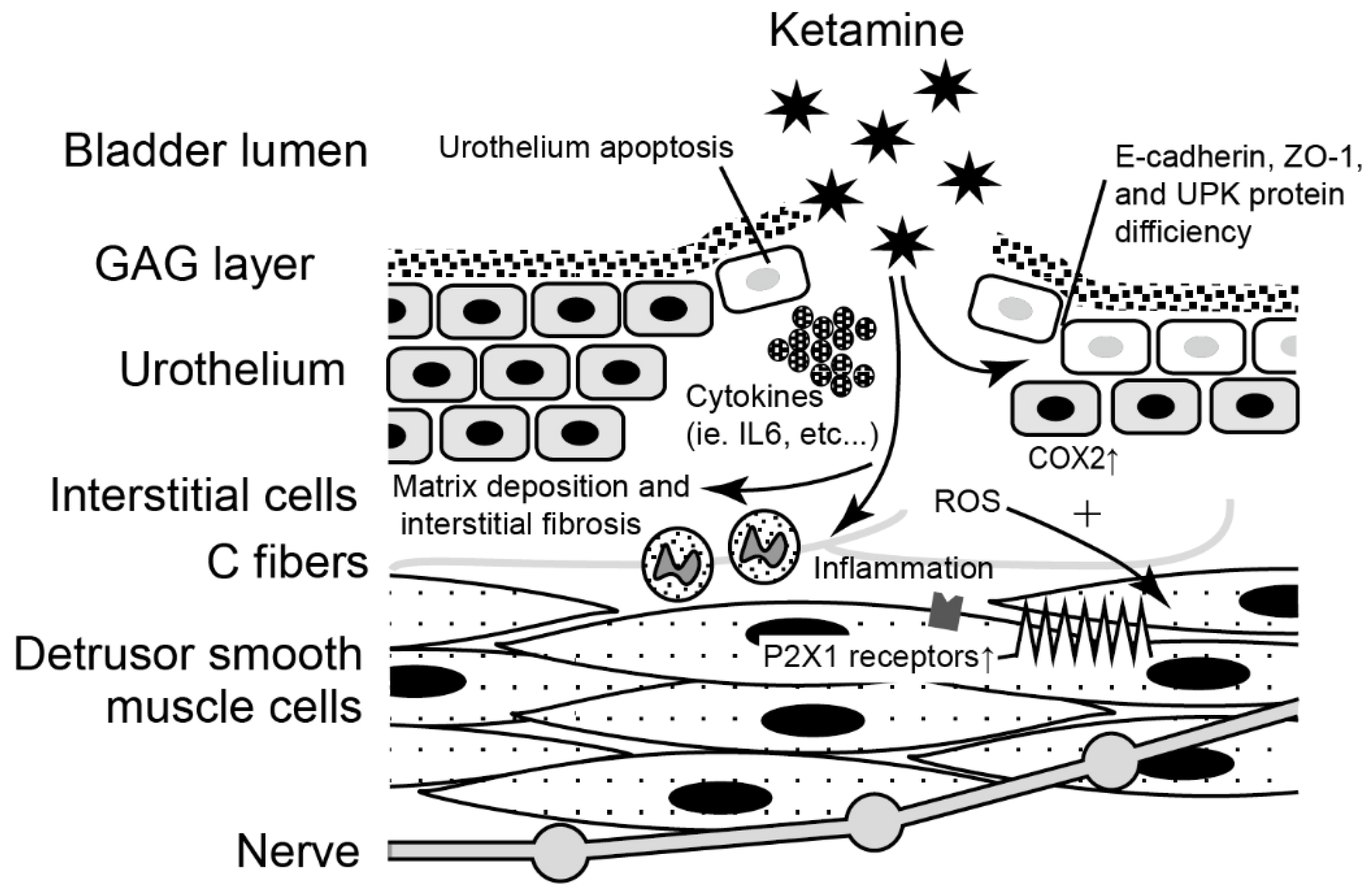
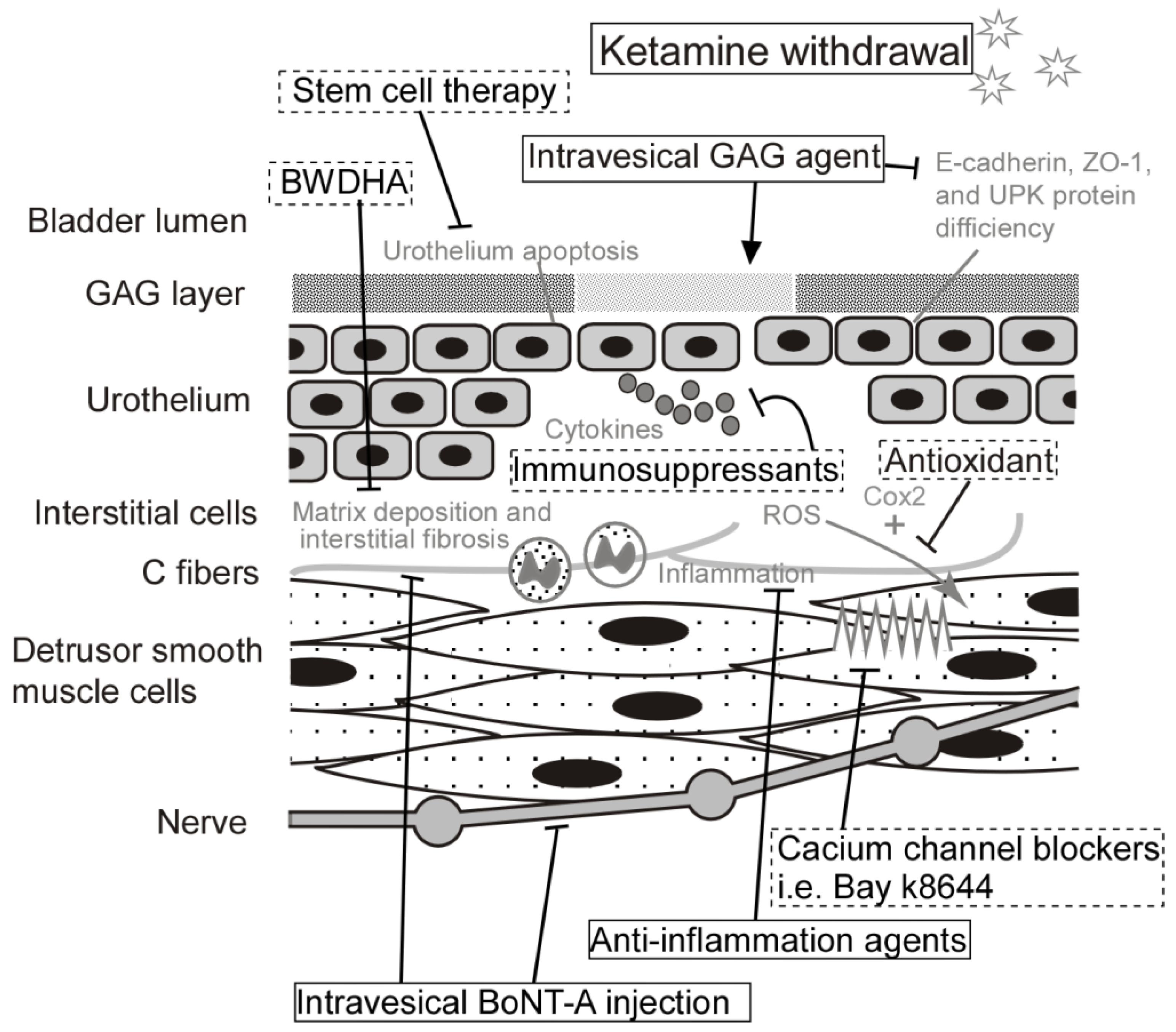
| Molecular Etiology | Human Study | Animal Study | In Vitro Study | Potential Treatment |
|---|---|---|---|---|
| Inflammation | Increased cytokine expressions, such as IL-1β, IL-6, CCL-2, CXCL, CXCL-10, NGF, and COX-2 [29,30] | Anti-inflammatory drugs Immunosuppressants Intravesical BoNT-A injection [31] | ||
| Urothelial junction-associated protein | Decreased expression of E-cadherin in the urothelial cells of KC bladder [24] | Decreased level of GAG, E-cadherin, ZO-1, and urothelial umbrella cells [22,32] | Intravesical instillation of GAG agents, such as Hyaluronic acid [32] | |
| Ion channels in the bladder mucosa | Higher presenting level of TRPV1 and TRPV4 in the bladder mucosa of KC bladder [33] |
| ||
| Oxidative stress | Antioxidant | |||
| Neurotransmission alternation |
|
| ||
| Ion channels in the bladder smooth muscle |
| Agonist of Cav1.2 (Bay k8644) [15] | ||
| Fibrosis-related genes | Upregulation of COL I, COL III, fibronectin, and TGF-β [39] | BWDHA [34] | ||
| Keratin family genes | Downregulation of keratin 6 a, 13, 14 [25] | |||
