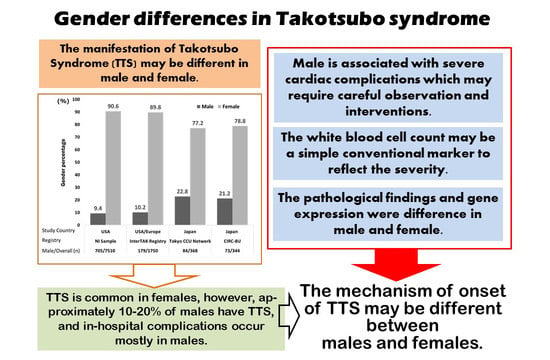
Gender Differences in Takotsubo Syndrome
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Proportion of Genders and Diagnosis of TTS
- (1)
- As TTS is a relatively rare disease, most large-scale reports have used data from multicenter registries. Krishnamoorthy et al. [29] used the US National Inpatient Sample registry (20% registration of non-federal hospitals) [30] and Templin et al. used the registry data (The International Takotsubo Registry) of nine countries in Europe and the United States [4]. Our previous report [2] used the Tokyo Cardiovascular Care Unit (CCU) Network database (for those accommodated in CCUs at 71 facilities in Tokyo), and it is possible that severely ill patients were registered. Yoshizawa et al. [31] used the registry of 10 hospitals affiliated with 8 medical schools in eastern Japan that contained 10,622 cases with acute coronary syndrome (the Cardiovascular Research Consortium-8 Universities: CIRC-8U).

- (2)
- From the 2008 modified Mayo Criteria [32], obstructive coronary artery disease is not an exclusion criterion for the diagnosis of TTS. Another major difference from the first edition of the Mayo Criteria is that after trauma, TTS can develop after an intracerebral hemorrhage (including a subarachnoid hemorrhage). However, it has been suggested that it may not be excluded and, therefore, not registered as TTS. In particular, male patients with coronary artery stenosis may be incorrectly diagnosed [13]. In addition, the subjective symptoms range from mild symptoms including chest discomfort to severe symptoms including respiratory distress from heart failure, shock, and left ventricular outflow tract stenosis. Mild cases might have been overlooked leading to the gender differences in TTS in each report. Currently, TTS is often diagnosed using the International Takotsubo Diagnostic Criteria (InterTAK Diagnostic Criteria) reported in 2018. Doctors have attempted to improve the accuracy of a TTS diagnosis using the InterTAK Diagnostic Score [33,34,35]. Recently, it has been reported that a differential diagnosis between TTS and acute myocardial infarction is possible due to the development of artificial intelligence in echocardiography [36]. Human monitoring is still necessary, but it is an area where further development is expected in the future [37].
3. Patient Backgrounds and Clinical Characteristics
3.1. Age
3.2. Preceding Stress and Symptoms at Admission
3.3. Examination of the Blood Tests
3.4. Examination of the Echocardiography
3.5. The Co-Existence of Coronary Artery Disease
3.6. Complications and Supportive Therapies during Hospitalization
3.7. Long-Term Outcomes and Therapies after Discharge
4. Research Reports Supporting the Gender Differences in TTS
4.1. Effects of Estrogen Concentration
4.2. Gene Expression, KEGG Analysis, and Pathological Analysis of the Heart Muscle
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Redfors, B.; Vedad, R.; Angerås, O.; Råmunddal, T.; Petursson, P.; Haraldsson, I.; Ali, A.; Dworeck, C.; Odenstedt, J.; Ioaness, D.; et al. Mortality in takotsubo syndrome is similar to mortality in myocardial infarction—a report from the SWEDEHEART registry. Int. J. Cardiol. 2015, 185, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Yoshikawa, T.; Maekawa, Y.; Ueda, T.; Isogai, T.; Sakata, K.; Nagao, K.; Yamamoto, T.; Takayama, M. Gender Differences in Patients with Takotsubo Cardiomyopathy: Multi-Center Registry from Tokyo CCU Network. PLoS ONE 2015, 10, e0136655. [Google Scholar] [CrossRef] [Green Version]
