Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago
Abstract
:Simple Summary
Abstract
1. Introduction
- (1)
- Assess how climate change might influence the bee diversity patterns in the Aegean Islands
- (2)
- Locate the bee species richness hotspots in the Aegean Islands
- (3)
- Investigate whether these hotspots might shift in the future and
- (4)
- Assess their overlap with the Natura 2000 protected areas network.
2. Materials and Methods
2.1. Occurrence Data
2.2. Environmental Data
2.3. Species Distribution Models
2.4. Biodiversity Hotspots Detection
2.5. Protected Areas Overlap
3. Results
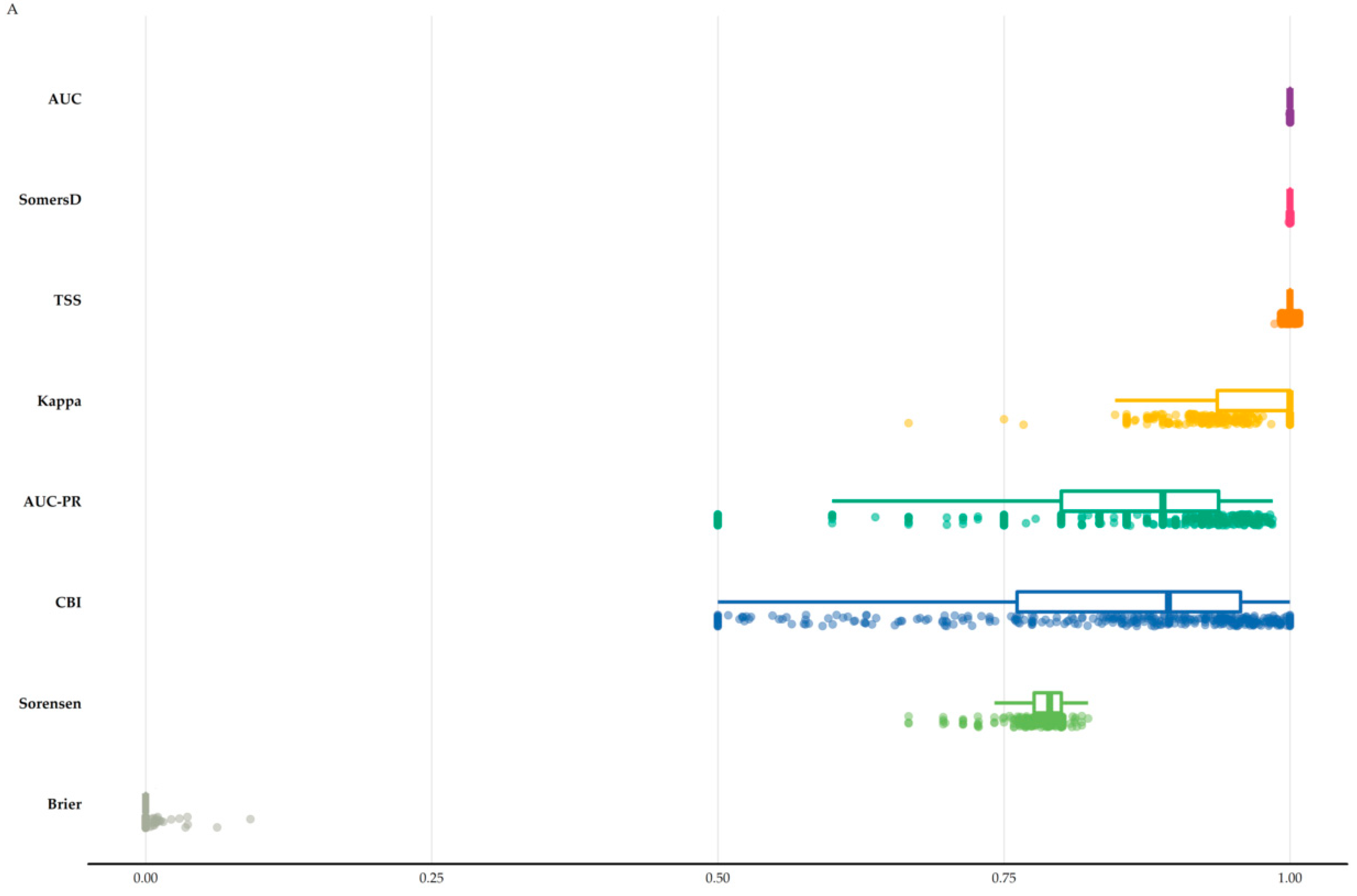
3.1. Species Distribution Models Perfomance
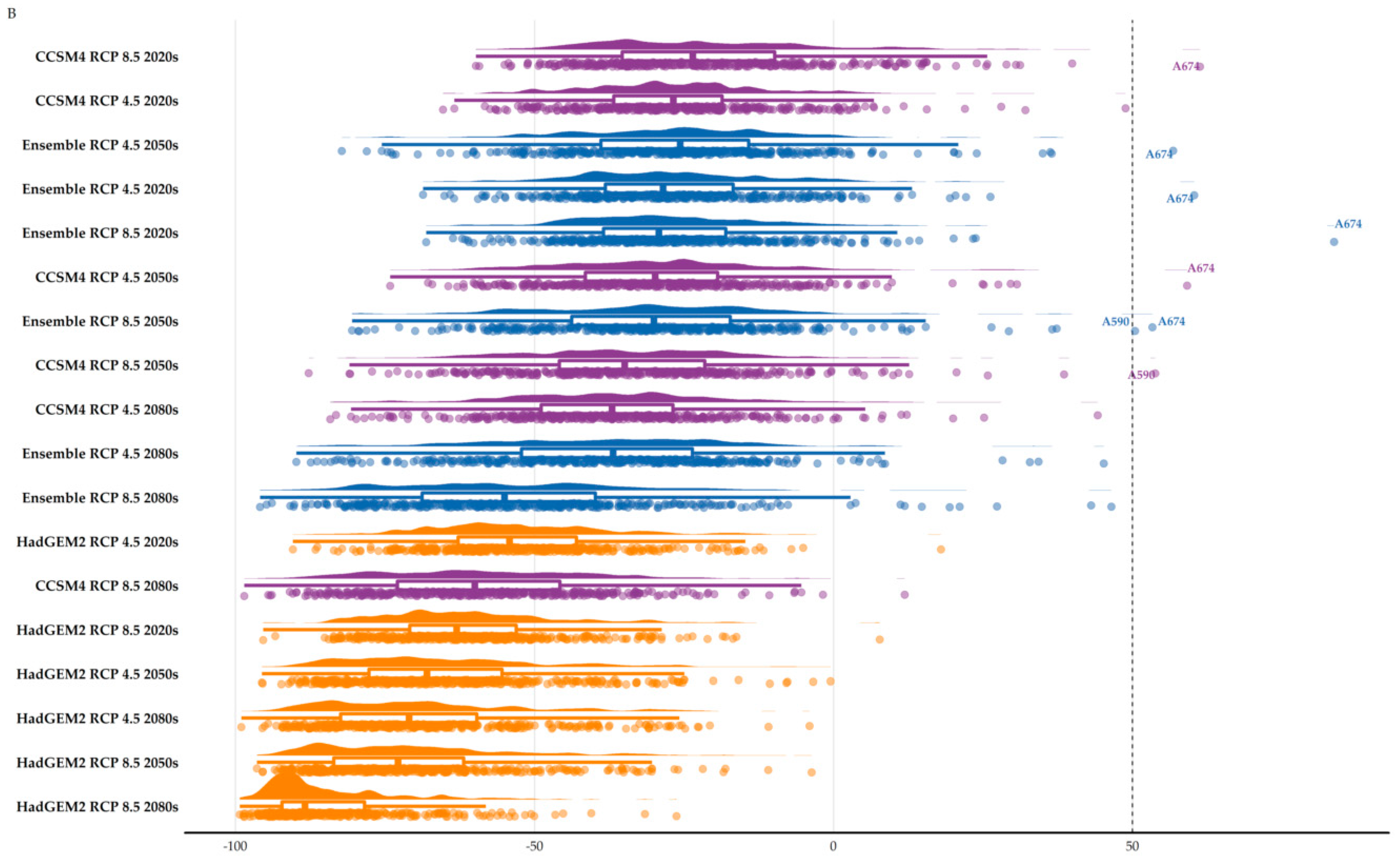
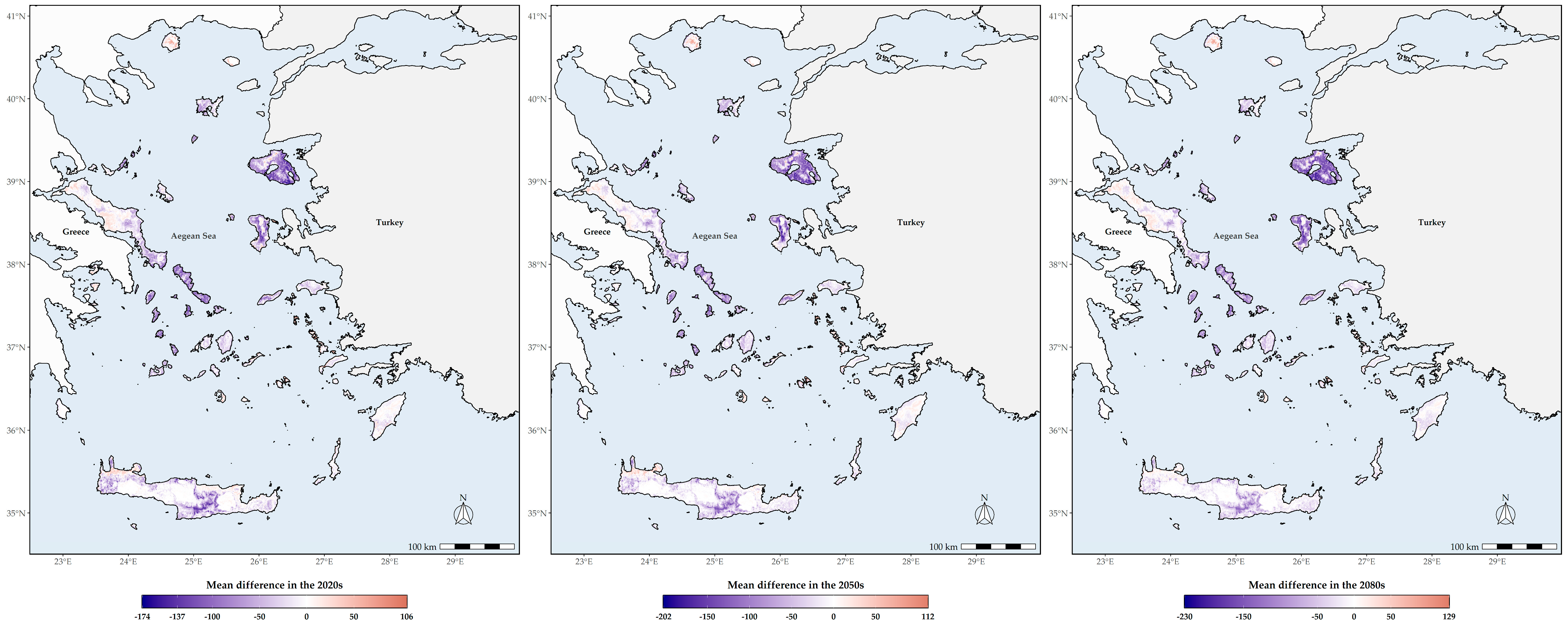
3.2. Area Range Change
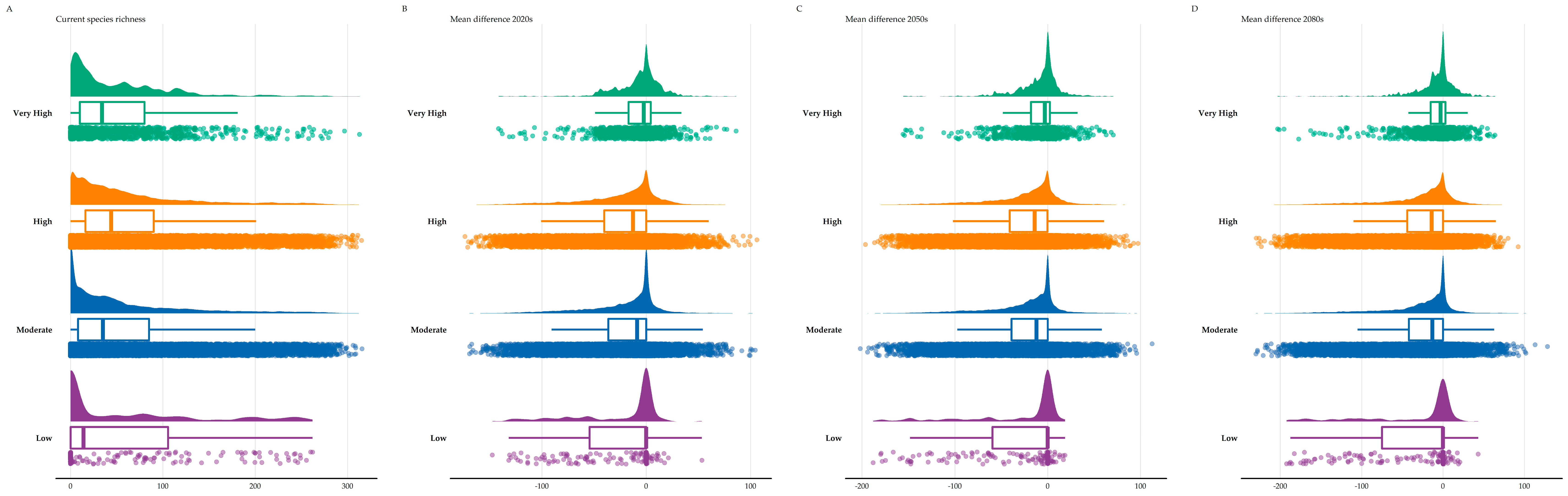
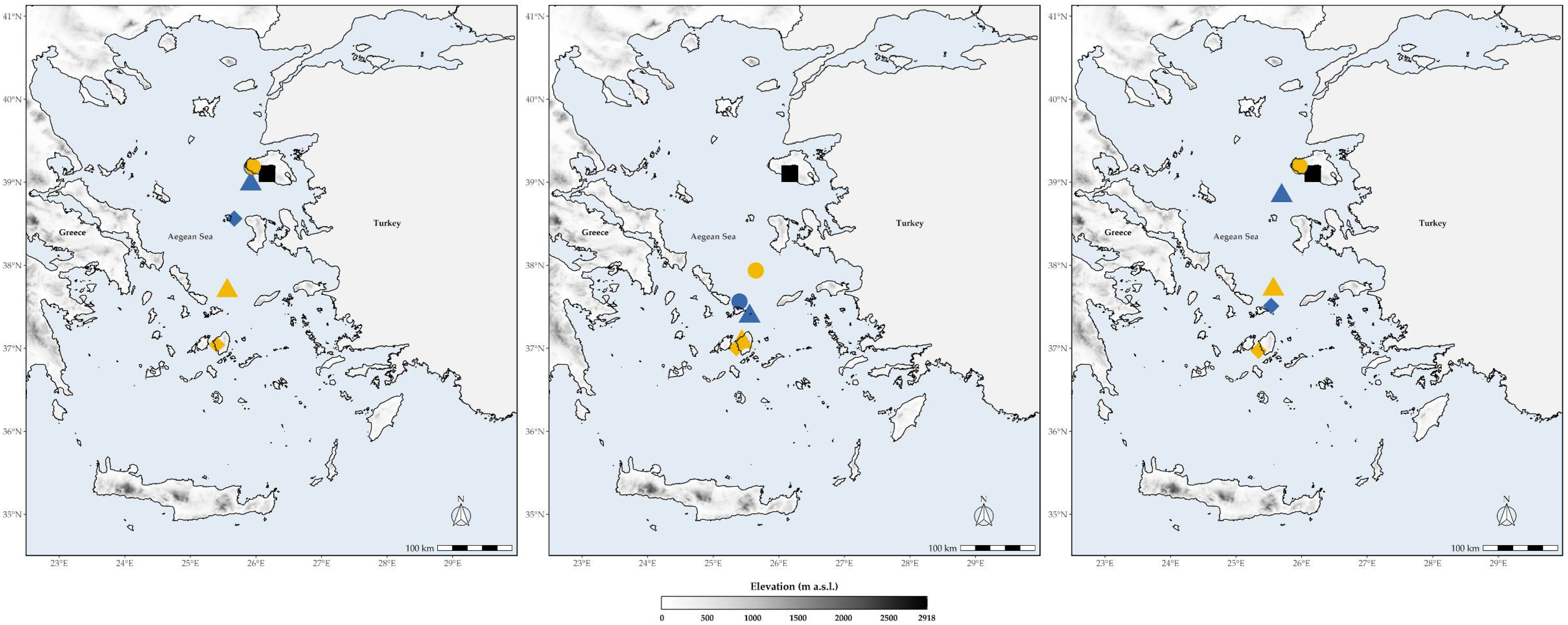
3.3. Biodiversity Hotspots