| Other signal pathways |
| |||
| ECM related genes | Upregulation of FN1, fibulin 2, fibrinogen-like 2, LAMC2, COL1A2, VCAN, AGT and C-type lectin domain family 4 member D [37] | |||
| Autophagy and angiogenesis | Ketamine induced dysregulation of autophagy and inhibition of angiogenesis (ketamine triggered PI3K/Akt/mTOR pathway) [40] | |||
| Cytosolic calcium concentration | Increased level of cytosolic calcium concentration [12] | Calcium channel blockers | ||
| Cell apoptosis | Stem cell therapy |
| Intravesical HA Instillation | Intravesical BoNT-A Injection | |
|---|---|---|
| Zeng, J [74] | ||
| Study design | Case series | Prospective study |
| Numbers of ketamine abusers |
| 36 (30 men, 6 women) |
| Age, years |
| 26.0 (19–38) |
| Duration of ketamine abuse, months |
| 12–60 |
| Drug administration |
| 200 U (injected into the bladder walls at 40 sites) followed by cystoscopic hydrodistention under a pressure of 80 cm and maintained the bladder capacity at 150–200 mL for 5 min |
| Outcomes |
| 1 month after BoNT-A treatment: nocturia↓, interval between micturition↑, void volume ↑, maximum flow rate ↑, bladder capacity↑, ICSI score ↓, and ICPI score ↓ |
