What Is behind the Correlation Analysis of Diarrheagenic E. coli Pathotypes?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Phenotypic and Genotypic Confirmation of DEC Isolates
2.3. Molecular Confirmation of DEC Pathotypes
2.4. Serotyping
2.5. Antimicrobial Susceptibility Testing
2.6. Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR) for DNA Fingerprinting of DEC
2.7. Statistical Analysis
3. Results
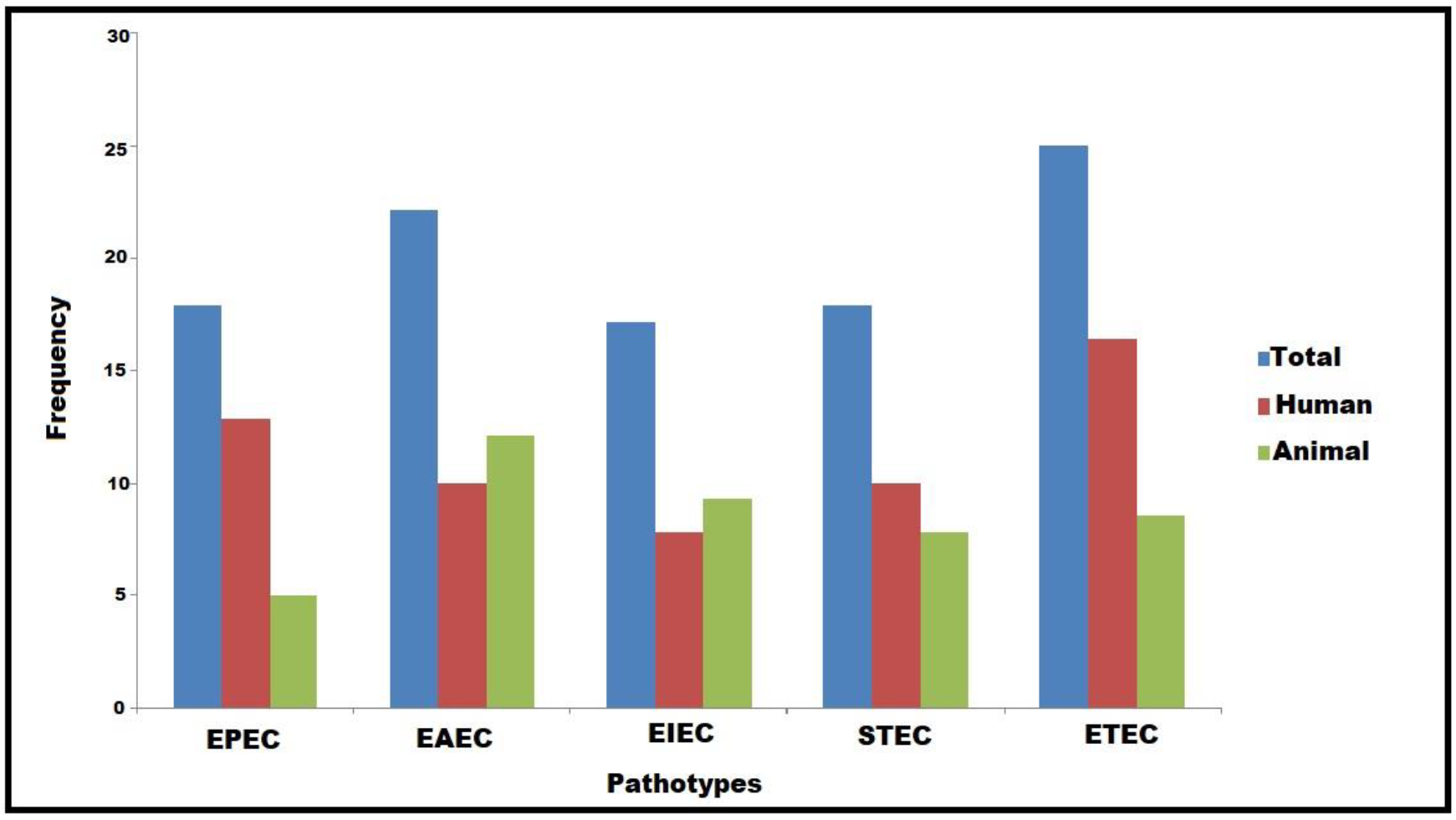
3.1. Characterization and Virulence-Associated Features of DEC Pathotypes
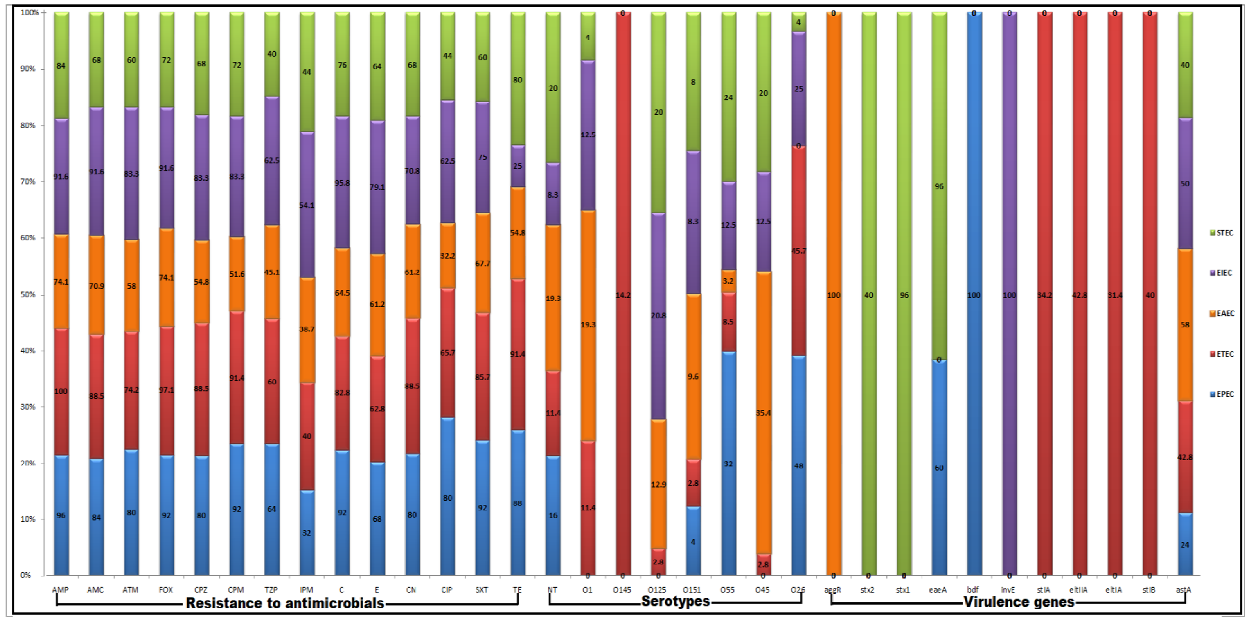
3.2. Serotyping of DEC Pathotypes
3.3. Antimicrobial Susceptibility Patterns of DEC Pathotypes
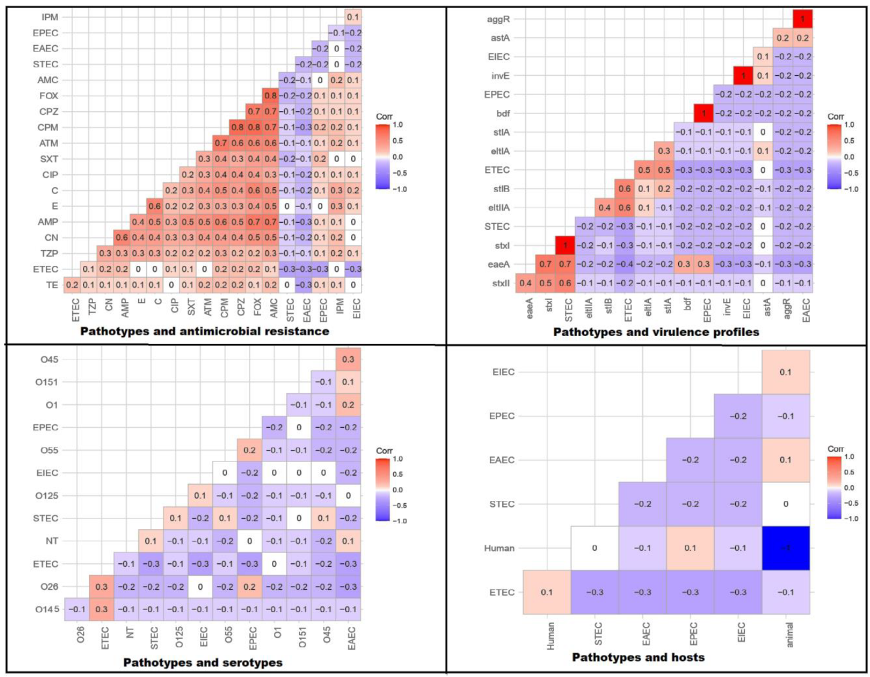
3.4. Correlation between Pathotypes and Antimicrobial Resistance, Serotypes, Virulence Gene Existence, and Host Types
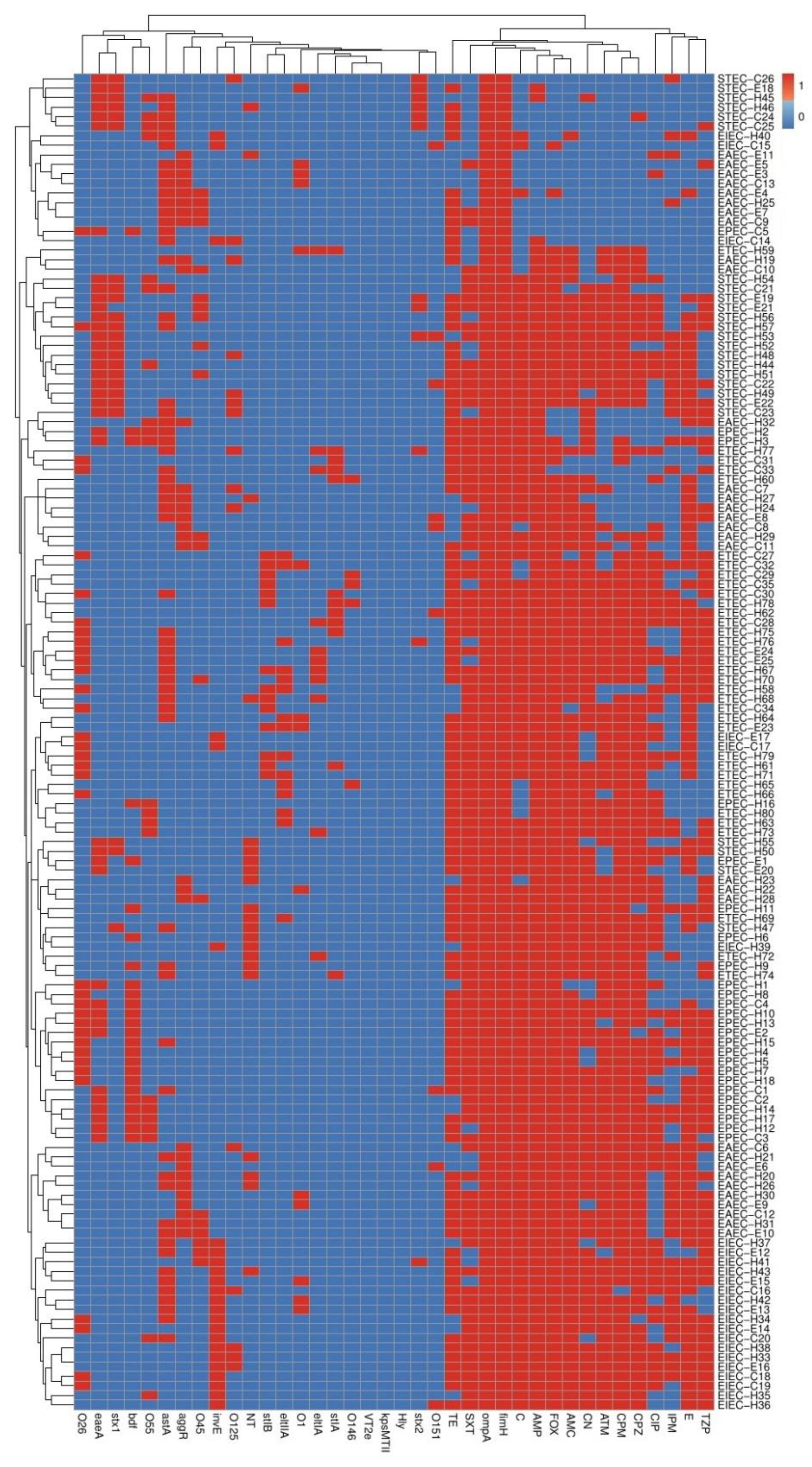
3.5. Phenotyping and Molecular Genotyping of DEC Isolates within and among Different Pathotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allocati, N.; Masulli, M.; Alexeyev, M.F.; Di-Ilio, C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health 2013, 10, 6235–6254. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Law, R.J.; Scholz, R.; Keeney, K.M.; Wlodarska, M.; Finlay, B.B. Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 2013, 26, 822–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elfaky, M.A.; Abdel-Hamid, M.I.; Khalifa, E.; Alshareef, W.A.; Mosbah, R.A.; Elazab, S.T.; Ghoneim, M.M.; Al-Sanea, M.M.; Bendary, M.M. Innovative next-generation therapies in combating multi-drug-resistant and multi-virulent Escherichia coli isolates: Insights from in vitro, in vivo, and molecular docking studies. Appl. Microbiol. Biotechnol. 2022, 106, 1691–1703. [Google Scholar] [CrossRef]
- Mosallam, F.M.; Helmy, E.A.; Bendary, M.M.; El-Batal, I.A. Potency of a novel synthesized Ag- eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021, 36, 1524–1537. [Google Scholar] [CrossRef]
- Jang, J.; Hur, H.G.; Sadowsky, M.J.; Byappanahalli, M.N.; Yan, T.; Ishii, S. Environmental Escherichia coli: Ecology and public health implications—A review. J. Appl. Microbiol. 2017, 123, 570–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robins-Browne, R.M.; Bordun, A.M.; Tauschek, M.; Bennett-Wood, V.R.; Russell, J.; Oppedisano, F.; Lister, N.A.; Bettelheim, K.A.; Fairley, C.K.; Sinclair, M.I.; et al. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg. Infect. Dis. 2004, 10, 1797–1805. [Google Scholar] [CrossRef]
- Pakbin, B.; Brück, W.M.; Rossen, J. Virulence Factors of Enteric Pathogenic Escherichia coli: A Review. Int. J. Mol. Sci. 2021, 22, 9922. [Google Scholar] [CrossRef]