3.4. Altitudinal and Latitudinal Shifts
3.5. Protected Areas Network Overlap
4. Discussion
4.1. Climate Change Impacts
- (1)
- intensifying land-use/land-cover change ([48,49]; https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 17 February 2022)),
- (2)
- increased aridity and subsequent desiccation stress,
- (3)
- phenological mismatches between pollinator activity and pollinated plants,
- (4)
- increased competition due to limited resources and the increased occurrence of invasive species, and finally,
- (5)
4.2. Species Richness Hotspots
4.3. Altitudinal and Latitudinal Shifts of Species Richness Hotspots
4.4. Conservation Implications-Assessment of the Effectiveness of the Greek Protected Areas Network
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garibaldi, L.A.; Muchhala, N.; Motzke, I.; Bravo-Monroy, L.; Olschewski, R.; Klein, A.M. Services from Plant-Pollinator Interactions in the Neotropics; DeClerk, F., Le Coq, J.F., Rapidel, B., Beer, J., Eds.; Routledge: London, UK, 2012; ISBN 9781136537615. [Google Scholar]
- Kevan, P.G.; Baker, H.G. Insects as Flower Visitors and Pollinators. Annu. Rev. Entomol. 1983, 28, 407–453. [Google Scholar] [CrossRef]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Geyle, H.M.; Braby, M.F.; Andren, M.; Beaver, E.P.; Bell, P.; Byrne, C.; Castles, M.; Douglas, F.; Glatz, R.V.; Haywood, B.; et al. Butterflies on the brink: Identifying the Australian butterflies (Lepidoptera) most at risk of extinction. Austral Entomol. 2021, 60, 98–110. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Nature, I.U. for C. of the IUCN Red List of Threatened Species: Summary Statistics. Available online: https://www.iucnredlist.org/resources/summary-statistics#SummaryTables (accessed on 22 December 2021).