- Imori, Y.; Kato, K.; Cammann, V.L.; Szawan, K.A.; Wischnewsky, M.; Dreiding, S.; Würdinger, M.; Schönberger, M.; Petkova, V.; Niederseer, D.; et al. Ethnic comparison in takotsubo syndrome: Novel insights from the International Takotsubo Registry. Clin. Res. Cardiol. 2021, 111, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isogai, T.; Yasunaga, H.; Matsui, H.; Tanaka, H.; Ueda, T.; Horiguchi, H.; Fushimi, K. Out-of-hospital versus in-hospital Takotsubo cardiomyopathy: Analysis of 3719 patients in the Diagnosis Procedure Combination database in Japan. Int. J. Cardiol. 2014, 176, 413–417. [Google Scholar] [CrossRef]
- Kato, K.; Lyon, A.R.; Ghadri, J.-R.; Templin, C. Takotsubo syndrome: Aetiology, presentation and treatment. Heart 2017, 103, 1461–1469. [Google Scholar] [CrossRef]
- Kato, K.; Kitahara, H.; Kobayashi, Y. Recurrence of Takotsubo Cardiomyopathy. Int. Cardiovasc. Forum J. 2016, 5, 50–52. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T. Takotsubo cardiomyopathy, a new concept of cardiomyopathy: Clinical features and pathophysiology. Int. J. Cardiol. 2015, 182, 297–303. [Google Scholar] [CrossRef]
- Isogai, T.; Matsui, H.; Tanaka, H.; Fushimi, K.; Yasunaga, H. Early β-blocker use and in-hospital mortality in patients with Takotsubo cardiomyopathy. Heart 2016, 102, 1029–1035. [Google Scholar] [CrossRef]
- Dote, K.; Sato, H.; Tateishi, H.; Uchida, T.; Ishihara, M. Myocardial stunning due to simultaneous multivessel coronary spasms: A review of 5 cases. J. Cardiol. 1990, 21, 203–214. [Google Scholar]
- Sato, H.; Tateishi, H.; Uchida, T. Takotsubo-type cardiomyopathy due to multivessel spasm. In Clinical Aspect of Myocardial Injury: From Ischaemia to Heart Failure; Kodama, K., Haze, K., Hon, M., Eds.; Kagakuhyouronsya Co.,: Tokyo, Japan, 1990; pp. 56–64. (In Japanese) [Google Scholar]
- Sharkey, S.W.; Lesser, J.R.; Maron, M.S.; Maron, B.J. Why not just call it tako-tsubo cardiomyopathy: A discussion of nomenclature. J. Am. Coll. Cardiol. 2011, 57, 1496–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templin, C.; Napp, L.C.; Ghadri, J.R. Takotsubo Syndrome: Underdiagnosed, Underestimated, but Understood? J. Am. Coll. Cardiol. 2016, 67, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Güthe, T.; Bhogal, P.; Cimpoca, A.; Ganslandt, O.; Bäzner, H.; Henkes, H. Aneurysmal subarachnoid hemorrhage as a trigger for Takotsubo syndrome: A comprehensive review. Rev. Cardiovasc. Med. 2021, 22, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur. Heart J. 2018, 39, 2032–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghadri, J.-R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, Y.; Kawamura, A.; Yuasa, S.; Nesto, R.W.; Fukuda, K. Direct Comparison of Takotsubo Cardiomyopathy between Japan and USA: 3-year Follow-up Study. Intern. Med. 2012, 51, 257–262. [Google Scholar] [CrossRef] [Green Version]