- Johnson, J.R.; O’Bryan, T.T.; Kuskowski, M.; Maslow, J.N. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 2001, 69, 5363–5374. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.; Palm, M.; Mustonen, V.; Warringer, J.; Farewell, A.; Parts, L.; Moradigaravand, D. Genomic Epidemiology and Evolution of Escherichia coli in Wild Animals in Mexico. mSphere 2021, 6, e00738-20. [Google Scholar] [CrossRef]
- Singh, P.; Metgud, S.C.; Roy, S.; Purwar, S. Evolution of diarrheagenic Escherichia coli pathotypes in India. J. Lab. Physicians 2019, 11, 346–351. [Google Scholar] [CrossRef]
- Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Al-Sanea, M.M.; Soliman, W.E.; Ghoneim, M.M.; Bendary, M.M. Molecular Detection of Fluoroquinolone Resistance among Multidrug-, Extensively Drug-, and Pan-Drug-Resistant Campylobacter Species in Egypt. Antibiotics 2021, 10, 1342. [Google Scholar] [CrossRef]
- Mamun, M.M.; Parvej, M.S.; Ahamed, S.; Hassan, J.; Nazir, K.; Nishikawa, Y.; Nishikawa, Y.; Rahman, M.T. Prevalence and characterization of shiga toxigenic Escherichia coli in broiler birds in Mymensingh. Bangl. J. Vet. Med. 2016, 14, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.M.; Mohamed, Z.K.; Klena, J.D.; Ahmed, S.F.; Moussa, T.A.; Ghenghesh, K.S. Molecular characterization of diarrheagenic Escherichia coli from Libya. Am. J. Trop. Med. Hyg. 2012, 86, 866–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, W.M.; Pollard, D.R.; Lior, H.; Tyler, S.D.; Rozee, K.R. Differentiation of genes coding for Escherichia coli verotoxin 2 and the verotoxin associated with porcine edema disease (VTe) by the polymerase chain reaction. J. Clin. Microbiol. 