- Nieto, A.; Roberts, S.P.M.; Kemp, J.; Rasmont, P.; Kuhlmann, M.; García Criado, M.; Biesmeijer, J.C.; Bogusch, P.; Dathe, H.H.; De la Rúa, P.; et al. European Red List of Bees; Publications Office of the European Union: Luxembourg, 2014; ISBN 978-92-79-44512-5. [Google Scholar]
- Cardoso, P.; Barton, P.S.; Birkhofer, K.; Chichorro, F.; Deacon, C.; Fartmann, T.; Fukushima, C.S.; Gaigher, R.; Habel, J.C.; Hallmann, C.A.; et al. Scientists’ warning to humanity on insect extinctions. Biol. Conserv. 2020, 242, 108426. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Wyckhuys, K.A.G. Further evidence for a global decline of the entomofauna. Austral Entomol. 2021, 60, 9–26. [Google Scholar] [CrossRef]
- Biesmeijer, J.C.; Roberts, S.P.M.; Reemer, M.; Ohlemüller, R.; Edwards, M.; Peeters, T.; Schaffers, A.P.; Potts, S.G.; Kleukers, R.; Thomas, C.D.; et al. Parallel Declines in Pollinators and Insect-Pollinated Plants in Britain and the Netherlands. Science 2006, 313, 351–354. [Google Scholar] [CrossRef]
- Koch, J.B.; General, D.E.M. A preliminary assessment of bumble bee (Hymenoptera: Apidae) habitat suitability across protected and unprotected areas in the Philippines. Ann. Entomol. Soc. Am. 2019, 112, 44–49. [Google Scholar] [CrossRef]
- Brühl, C.A.; Bakanov, N.; Köthe, S.; Eichler, L.; Sorg, M.; Hörren, T.; Mühlethaler, R.; Meinel, G.; Lehmann, G.U.C. Direct pesticide exposure of insects in nature conservation areas in Germany. Sci. Rep. 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Falcón-Brindis, A.; León-Cortés, J.L.; Montañez-Reyna, M. How effective are conservation areas to preserve biodiversity in Mexico? Perspect. Ecol. Conserv. 2021, 19, 399–410. [Google Scholar] [CrossRef]
- De Palma, A.; Abrahamczyk, S.; Aizen, M.A.; Albrecht, M.; Basset, Y.; Bates, A.; Blake, R.J.; Boutin, C.; Bugter, R.; Connop, S.; et al. Predicting bee community responses to land-use changes: Effects of geographic and taxonomic biases. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Marshall, L.; Biesmeijer, J.C.; Rasmont, P.; Vereecken, N.J.; Dvorak, L.; Fitzpatrick, U.; Francis, F.; Neumayer, J.; Ødegaard, F.; Paukkunen, J.P.T.; et al. The interplay of climate and land use change affects the distribution of EU bumblebees. Glob. Chang. Biol. 2018, 24, 101–116. [Google Scholar] [CrossRef]
- Newbold, T.; Oppenheimer, P.; Etard, A.; Williams, J.J. Tropical and Mediterranean biodiversity is disproportionately sensitive to land-use and climate change. Nat. Ecol. Evol. 2020, 4, 1630–1638. [Google Scholar] [CrossRef]
- Miličić, M.; Popov, S.; Branco, V.V.; Cardoso, P. Insect threats and conservation through the lens of global experts. Conserv. Lett. 2021, 14, 1–14. [Google Scholar] [CrossRef]
- Yang, L.H.; Postema, E.G.; Hayes, T.E.; Lippey, M.K.; MacArthur-Waltz, D.J. The complexity of global change and its effects on insects. Curr. Opin. Insect Sci. 2021, 47, 90–102. [Google Scholar] [CrossRef]
- Poniatowski, D.; Beckmann, C.; Löffler, F.; Münsch, T.; Helbing, F.; Samways, M.J.; Fartmann, T. Relative impacts of land-use and climate change on grasshopper range shifts have changed over time. Glob. Ecol. Biogeogr. 2020, 29, 2190–2202. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- McCain, C.M.; Garfinkel, C.F. Climate change and elevational range shifts in insects. Curr. Opin. Insect Sci. 2021, 47, 111–118. [Google Scholar] [CrossRef]
- Kehoe, R.; Frago, E.; Sanders, D. Cascading extinctions as a hidden driver of insect decline. Ecol. Entomol. 2021, 46, 743–756. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Kreft, H.; Irl, S.D.H.; Norder, S.; Ah-Peng, C.; Borges, P.A.V.; Burns, K.C.; de Nascimento, L.; Meyer, J.-Y.; Montes, E. Scientists’ warning–The outstanding biodiversity of islands is in peril. Glob. Ecol. Conserv. 2021, 31, e01847. [Google Scholar] [CrossRef]
- Tabor, J.A.; Koch, J.B. Ensemble models predict invasive bee habitat suitability will expand under future climate scenarios in hawai’i. Insects 2021, 12, 443. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Rigal, F.; Ros-Prieto, A.; Cardoso, P. Increase of insular exotic arthropod diversity is a fundamental dimension of the current biodiversity crisis. Insect Conserv. Divers. 2020, 13, 508–518. [Google Scholar] [CrossRef]
- Kenis, M.; Auger-Rozenberg, M.-A.; Roques, A.; Timms, L.; Péré, C.; Cock, M.J.W.; Settele, J.; Augustin, S.; Lopez-Vaamonde, C. Ecological effects of invasive alien insects. Biol. Invasions 2009, 11, 21–45. [Google Scholar] [CrossRef]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef]
- Lázaro, A.; Müller, A.; Ebmer, A.W.; Dathe, H.H.; Scheuchl, E.; Schwarz, M.; Risch, S.; Pauly, A.; Devalez, J.; Tscheulin, T.; et al. Impacts of beekeeping on wild bee diversity and pollination networks in the Aegean Archipelago. Ecography (Cop.) 2021, 44, 1353–1365. [Google Scholar] [CrossRef]
- Herrera, C.M. Gradual replacement of wild bees by honeybees in flowers of the Mediterranean Basin over the last 50 years. Proc. R. Soc. B Biol. Sci. 2020, 287, 20192657. [Google Scholar] [CrossRef] [Green Version]
- Goulson, D.; Sparrow, K.R. Evidence for competition between honeybees and bumblebees; effects on bumblebee worker size. J. Insect Conserv. 2009, 13, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Lindström, S.A.M.; Herbertsson, L.; Rundlöf, M.; Bommarco, R.; Smith, H.G. Experimental evidence that honeybees depress wild insect densities in a flowering crop. Proc. R. Soc. B Biol. Sci. 2016, 283, 20161641. [Google Scholar] [CrossRef] [Green Version]
- Shavit, O.; Dafni, A.; Ne’Eman, G. Competition between honeybees (Apis mellifera) and native solitary bees in the Mediterranean region of Israel-implications for conservation. Isr. J. Plant Sci. 2009, 57, 171–183. [Google Scholar] [CrossRef]
- Valido, A.; Rodríguez-Rodríguez, M.C.; Jordano, P. Honeybees disrupt the structure and functionality of plant-pollinator networks. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Petanidou, T.; Kallimanis, A.S.; Lazarina, M.; Tscheulin, T.; Devalez, J.; Stefanaki, A.; Hanlidou, E.; Vujić, A.; Kaloveloni, A.; Sgardelis, S.P. Climate drives plant–pollinator interactions even along small-scale climate gradients: The case of the Aegean. Plant Biol. 2018, 20, 176–183. [Google Scholar] [CrossRef]
- Giannini, T.C.; Chapman, D.S.; Saraiva, A.M.; Alves-dos-Santos, I.; Biesmeijer, J.C. Improving species distribution models using biotic interactions: A case study of parasites, pollinators and plants. Ecography (Cop.) 2013, 36, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Krechemer, F.D.S.; Marchioro, C.A. Past, present and future distributions of bumblebees in South America: Identifying priority species and areas for conservation. J. Appl. Ecol. 2020, 57, 1829–1839. [Google Scholar] [CrossRef]
- Martínez-López, O.; Koch, J.B.; Martínez-Morales, M.A.; Navarrete-Gutiérrez, D.; Enríquez, E.; Vandame, R. Reduction in the potential distribution of bumble bees (Apidae: Bombus) in Mesoamerica under different climate change scenarios: Conservation implications. Glob. Chang. Biol. 2021, 27, 1772–1787. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Raus, T.; Bergmeier, E.; Constantinidis, T.; Iatrou, G.; Kokkini, S.; Strid, A.; Tzanoudakis, D. Vascular plants of Greece: An annotated checklist. Englera 2013, 6, 301–347. [Google Scholar] [CrossRef] [Green Version]