- Gianni, M.; Dentali, F.; Grandi, A.M.; Sumner, G.; Hiralal, R.; Lonn, E. Apical ballooning syndrome or takotsubo cardiomyopathy: A systematic review. Eur. Heart J. 2006, 27, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Akashi, Y.J.; Nakazawa, K.; Sakakibara, M.; Miyake, F.; Koike, H.; Sasaka, K. The clinical features of takotsubo cardiomyopathy. QJM 2003, 96, 563–573. [Google Scholar] [CrossRef]
- Izumo, M.; Nalawadi, S.; Shiota, M.; Das, J.; Dohad, S.; Kuwahara, E.; Fukuoka, Y.; Siegel, R.J.; Shiota, T. Mechanisms of acute mitral regurgitation in patients with takotsubo cardiomyopathy: An echocardiographic study. Circ. Cardiovasc. Imaging 2011, 4, 392–398. [Google Scholar] [CrossRef]
- Izumo, M.; Shiota, M.; Nalawadi, S.; Das, J.; Dohad, S.; Kuwahara, E.; Fukuoka, Y.; Siegel, R.J.; Shiota, T. Determinants of Secondary Pulmonary Hypertension in Patients with Takotsubo Cardiomyopathy. Echocardiography 2015, 32, 1608–1613. [Google Scholar] [CrossRef]
- Kagiyama, N.; Okura, H.; Tamada, T.; Imai, K.; Yamada, R.; Kume, T.; Hayashida, A.; Neishi, Y.; Kawamoto, T.; Yoshida, K. Impact of right ventricular involvement on the prognosis of takotsubo cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, T.; Yoshikawa, T.; Maekawa, Y.; Ueda, T.; Isogai, T.; Konishi, Y.; Sakata, K.; Nagao, K.; Yamamoto, T.; Takayama, M. Characterization of predictors of in-hospital cardiac complications of takotsubo cardiomyopathy: Multi-center registry from Tokyo CCU Network. J. Cardiol. 2014, 63, 269–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobue, Y.; Watanabe, E.; Ichikawa, T.; Koshikawa, M.; Yamamoto, M.; Harada, M.; Ozaki, Y. Physically triggered Takotsubo cardiomyopathy has a higher in-hospital mortality rate. Int. J. Cardiol. 2017, 235, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Nef, H.M.; Lyon, A.R. Epidemiology and pathophysiology of Takotsubo syndrome. Nat. Rev. Cardiol. 2015, 12, 387–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueyama, T.; Hano, T.; Kasamatsu, K.; Yamamoto, K.; Tsuruo, Y.; Nishio, I. Estrogen attenuates the emotional stress-induced cardiac responses in the animal model of Tako-tsubo (Ampulla) cardiomyopathy. J Cardiovasc. Pharmacol. 2003, 42 (Suppl. S1), S117–S119. [Google Scholar] [CrossRef]
- Ueyama, T.; Ishikura, F.; Matsuda, A.; Asanuma, T.; Ueda, K.; Ichinose, M.; Kasamatsu, K.; Hano, T.; Akasaka, T.; Tsuruo, Y.; et al. Chronic estrogen supplementation following ovariectomy improves the emotional stress-induced cardiovascular responses by indirect action on the nervous system and by direct action on the heart. Circ. J. 2007, 71, 565–573. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Komiyama, T.; Matsumoto, S.; Kajiwara, H.; Kobayashi, H.; Ikari, Y. Examination of gender differences in patients with takotsubo syndrome according to left ventricular biopsy: Two case reports. J. Med. Case. Rep. 2021, 15, 281. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Garg, J.; Sharma, A.; Palaniswamy, C.; Shah, N.; Lanier, G.; Patel, N.C.; Lavie, C.J.; Ahmad, H. Gender Differences and Predictors of Mortality in Takotsubo Cardiomyopathy: Analysis from the National Inpatient Sample 2009–2010 Database. Cardiology 2015, 132, 131–136. [Google Scholar] [CrossRef]