1990, 28, 2351–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piva, I.C.; Pereira, A.L.; Ferraz, L.R.; Silva, R.S.; Vieira, A.C.; Blanco, J.E.; Blanco, M.; Blanco, J.; Giugliano, L.G. Virulence Markers of Enteroaggregative Escherichia coli Isolated from Children and Adults with Diarrhea in Brasília. Braz. J. Clin. Microbiol. 2003, 41, 1827–1832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, C.; Janßen, T.; Kießling, S.; Philipp, H.C.; Wieler, L.H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005, 49, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Dipineto, L.; Santaniello, A.; Fontanella, M.; Lagos, K.; Fioretti, A.; Menna, L.F. Presence of shiga toxin-producing Escherichia coli O157,H7 in living layer hens. Lett. Appl. Microbiol. 2006, 43, 293–295. [Google Scholar] [CrossRef]
- Ewers, C.; Li, G.; Wilking, H.; Kiebling, S.; Alt, K.; Antáo, E.M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian pathogenic uropathogenic and newborn meningitis-causing Escherichia coli, How closely related are they? Inter. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Müller, D.; Greune, L.; Heusipp, G.; Karch, H.; Fruth, A.; Tschäpe, H.; Schmidt, M.A. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 2007, 73, 3380–3390. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.X.; Thong, K.L. Multiplex PCR for simultaneous detection of virulence genes in Escherichia coli. Malays. J. Sci. 2009, 28, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ghanbarpour, R.; Salehi, M. Determination of adhesin encoding genes in Escherichia coli isolates from omphalitis of chicks. Amer. J. Ani. Vet. Sci. 2010, 5, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, P.; Kargar, M.; Doosti, A.; Mardaneh, J.; Dehyadegari, M.A.; Ghorbani-Dalini, S. Multiplex real-time PCR assay for the detection of LT STIa and STIb genes in enterotoxigenic Escherichia coli. Int. J. Entric Pathog. 2014, 2, 16431. [Google Scholar] [CrossRef]
- Yun, Z.; Zeng, L.; Huang, W. Detection and categorization of diarrheagenic Escherichia coli with auto-microfluidic thin-film chip Method. Sci. Rep. 2018, 8, 12926. [Google Scholar] [CrossRef] [PubMed]
- Omolajaiye, S.A.; Afolabi, K.O.; Iweriebor, B.C. Pathotyping and antibiotic resistance profiling of Escherichia coli isolates from children with acute diarrhea in amatole district municipality of eastern cape South Africa. BioMed Res. Int. 2020, 20, 10. [Google Scholar] [CrossRef]
- Guion, C.E.; Ochoa, T.J.; Walker, C.M.; Barletta, F.; Cleary, T.G. Detection of diarrheagenic Escherichia coli by use of melting-curve analysis and real-time multiplex PCR. J. Clin. Microbiol. 2008, 46, 1752–1757. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Document M100-S15; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. Approved Standard. CLSI: Wayne, PA, USA, 2018.