- Vujić, A.; Speight, M.; de Courcy, M.; Rojo, S.; Ståhls, G.; Radenković, S.; Likov, L.; Miličić, M.; Pérez-Bañón, C.; Falk, S. Atlas of the Hoverflies (Diptera: Syrphidae) of Greece; Brill Publishers: Leiden, The Netherlands, 2020; ISBN 978-90-04-33466-3. [Google Scholar]
- Legakis, A.; Constantinidis, T.; Petrakis, P. V Biodiversity in Greece. In Global Biodiversity; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 71–113. ISBN 0429487754. [Google Scholar]
- Michener, C.D. Biogeography of the Bees. Ann. Mo. Bot. Gard. 1979, 66, 277. [Google Scholar] [CrossRef]
- Ollerton, J.; Johnson, S.D.; Hingston, A.B. Geographic Variation in Diversity and Specificity of Pollination Systems; Waser, N., Ollerton, J., Eds.; University of Chicago Press: Chicago, IL, USA, 2006; pp. 283–308. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Kallimanis, A.; Strid, A.; Dimopoulos, P. Plant Endemism Centres and Biodiversity Hotspots in Greece. Biology 2021, 10, 72. [Google Scholar] [CrossRef]
- Kaltsas, D.; Trichas, A.; Kougioumoutzis, K.; Chatzaki, M. Ground beetles respond to grazing at assemblage level, rather than species-specifically: The case of Cretan shrublands. J. Insect Conserv. 2013, 17, 681–697. [Google Scholar] [CrossRef]
- Tsani, S.Z. Energy consumption and economic growth: A causality analysis for Greece. Energy Econ. 2010, 32, 582–590. [Google Scholar] [CrossRef]
- Kopsidis, M.; Ivanov, M. Industrialization and De-industrialization in Southeast Europe, 1870–2010. In The Spread of Modern Industry to the Periphery since 1871; Oxford University Press: New York, NY, USA, 2017; pp. 91–114. ISBN 978-0-19-875364-3. [Google Scholar] [CrossRef]
- Barredo, J.I.; Mauri, A.; Caudullo, G.; Dosio, A. Assessing Shifts of Mediterranean and Arid Climates Under RCP4.5 and RCP8.5 Climate Projections in Europe. Pure Appl. Geophys. 2018, 175, 3955–3971. [Google Scholar] [CrossRef]
- Ruiz-Labourdette, D.; Schmitz, M.F.; Pineda, F.D. Changes in tree species composition in Mediterranean mountains under climate change: Indicators for conservation planning. Ecol. Indic. 2013, 24, 310–323. [Google Scholar] [CrossRef]
- Kaloveloni, A.; Tscheulin, T.; Petanidou, T. Geography, climate, ecology: What is more important in determining bee diversity in the Aegean Archipelago? J. Biogeogr. 2018, 45, 2690–2700. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Tiniakou, A. Ecological factors driving plant species diversity in the South Aegean Volcanic Arc and other central Aegean islands. Plant Ecol. Divers. 2014, 8, 173–186. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Valli, A.T.; Georgopoulou, E.; Simaiakis, S.M.; Triantis, K.A.; Trigas, P. Network biogeography of a complex island system: The Aegean Archipelago revisited. J. Biogeogr. 2017, 44, 651–660. [Google Scholar] [CrossRef]
- Triantis, K.; Kougioumoutzis, K.; Legakis, A.; Anastasiou, I.; Andriopoulos, P.; Georgiadis, C.; Lymberakis, P.; Oikonomou, A.; Probonas, N.; Proios, K. The zoogeographic regions of the Aegean Sea: A multi-taxon approach. In Biogeography and Biodiversity of the Aegean. In Honour of Prof. Moysis Mylonas; Sfenthourakis, S., Triantis, K., Parmakelis, A., Pafilis, P., Poulakakis, N., Eds.; Broken Hill Publishers: Nicosia, Cyprus, 2018; pp. 279–290. ISBN 992556378X. [Google Scholar]
- Rixen, C.; Wipf, S.; Frei, E.; Stöckli, V. Faster, higher, more? Past, present and future dynamics of alpine and arctic flora under climate change. Alp. Bot. 2014, 124, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Fassou, G.; Kougioumoutzis, K.; Iatrou, G.; Trigas, P.; Papasotiropoulos, V. Genetic diversity and range dynamics of Helleborus odorus subsp. cyclophyllus under different climate change scenarios. Forests 2020, 11, 620. [Google Scholar] [CrossRef]
- Stathi, E.; Kougioumoutzis, K.; Abraham, E.M.; Trigas, P.; Ganopoulos, I.; Avramidou, E.V.; Tani, E. Population genetic variability and distribution of the endangered Greek endemic Cicer graecum under climate change scenarios. AoB Plants 2020, 12, plaa007. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Plant Diversity Patterns and Conservation Implications under Climate-Change Scenarios in the Mediterranean: The Case of Crete (Aegean, Greece). Diversity 2020, 12, 270. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Trigas, P.; Strid, A.; Dimopoulos, P. Spatial Phylogenetics, Biogeographical Patterns and Conservation Implications of the Endemic Flora of Crete (Aegean, Greece) under Climate Change Scenarios. Biology 2020, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Charitonidou, M.; Kougioumoutzis, K.; Halley, J.M. An orchid in retrograde: Climate-driven range shift patterns of ophrys helenae in Greece. Plants 2021, 10, 470. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation genetics of four critically endangered greek endemic plants: A preliminary assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Minachilis, K.; Kougioumoutzis, K.; Petanidou, T. Climate change effects on multi-taxa pollinator diversity and distribution along the elevation gradient of Mount Olympus, Greece. Ecol. Indic. 2021, 132, 108335. [Google Scholar] [CrossRef]
- Kaloveloni, A.; Tscheulin, T.; Vujić, A.; Radenković, S.; Petanidou, T. Winners and losers of climate change for the genus Merodon (Diptera: Syrphidae) across the Balkan Peninsula. Ecol. Modell. 2015, 313, 201–211. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Strid, A.; Raus, T.; Dimopoulos, P. Climate-Change Impacts on the Southernmost Mediterranean Arctic-Alpine Plant Populations. Sustainability 2021, 13, 13778. [Google Scholar] [CrossRef]
- Kokkoris, I.P.; Mallinis, G.; Bekri, E.S.; Vlami, V.; Zogaris, S.; Chrysafis, I.; Mitsopoulos, I.; Dimopoulos, P. National Set of MAES Indicators in Greece: Ecosystem Services and Management Implications. Forests 2020, 11, 595. [Google Scholar] [CrossRef]
- Apostolopoulou, E.; Pantis, J.D. Conceptual gaps in the national strategy for the implementation of the European Natura 2000 conservation policy in Greece. Biol. Conserv. 2009, 142, 221–237. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Kallimanis, A.S.; Sgardelis, S.P.; Pantis, J.D. Does higher taxon diversity reflect richness of conservation interest species? The case for birds, mammals, amphibians, and reptiles in Greek protected areas. Ecol. Indic. 2008, 8, 664–671. [Google Scholar] [CrossRef]
- Tsianou, M.A.; Mazaris, A.D.; Kallimanis, A.S.; Deligioridi, P.S.K.; Apostolopoulou, E.; Pantis, J.D. Identifying the criteria underlying the political decision for the prioritization of the Greek Natura 2000 conservation network. Biol. Conserv. 2013, 166, 103–110. [Google Scholar] [CrossRef]
- Trigas, P.; Tsiftsis, S.; Tsiripidis, I.; Iatrou, G. Distribution Patterns and Conservation Perspectives of the Endemic Flora of Peloponnese (Greece). Folia Geobot. 2012, 47, 421–439. [Google Scholar] [CrossRef]
- Spiliopoulou, K.; Dimitrakopoulos, P.G.; Brooks, T.M.; Kelaidi, G.; Paragamian, K.; Kati, V.; Oikonomou, A.; Vavylis, D.; Trigas, P.; Lymberakis, P. The Natura 2000 network and the ranges of threatened species in Greece. Biodivers. Conserv. 2021, 30, 945–961. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction Risk Assessment of the Greek Endemic Flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- CBD Decision adopted by the Conference of the Parties to the Convention on Biological Diversity. XIII/28. Indicators for the Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets 2016. Available online: https://www.cbd.int/doc/decisions/cop-13/cop-13-dec-28-en.pdf (accessed on 12 February 2022).