- Brinjikji, W.; El-Sayed, A.M.; Salka, S. In-hospital mortality among patients with takotsubo cardiomyopathy: A study of the National Inpatient Sample 2008 to 2009. Am. Heart J. 2012, 164, 215–221. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Itoh, T.; Morino, Y.; Taniai, S.; Ishibashi, Y.; Komatsu, T.; Taguchi, I.; Nishinari, M.; Ako, J.; Kyono, H.; et al. Gender Differences in the Circadian and Seasonal Variations in Patients with Takotsubo Syndrome: A Multicenter Registry at Eight University Hospitals in East Japan. Intern. Med. 2021, 60, 2749–2755. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Ghadri, J.R.; Cammann, V.L.; Jurisic, S.; Seifert, B.; Napp, L.C.; Diekmann, J.; Bataiosu, D.R.; D’Ascenzo, F.; Ding, K.J.; Sarcon, A.; et al. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: Results from the International Takotsubo Registry. Eur. J. Heart Fail. 2017, 19, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samul-Jastrzębska, J.; Roik, M.; Wretowski, D.; Łabyk, A.; Ślubowska, A.; Bizoń, A.; Paczyńska, M.; Kurnicka, K.; Pruszczyk, P.; Ciurzyński, M. Evaluation of the InterTAK Diagnostic Score in differentiating Takotsubo syndrome from acute coronary syndrome. A single center experience. Cardiol. J. 2021, 28, 416–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keramida, K.; Backs, J.; Bossone, E.; Citro, R.; Dawson, D.; Omerovic, E.; Parodi, G.; Schneider, B.; Ghadri, J.R.; Van Laake, L.W.; et al. Takotsubo syndrome in heart failure and World Congress on Acute Heart Failure 2019: Highlights from the experts. ESC Heart Fail. 2020, 7, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laumer, F.; Di Vece, D.; Cammann, V.L.; Würdinger, M.; Petkova, V.; Schönberger, M.; Schönberger, A.; Mercier, J.C.; Niederseer, D.; Seifert, B.; et al. Assessment of Artificial Intelligence in Echocardiography Diagnostics in Differentiating Takotsubo Syndrome From Myocardial Infarction. JAMA Cardiol. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Wehbe, R.M.; Thomas, J.D. Validating Deep Learning to Distinguish Takotsubo Syndrome From Acute Myocardial Infarction-Beware of Shortcuts, Human Supervision Required. JAMA Cardiol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Silverio, A.; Parodi, G.; Scudiero, F.; Bossone, E.; Di Maio, M.; Vriz, O.; Bellino, M.; Zito, C.; Provenza, G.; Radano, I.; et al. Beta-blockers are associated with better long-term survival in patients with Takotsubo syndrome. Heart 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Butt, J.H.; Bang, L.E.; Rørth, R.; Schou, M.; Kristensen, S.L.; Yafasova, A.; Havers-Borgersen, E.; Vinding, N.E.; Jessen, N.; Kragholm, K.; et al. Long-term Risk of Death and Hospitalization in Patients With Heart Failure and Takotsubo Syndrome: Insights From a Nationwide Cohort. J. Card. Fail. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Singh, T.; Khan, H.; Gamble, D.T.; Scally, C.; Newby, D.E.; Dawson, D. Takotsubo Syndrome: Pathophysiology, Emerging Concepts, and Clinical Implications. Circulation 2022, 145, 1002–1019. [Google Scholar] [CrossRef]
- Gorini, A.; Galli, F.; Giuliani, M.; Pierobon, A.; Werba, J.P.; Adriano, E.P.; Trabattoni, D. Psychological Characteristics of Patients with Takotsubo Syndrome and Patients with Acute Coronary Syndrome: An Explorative Study toward a Better Personalized Care. J. Pers. Med. 2022, 12, 38. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Gili, S.; Bertaina, M.; Iannaccone, M.; Cammann, V.L.; Di Vece, D.; Kato, K.; Saglietto, A.; Szawan, K.A.; Frangieh, A.H.; et al. Impact of aspirin on takotsubo syndrome: A propensity score-based analysis of the InterTAK Registry. Eur. J. Heart Fail. 2020, 22, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Amadio, P.; Porro, B.; Cavalca, V.; Barbieri, S.S.; Eligini, S.; Fiorelli, S.; Di Minno, A.; Gorini, A.; Giuliani, M.; Werba, J.P.; et al. Persistent long-term platelet activation and endothelial perturbation in women with Takotsubo syndrome. Biomed. Pharm. 2021, 136, 111259. [Google Scholar] [CrossRef] [PubMed]