- Magiorakos, A.P. Multidrug-resistant extensively drug-resistant and pandrug-resistant bacteria, an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Ramazanzadeh, R.; Zamani, S.; Zamani, S. Genetic diversity in clinical isolates of Escherichia coli by enterobacterial repetitive intergenic consensus (ERIC)-PCR technique in Sanandaj hospitals. Iran J. Microbiol. 2013, 5, 126–131. [Google Scholar] [PubMed]
- Ghaly, M.; Shaheen, A.; Bouhy, A.; Bendary, M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020, 202, 1231–1240. [Google Scholar] [CrossRef]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Characterization of Methicillin Resistant Staphylococcus aureus isolated from human and animal samples in Egypt. Cell. Mol. Biol. 2016, 29, 94–100. [Google Scholar]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55. [Google Scholar] [PubMed]
- Ghaly, M.F.; Nasr, Z.M.; Abousaty, A.I.; Seadawy, H.G.; Shaheen, M.A.A.; Albogami, S.; Al-Sanea, M.M.; Bendary, M.M. Alternative and Complementary Therapies against Foodborne Salmonella Infections. Antibiotics 2021, 10, 1453. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, S.; Farhadifar, F.; Roshani, D.; Ahmadi, A.; Haghi, F. Study on the presence of resistant diarrheagenic pathotypes in Escherichia coli isolated from patients with urinary tract infection. Gastroenterol. Hepatol. Bed Bench 2019, 12, 348–357. [Google Scholar]
- Lamprecht, C.; Romanis, M.; Huisamen, N.; Carinus, A.; Schoeman, N.; Sigge, G.O. Escherichia coli with virulence factors and multidrug resistance in the Plankenburg River. S. Afr. J. Sci. 2014, 110, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fallah, N.; Ghaemi, M.; Ghazvini, K.; Rad, M.; Jamshidi, A. Occurrence pathotypes and antimicrobial resistance profiles of diarrheagenic Escherichia coli strains in animal source food products from public markets in Mashhad Iran. Food Control 2021, 121, 107640. [Google Scholar] [CrossRef]
- Askari, A.; Ghanbarpour, R.; Akhtardanesh, B.; Aflatoonian, M.R.; Sharifi, H.; Jajarmi, M.; Molaei, R. Detection of zoonotic diarrheagenic pathotypes of Escherichia coli in healthy household dogs. Iran. J. Microbiol. 2020, 12, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Bordier, M.; Binot, A.; Pauchard, Q.; Nguyen, D.T.; Trung, T.N.; Fortané, N.; Goutard, F.L. Antibiotic resistance in Vietnam, moving towards a One Health surveillance system. BMC Public Health 2018, 18, 1–14. [Google Scholar] [CrossRef]
- Ferdous, J.; Rashid, R.B.; Sultana, R.; Saima, S.; Jahan Prima, M.; Begum, A.; Mackie Jensen, P.K. Is It Human or Animal? The Origin of Pathogenic, E. coli in the Drinking Water of a Low-Income Urban Community in Bangladesh. Trop. Med. Infect. Dis. 2021, 6, 181. [Google Scholar] [CrossRef]
- Iman, S. Escherichia coli pathotypes associated with diarrhea in human and domestic animals. Am. J. Anim. Vet. Sci. 2014, 155, 161. [Google Scholar]