- Petanidou, T.; Ellis, W.N. Interdependence of native bee faunas and floras in changing Mediterranean communities. In The Conservation of Bees; Mathesos, A., Ed.; Academic Press: London, UK, 1997; pp. 201–226. [Google Scholar]
- Petanidou, T.; Ellis, W.N. Pollinating Fauna of a Phryganic Ecosystem: Composition and Diversity. Biodivers. Lett. 1993, 1, 9. [Google Scholar] [CrossRef]
- Petanidou, T.; Lamborn, E. A land for flowers and bees: Studying pollination ecology in Mediterranean communities. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2005, 139, 279–294. [Google Scholar] [CrossRef]
- Petanidou, T.; Ståhls, G.; Vujić, A.; Olesen, J.M.; Rojo, S.; Thrasyvoulou, A.; Sgardelis, S.; Kallimanis, A.S.; Kokkini, S.; Tscheulin, T. Investigating plant—Pollinator relationships in the Aegean: The approaches of the project POL-AEGIS (The pollinators of the Aegean archipelago: Diversity and threats). J. Apic. Res. 2013, 52, 106–117. [Google Scholar] [CrossRef] [Green Version]
- Robertson, M.P.; Visser, V.; Hui, C. Biogeo: An R package for assessing and improving data quality of occurrence record datasets. Ecography (Cop.) 2016, 39, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography (Cop.) 2015, 38, 541–545. [Google Scholar] [CrossRef]
- van Proosdij, A.S.J.; Sosef, M.S.M.; Wieringa, J.J.; Raes, N. Minimum required number of specimen records to develop accurate species distribution models. Ecography (Cop.) 2016, 39, 542–552. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hamann, A.; Wang, T.; Spittlehouse, D.L.; Murdock, T.Q. A Comprehensive, High-Resolution Database of Historical and Projected Climate Surfaces for Western North America. Bull. Am. Meteorol. Soc. 2013, 94, 1307–1309. [Google Scholar] [CrossRef]
- Marchi, M.; Castellanos-Acuña, D.; Hamann, A.; Wang, T.; Ray, D.; Menzel, A. ClimateEU, scale-free climate normals, historical time series, and future projections for Europe. Sci. Data 2020, 7, 428. [Google Scholar] [CrossRef]
- Wang, T.; Hamann, A.; Spittlehouse, D.L.; Murdock, T.Q. ClimateWNA—High-Resolution Spatial Climate Data for Western North America. J. Appl. Meteorol. Climatol. 2012, 51, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.; Philipps, S.; Leathwick, J.; Elith, J. dismo: Species Distribution Modeling, R package version 1.1-4. 2017.
- Title, P.O.; Bemmels, J.B. ENVIREM: An expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography (Cop.) 2018, 41, 291–307. [Google Scholar] [CrossRef] [Green Version]
- Hijmans, R.J. Package ‘raster’-Geographic Data Analysis and Modeling. CRAN Repos 2019. [Google Scholar]
- Evans, J.S. spatialEco, R package version 1.2-0. 2019.
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography (Cop.) 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography (Cop.) 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Guisan, A.; Nobis, M.P.; Bergamini, A. Including environmental niche information to improve IUCN Red List assessments. Divers. Distrib. 2017, 23, 484–495. [Google Scholar] [CrossRef] [Green Version]
- Breiner, F.T.; Guisan, A.; Bergamini, A.; Nobis, M.P. Overcoming limitations of modelling rare species by using ensembles of small models. Methods Ecol. Evol. 2015, 6, 1210–1218. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography (Cop.) 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. The effect of sample size on the accuracy of species distribution models: Considering both presences and pseudo-absences or background sites. Ecography (Cop.) 2019, 42, 535–548. [Google Scholar] [CrossRef] [Green Version]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. blockCV: An r package for generating spatially or environmentally separated folds for k-fold cross-validation of species distribution models. Methods Ecol. Evol. 2019, 10, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/ absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Modell. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Measuring and comparing the accuracy of species distribution models with presence-absence data. Ecography (Cop.) 2011, 34, 232–243. [Google Scholar] [CrossRef]
- Sofaer, H.R.; Hoeting, J.A.; Jarnevich, C.S. The area under the precision-recall curve as a performance metric for rare binary events. Methods Ecol. Evol. 2019, 10, 565–577. [Google Scholar] [CrossRef]
- Broennimann, O.; Di Cola, V.; Guisan, A. ecospat: Spatial Ecology Miscellaneous Methods, R package version 3.2. 2021.
- Hammer, B.; Frasco, M. metrics: Evaluation Metrics for Machine Learning, R package version 0.1.4. 2018.
- Márcia Barbosa, A.; Real, R.; Muñoz, A.R.; Brown, J.A. New measures for assessing model equilibrium and prediction mismatch in species distribution models. Divers. Distrib. 2013, 19, 1333–1338. [Google Scholar] [CrossRef]
- Schwarz, J.; Heider, D. GUESS: Projecting machine learning scores to well-calibrated probability estimates for clinical decision-making. Bioinformatics 2019, 35, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Signorell, A.; Aho, K.; Anderegg, N.; Aragon, T.; Arppe, A.; Baddeley, A.; Bolker, B.; Caeiro, F.; Champely, S.; Chessel, D. DescTools: Tools for Descriptive Statistics, R package version 0.99-40. 2021.
- Smith, A.B. enmSdm: Tools for Modeling Species Niches and Distributions, R package version 0.5.1.5. 2020.
- Yan, Y. MLmetrics: Machine Learning Evaluation Metrics, R package version 1.1.1. 2016.