- Brenner, R.; Weilenmann, D.; Maeder, M.T.; Jörg, L.; Bluzaite, I.; Rickli, H.; De Pasquale, G.; Ammann, P. Clinical characteristics, sex hormones, and long-term follow-up in Swiss postmenopausal women presenting with Takotsubo cardiomyopathy. Clin. Cardiol. 2012, 35, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.J.; Dragon, J.A.; Moussawi, M.; Huber, S.A. Sex-specific signaling through Toll-Like Receptors 2 and 4 contributes to survival outcome of Coxsackievirus B3 infection in C57Bl/6 mice. Biol. Sex Differ. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napp, L.C.; Westenfeld, R.; Møller, J.E.; Pappalardo, F.; Ibrahim, K.; Bonello, L.; Wilkins, C.; Pershad, A.; Mannino, S.F.; Schreiber, T.L.; et al. Impella mechanical circulatory support for Takotsubo syndrome with shock: A retrospective multicenter analysis. Cardiovasc. Revasc. Med. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Nakamori, T.; Nakamura, M.; Imamura, T. Implication of percutaneous left ventricular assist device for Takotsubo syndrome. Cardiovasc. Revasc. Med. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Imori, Y.; Yoshikawa, T.; Murakami, T.; Isogai, T.; Yamaguchi, T.; Maekawa, Y.; Sakata, K.; Mochizuki, H.; Arao, K.; Otsuka, T.; et al. Impact of Trigger on Outcome of Takotsubo Syndrome―Multi-Center Registry From Tokyo Cardiovascular Care Unit Network. Circ. Rep. 2019, 1, 493–501. [Google Scholar] [CrossRef] [Green Version]
- John, K.; Lal, A.; Mishra, A. A review of the presentation and outcome of takotsubo cardiomyopathy in COVID-19. Monaldi. Arch. Chest Dis. 2021, 91, 33759445. [Google Scholar] [CrossRef]
- Hegde, S.; Khan, R.; Zordok, M.; Maysky, M. Characteristics and outcome of patients with COVID-19 complicated by Takotsubo cardiomyopathy: Case series with literature review. Open Heart 2020, 7, e001360. [Google Scholar] [CrossRef]
| Country | Registry | Study Period | All, Years | Male, Years | Female, Years | p-Value | Ref. |
|---|---|---|---|---|---|---|---|
| USA | NI Sample | 2009–2010 | 65.6 (64.9–66.2) | 59.5 (56.6–62.3) | 66.2 (65.5–66.8) | <0.001 | [29] |
| USA/Europe | InterTAK Registry | 1998–2014 | 66.4 ± 13.1 | 62.9 ± 13.1 | 66.8 ± 13.0 | <0.001 | [4] |
| Japan | Tokyo CCU Network | 2010–2012 | 76 (67–82) | 72 (64–81) | 76 (68–83) | 0.040 | [2] |
| Japan | CIRC-8U | 1997–2014 | 71.6 ± 11.2 | 71.8 ± 10.4 | 71.5 ± 11.4 | 0.899 | [31] |
| Registry | Male | Female | p-Value | Ref. | |
|---|---|---|---|---|---|
| InterTAK Registry | Physical stress, % | 50.8 | 34.3 | <0.001 | [4] |
| Emotional stress, % | 14.5 | 29.2 | <0.001 | ||
| Absence of stress, % | 25.7 | 28.8 | 0.39 | ||
| Tokyo CCU Network | Physical stress, % | 50.0 | 31.3 | 0.002 | [2] |
| Emotional stress, % | 19.0 | 31.0 | 0.039 | ||
| Absence of stress, % | 31.0 | 37.7 | 0.260 | ||
| CIRC-8U | Physical stress, % | 64 | 46 | 0.007 | [31] |