| At 1 month after intravesical instillation of HA: VAS ↓ IPSS voiding subscore ↓ ICSI scores↓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-L.; Wu, S.-T.; Cha, T.-L.; Sun, G.-H.; Meng, E. Molecular Pathophysiology and Potential Therapeutic Strategies of Ketamine-Related Cystitis. Biology 2022, 11, 502. https://doi.org/10.3390/biology11040502
Chen C-L, Wu S-T, Cha T-L, Sun G-H, Meng E. Molecular Pathophysiology and Potential Therapeutic Strategies of Ketamine-Related Cystitis. Biology. 2022; 11(4):502. https://doi.org/10.3390/biology11040502
Chicago/Turabian StyleChen, Chin-Li, Sheng-Tang Wu, Tai-Lung Cha, Guang-Huan Sun, and En Meng. 2022. "Molecular Pathophysiology and Potential Therapeutic Strategies of Ketamine-Related Cystitis" Biology 11, no. 4: 502. https://doi.org/10.3390/biology11040502
APA StyleChen, C. -L., Wu, S. -T., Cha, T. -L., Sun, G. -H., & Meng, E. (2022). Molecular Pathophysiology and Potential Therapeutic Strategies of Ketamine-Related Cystitis. Biology, 11(4), 502. https://doi.org/10.3390/biology11040502