- Raes, N.; ter Steege, H. A null-model for significance testing of presence-only species distribution models. Ecography (Cop.) 2007, 30, 727–736. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography (Cop.) 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography (Cop.) 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Miller, D.L.; Roberts, J.J.; Mannocci, L.; Harris, C.M.; Thomas, L. dsmextra: Extrapolation assessment tools for density surface models. Methods Ecol. Evol. 2020, 11, 1464–1469. [Google Scholar] [CrossRef]
- Mannocci, L.; Roberts, J.J.; Halpin, P.N.; Authier, M.; Boisseau, O.; Bradai, M.N.; Cañadas, A.; Chicote, C.; David, L.; Di-Méglio, N.; et al. Assessing cetacean surveys throughout the Mediterranean Sea: A gap analysis in environmental space. Sci. Rep. 2018, 8, 3126. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Carnaval, A.C. A tale of two niches: Methods, concepts, and evolution. Front. Biogeogr. 2019, 11, 11. [Google Scholar] [CrossRef] [Green Version]
- Linder, H.P. Plant diversity and endemism in sub-Saharan tropical Africa. J. Biogeogr. 2001, 28, 169–182. [Google Scholar] [CrossRef]
- Linder, H.P. On areas of endemism, with an example from the African restionaceae. Syst. Biol. 2001, 50, 892–912. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, C.E.; Laffan, S.W.; Miller, J.T. Spatial distribution of species richness and endemism of the genus Acacia in Australia. Aust. J. Bot. 2011, 59, 601. [Google Scholar] [CrossRef]
- Guerin, G.R. biomapME: Biodiversity Mapping and Macroecology, R package v2.0. 2020.
- Daru, B.H.; Elliott, T.L.; Park, D.S.; Davies, T.J. Understanding the Processes Underpinning Patterns of Phylogenetic Regionalization. Trends Ecol. Evol. 2017, 32, 845–860. [Google Scholar] [CrossRef]
- Daru, B.H.; Karunarathne, P.; Schliep, K. phyloregion: R package for biogeographical regionalization and macroecology. Methods Ecol. Evol. 2020, 11, 1483–1491. [Google Scholar] [CrossRef]
- Daru, B.H.; Farooq, H.; Antonelli, A.; Faurby, S. Endemism patterns are scale dependent. Nat. Commun. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Zizka, A.; Antonelli, A.; Silvestro, D. sampbias, a method for quantifying geographic sampling biases in species distribution data. Ecography (Cop.) 2021, 44, 25–32. [Google Scholar] [CrossRef]
- Kennedy, C.M.; Oakleaf, J.R.; Theobald, D.M.; Baruch-Mordo, S.; Kiesecker, J. Managing the middle: A shift in conservation priorities based on the global human modification gradient. Glob. Chang. Biol. 2019, 25, 811–826. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J. wdpar: Interface to the World Database on Protected Areas, R package version 1.0.5. 2020.
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, A.; Steffan-Dewenter, I.; Westphal, C.; Messinger, O.; Potts, S.G.; Roberts, S.P.M.; Settele, J.; Szentgyörgyi, H.; Vaissière, B.E.; Vaitis, M.; et al. Assessing bee species richness in two Mediterranean communities: Importance of habitat type and sampling techniques. Ecol. Res. 2011, 26, 969–983. [Google Scholar] [CrossRef]
- Gonzalez, V.H.; Cobos, M.E.; Jaramillo, J.; Ospina, R. Climate change will reduce the potential distribution ranges of Colombia’s most valuable pollinators. Perspect. Ecol. Conserv. 2021, 19, 195–206. [Google Scholar] [CrossRef]
- Diamond, S.E. Contemporary climate-driven range shifts: Putting evolution back on the table. Funct. Ecol. 2018, 32, 1652–1665. [Google Scholar] [CrossRef] [Green Version]
- Outhwaite, C.L.; Gregory, R.D.; Chandler, R.E.; Collen, B.; Isaac, N.J.B. Complex long-term biodiversity change among invertebrates, bryophytes and lichens. Nat. Ecol. Evol. 2020, 4, 384–392. [Google Scholar] [CrossRef]
- Van Klink, R.; Bowler, D.E.; Gongalsky, K.B.; Swengel, A.B.; Gentile, A.; Chase, J.M. Meta-analysis reveals declines in terrestrial but increases in freshwater insect abundances. Science 2020, 368, 417–420. [Google Scholar] [CrossRef]
- Rasmont, P. Atlas of the European Bees: Genus Melecta, 2nd ed.; Rasmont, P., Ed.; Mons: Gembloux, Belgium, 2016; Available online: http://www.atlashymenoptera.net/page.aspx?id=256 (accessed on 22 December 2021).
- Kerr, J.T.; Pindar, A.; Galpern, P.; Packer, L.; Potts, S.G.; Roberts, S.M.; Rasmont, P.; Schweiger, O.; Colla, S.R.; Richardson, L.L. Climate change impacts on bumblebees converge across continents. Science 2015, 349, 177–180. [Google Scholar] [CrossRef]
- Miličić, M.; Vujić, A.; Cardoso, P. Effects of climate change on the distribution of hoverfly species (Diptera: Syrphidae) in Southeast Europe. Biodivers. Conserv. 2018, 27, 1173–1187. [Google Scholar] [CrossRef] [Green Version]
- Françoso, E.; Zuntini, A.R.; Arias, M.C. Combining phylogeography and future climate change for conservation of Bombus morio and B. pauloensis (Hymenoptera: Apidae). J. Insect Conserv. 2019, 23, 63–73. [Google Scholar] [CrossRef]
- Naeem, M.; Liu, M.; Huang, J.; Ding, G.; Potapov, G.; Jung, C.; An, J. Vulnerability of East Asian bumblebee species to future climate and land cover changes. Agric. Ecosyst. Environ. 2019, 277, 11–20. [Google Scholar] [CrossRef]
- Rasmont, P.; Franzén, M.; Lecocq, T.; Harpke, A.; Roberts, S.P.M.; Biesmeijer, J.C.; Castro, L.; Cederberg, B.; Dvorak, L.; Fitzpatrick, Ú. Climatic Risk and Distribution Atlas of European Bumblebees; Pensoft Publishers: Sofia, Bulgaria, 2015; Volume 10, ISBN 9546427683. [Google Scholar]
- Colla, S.R.; Gadallah, F.; Richardson, L.; Wagner, D.; Gall, L. Assessing declines of North American bumble bees (Bombus spp.) using museum specimens. Biodivers. Conserv. 2012, 21, 3585–3595. [Google Scholar] [CrossRef]
- Jactel, H.; Koricheva, J.; Castagneyrol, B. Responses of forest insect pests to climate change: Not so simple. Curr. Opin. Insect Sci. 2019, 35, 103–108. [Google Scholar] [CrossRef]
- Kalaentzis, K.; Kazilas, C.; Agapakis, G.; Kocarek, P. Hidden in plain sight: First records of the alien earwig Euborellia femoralis (Dohrn, 1863) in Europe. BioInvasions Rec. 2021, 10, 1022–1031. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Rackham, O.; Moody, J. The Making of the Cretan Landscape; Manchester University Press: Manchester, UK, 1996; ISBN 071903647X. [Google Scholar]
- Bambaradeniya, C.N.B.; Amerasinghe, F.P. Biodiversity Associated with the Rice Field Agroecosystem in Asian Countries: A Brief Review; Working Paper 63; International Water Management Insitute: Colombo, Sri Lanka, 2004. [Google Scholar]