| Emotional stress, % | 10 | 26 | 0.004 | ||
| Absence of stress, % | 26 | 28 | 0.764 |
| Registry | Male | Female | p-Value | Ref. | |
|---|---|---|---|---|---|
| InterTAK Registry | WBC (/μL) | 10,680 (7650–15,600) | 9690 (7400–12,480) | 0.013 | [4] |
| CRP (mg/L) | 5.00 (2.00–23.75) | 3.80 (1.13–11.00) | 0.021 | ||
| Tokyo CCU Network | WBC (/μL) | 9100 (7100–11,970) | 8100 (6400–11,000) | 0.091 | [2] |
| Peak CK (IU/L) | 471 (198–713) | 258 (143–394) | 0.012 | ||
| BNP (pg/mL) | 233 (75–521) | 199 (76–627) | 0.855 | ||
| CRP (mg/dL) | 0.56 (0.1–3.0) | 0.32 (0.1–2.1) | 0.055 | ||
| CIRC-8U | WBC (/μL) | 10,685 ± 4185 | 9704 ± 4853 | 0.011 | [31] |
| Peak CK (IU/L) | 799 ± 1838 | 779 ± 2180 | 0.065 | ||
| CRP (mg/dL) | 5.6 ± 7.1 | 2.7 ± 5.7 | <0.001 |
| Registry | Male | Female | p-Value | Ref. | |
|---|---|---|---|---|---|
| InterTAK Registry | Apical type, % | 81.6 | 81.7 | 0.96 | [4] |
| Midventricular type, % | 12.8 | 14.8 | 0.49 | ||
| LVEF (%) | 39.0 ± 11.5 | 41.3 ± 11.8 | 0.017 | ||
| Tokyo CCU Network | Apical type, % | 90.5 | 90.8 | 0.918 | [2] |
| Midventricular type, % | N/A | N/A | N/A | ||
| LVEF (%) | 48 (40–60) | 50 (40–64) | 0.500 | ||
| LVOTO, % | 4.8 | 9.2 | 0.196 | ||
| CIRC-8U | Apical type *, % | 93.6 | 91.0 | NS | [31] |
| Midventricular type *, % | 2.1 | 4.0 | NS | ||
| LVEF (%) | 44.7 ± 13.2 | 46.2 ± 13.0 | 0.544 | ||
| LVOTO *, % | 0 | 6 | 0.162 |
| Registry | Male | Female | p-Value | Ref. | |
|---|---|---|---|---|---|
| NI Sample | Mortality, % | 4.8 | 2.1 | 0.04 | [29] |
| Respiratory failure, % | 18.2 | 12.6 | 0.06 | ||
| Ventricular arrhythmias, % | 7.7 | 5.4 | 0.27 | ||
| InterTAK Registry | Mortality, % | 7.3 | 3.8 | 0.025 | [4] |
| Respiratory support, % | 29.5 | 16.0 | <0.001 | ||
| Catecholamine use, % | 21.0 | 11.2 | <0.001 | ||
| Tokyo CCU Network | Mortality, % | 9.5 | 5.3 | NS | [2] |
| Heart failure *, % | 20.2 | 10.6 | <0.05 | ||
| Ventricular arrhythmias, % | 8.3 | 3.9 | NS | ||
| Respiratory support, % | 28.6 | 12.7 | <0.05 | ||
| Catecholamine use, % | 11.9 | 12.3 | NS | ||
| CIRC-8U | Mortality, % | 18 | 7 | 0.005 | [31] |
| Cardiovascular death, % | 4 | 3 | 0.704 | ||
| Death by other reasons, % | 14 | 4 | 0.003 | ||
| Heart failure, % | 34 | 29 | 0.388 | ||
| Ventricular arrhythmias, % | 5 | 4 | 0.510 |
| Category | Term | Count | % | p-Value | Bonferroni | Benjamini |
|---|---|---|---|---|---|---|
| KEGG_PATHWAY | hsa04512: ECM-receptor interaction | 17 | 2.007084 | 1.23 × 10−7 | 2.96 × 10−5 | 2.96 × 10−5 |
| KEGG_PATHWAY | hsa04514: Cell adhesion molecules (CAMs) | 17 | 2.007084 | 8.61 × 10−5 | 0.020544 | 0.010326 |
| KEGG_PATHWAY | hsa04060: Cytokine–cytokine-receptor interaction | 19 | 2.243211 | 0.004895 | 0.693482 | 0.325753 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murakami, T.; Komiyama, T.; Kobayashi, H.; Ikari, Y. Gender Differences in Takotsubo Syndrome. Biology 2022, 11, 653. https://doi.org/10.3390/biology11050653
Murakami T, Komiyama T, Kobayashi H, Ikari Y. Gender Differences in Takotsubo Syndrome. Biology. 2022; 11(5):653. https://doi.org/10.3390/biology11050653
Chicago/Turabian StyleMurakami, Tsutomu, Tomoyoshi Komiyama, Hiroyuki Kobayashi, and Yuji Ikari. 2022. "Gender Differences in Takotsubo Syndrome" Biology 11, no. 5: 653. https://doi.org/10.3390/biology11050653