- Ollerton, J.; Erenler, H.; Edwards, M.; Crockett, R. Extinctions of aculeate pollinators in Britain and the role of large-scale agricultural changes. Science 2014, 346, 1360–1362. [Google Scholar] [CrossRef] [Green Version]
- Kaiser-Bunbury, C.N.; Traveset, A.; Hansen, D.M. Conservation and restoration of plant–animal mutualisms on oceanic islands. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 131–143. [Google Scholar] [CrossRef]
- Koh, L.P.; Sodhi, N.S.; Brook, B.W. Co-extinctions of tropical butterflies and their hostplants. Biotropica 2004, 36, 272–274. [Google Scholar] [CrossRef]
- Giannini, T.C.; Costa, W.F.; Borges, R.C.; Miranda, L.; da Costa, C.P.W.; Saraiva, A.M.; Fonseca, V.L.I. Climate change in the Eastern Amazon: Crop-pollinator and occurrence-restricted bees are potentially more affected. Reg. Environ. Chang. 2020, 20, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lautenbach, S.; Seppelt, R.; Liebscher, J.; Dormann, C.F. Spatial and temporal trends of global pollination benefit. PLoS ONE 2012, 7, e35954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontogeorgos, A.; Chatzitheodoridis, F. David and Goliath: An Investigation between Greece–Germany Bilateral Trade for Agricultural Products. In Global, Regional and Local Perspectives on the Economies of Southeastern Europe; Springer: Cham, Switzerland, 2021; pp. 33–52. [Google Scholar]
- Trigas, P.; Iatrou, G.; Karetsos, G. Species diversity, endemism and conservation of the family Caryophyllaceae in Greece. Biodivers. Conserv. 2007, 16, 357–376. [Google Scholar] [CrossRef]
- Panitsa, M.; Kagiampaki, A.; Kougioumoutzis, K. Plant diversity and biogeography of the Aegean Archipelago: A New Synthesis. In Biogeography and Biodiversity of the Aegean. In honour of Prof. Moysis Mylonas; Sfenthourakis, S., Pafilis, P., Parmakelis, A., Poulakakis, N., Triantis, K., Eds.; Broken Hill Publishers Ltd.: Nicosia, Cyprus, 2018; pp. 223–244. ISBN 9789925563784. [Google Scholar]
- Hausdorf, B.; Hennig, C. The influence of recent geography, palaeogeography and climate on the composition of the fauna of the central Aegean Islands. Biol. J. Linn. Soc. 2005, 84, 785–795. [Google Scholar] [CrossRef]
- Welter-Schultes, F.W.; Williams, M.R. History, island area and habitat availability determine land snail species richness of Aegean islands. J. Biogeogr. 1999, 26, 239–249. [Google Scholar] [CrossRef]
- Pitta, E.; Kassara, C.; Trichas, A.; Sfenthourakis, S.; Chatzaki, M. Community variation of spiders, beetles and isopods in three small island groups of the Aegean Sea: The interplay between history and ecology. J. Biogeogr. 2017, 44, 1077–1087. [Google Scholar] [CrossRef]
- Stahls, G.; Vujić, A.; Petanidou, T.; Cardoso, P.; Radenković, S.; Ačanski, J.; Perez Banon, C.; Rojo, S. Phylogeographic patterns of Merodon hoverflies in the Eastern Mediterranean region: Revealing connections and barriers. Ecol. Evol. 2016, 6, 2226–2245. [Google Scholar] [CrossRef] [Green Version]
- Fattorini, S. Biogeography of the tenebrionid beetles (Coleoptera, Tenebrionidae) on the Aegean Islands (Greece). J. Biogeogr. 2002, 29, 49–67. [Google Scholar] [CrossRef]
- Simaiakis, S.M.; Rijsdijk, K.F.; Koene, E.F.M.; Norder, S.J.; Van Boxel, J.H.; Stocchi, P.; Hammoud, C.; Kougioumoutzis, K.; Georgopoulou, E.; Van Loon, E.; et al. Geographic changes in the Aegean Sea since the Last Glacial Maximum: Postulating biogeographic effects of sea-level rise on islands. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 71, 108–119. [Google Scholar] [CrossRef]
- Hammoud, C.; Kougioumoutzis, K.; Rijsdijk, K.F.; Simaiakis, S.M.; Norder, S.J.; Foufopoulos, J.; Georgopoulou, E.; Van Loon, E.E. Past connections with the mainland structure patterns of insular species richness in a continental-shelf archipelago (Aegean Sea, Greece). Ecol. Evol. 2021, 11, 5441–5458. [Google Scholar] [CrossRef]
- Breeze, T.D.; Gallai, N.; Garibaldi, L.A.; Li, X.S. Economic measures of pollination services: Shortcomings and future directions. Trends Ecol. Evol. 2016, 31, 927–939. [Google Scholar] [CrossRef]
- Jetz, W.; Rahbek, C.; Colwell, R.K. The coincidence of rarity and richness and the potential signature of history in centres of endemism. Ecol. Lett. 2004, 7, 1180–1191. [Google Scholar] [CrossRef]
- Forest, F.; Grenyer, R.; Rouget, M.; Davies, T.J.; Cowling, R.M.; Faith, D.P.; Balmford, A.; Manning, J.C.; Procheş, Ş.; Van Der Bank, M.; et al. Preserving the evolutionary potential of floras in biodiversity hotspots. Nature 2007, 445, 757–760. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Recognising and Reporting Other Effective Area-Based Conservation Measures; IUCN, International Union for Conservation of Nature: Gland, Switzerland, 2019; ISBN 9782831720258. [Google Scholar]
- IUCN. A Global Standard for the Identification of Key Biodiversity Areas, Version 1.0; IUCN: Gland, Switzerland, 2016; Volume 1, ISBN 978-2-8317-1835-4. [Google Scholar]
- Müller, A.; Schneider, U.A.; Jantke, K. Evaluating and expanding the European Union’s protected-area network toward potential post-2020 coverage targets. Conserv. Biol. 2020, 34, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.A.; Komar, O.; Chazdon, R.; Ferguson, B.G.; Finegan, B.; Griffith, D.M.; Martínez-Ramos, M.; Morales, H.; Nigh, R.; Soto-Pinto, L. Integrating agricultural landscapes with biodiversity conservation in the Mesoamerican hotspot. Conserv. Biol. 2008, 22, 8–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kougioumoutzis, K.; Kaloveloni, A.; Petanidou, T. Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago. Biology 2022, 11, 552. https://doi.org/10.3390/biology11040552
Kougioumoutzis K, Kaloveloni A, Petanidou T. Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago. Biology. 2022; 11(4):552. https://doi.org/10.3390/biology11040552
Chicago/Turabian StyleKougioumoutzis, Konstantinos, Aggeliki Kaloveloni, and Theodora Petanidou. 2022. "Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago" Biology 11, no. 4: 552. https://doi.org/10.3390/biology11040552
APA StyleKougioumoutzis, K., Kaloveloni, A., & Petanidou, T. (2022). Assessing Climate Change Impacts on Island Bees: The Aegean Archipelago. Biology, 11(4), 552. https://doi.org/10.3390/biology11040552