- Monaghan, Á.; Byrne, B.; Fanning, S.; Sweeney, T.; McDowell, D.; Bolton, D.J. Serotypes and virulence profiles of non-O157 Shiga toxin-producing Escherichia coli isolates from bovine farms. Appl. Environ. Microbiol. 2011, 77, 8662–8668. [Google Scholar] [CrossRef] [Green Version]
- Sharada, R.; Ruban, S.W.; Thiyageeswaran, M. Isolation characterization and antibiotic resistance pattern of E. coli isolated from poultry. Eur. J. Sci. Res. 2010, 5, 18–22. [Google Scholar]
- Oltani, M.; Peighambari, S.M.; Askari, M.; Sadrzadeh, A. Molecular typing of avian Escherichia coli isolates by enterobacterial repetitive intergenic consensus sequences-polymerase chain reaction (ERIC-PCR). Iran. J. Vet. Med. 2012, 6, 143–148. [Google Scholar]
- Brocchi, M.; Ferreira, A.; Lancellotti, M.; Stehling, E.G.; Campos, T.A.; Nakazato, G. Typing of avian pathogenic Escherichia coli strains by REP-PCR. Pesq. Vet. Bras. 2006, 26, 69–73. [Google Scholar] [CrossRef]


| Target Gene | Specificity | Primer Sequence (5′-3′) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| 16S rRNA | RNA component of the 30S ribosomal subunit | F: GACCTCGGTTTAGTTCACAGA R: CACACGCTGACGCTGACCA | 585 | [12] |
| ompA | Outer membrane protein | F: AGCTATCGCGATTGCAGTG R: GGTGTTGCCAGTAACCGG | 919 | [18] |
| kpsMTII | Adhesion | F: CAGGTAGCGTCGAACTGTA R: CATCCAGACGATAAGCATGAGCA | 280 | [18] |
| hly | Hemolysin | F: AACAAGGATAAGCACTGTTCTGGCT R: ACCATATAAGCGGTCATTCCCGTCA | 117 | [15] |
| stx2 | Shiga toxin 2 | F: CCATGACAACGGACAGCAGTT R: CCTGTCAACTGAGCAGCACTTTG | 779 | [17] |
| stx1 | Shiga toxin 1 | F: ACACTGGATGATCTCAGTGG R: CTGAATCCCCCTCCATTATG | 614 | [17] |
| fimH | Adhesion | F: TGCAGAACGGATAAGCCGTGG R: GCAGTCACCTGCCCTCCGGTA | 508 | [21] |
| vt2e | Vero toxin | F: CCTTAACTAAAAGGAATATA R: CTGGTGGTGTATGATTAATA | 230 | [14] |
| astA | Enterotoxin | F: TGCCATCAACACAGTATATCC R: TCAGGTCGCGAGTGACGGC | 116 | [16] |
| invE | Transcriptional regulation of invasion | F: CGATAGATGGCGAGAAATTATATCCCG R: CGATCAAGAATCCCTAACAGAAGAATCAC | 766 | [22] |
| aggR | Transcriptional activator of adherence fimbriae | F: ACGCAGAGTTGCCTGATAAAG R: AATACAGAATCGTCAGCATCAGC | 400 | [19] |
| eaeA | Intimin | F: GTAAAGTCCGTTACCCCAACCTG R: GCACACGGAGCTCCTCAGTCTCC | 218 | [24] |
| eltIA | Heat-labile toxin I | F: TTACGGCGTTACTATCCTCTCTA R: GGTCTCGGTCAGATATGTGATTC | 275 | [20] |
| eltIIA | Heat-labile toxin II | F: ATATCATTTTCTGTTTCAGCAAA R: CAATAAAATCATCTTCGCTCATG | 720 | [20] |
| stIA | Heat-stable toxin A | F: TTTCCCCTCTTTTAGTCAGTCAA R: GCAGGATTACAACACAATTCACAGCAG | 159 | [25] |
| stIB | Heat-stable toxin B | F: TGCTAAACCAGTAGAGTCTTCAAAA R: GCAGGATTACAACACAATTCACAGC | 138 | [25] |
| bfp | Bundle-forming pilus | F: GACACCTCATTGCTGAAGTCG R: CCAGAACACCTCCGTTATGC | 910 | [19] |
| Antimicrobial Agent | Symbol | Conc. (ug) | Interpretative Categories | |||
|---|---|---|---|---|---|---|
| Zone Diameter Breakpoints (mm) | MIC Breakpoints (μg/mL) | |||||
| Resistance | Sensitive | Resistance | Sensitive | |||
| Ampicillin | AMP | 10 | ≤13 | ≥17 | ≥32 | ≤8 |
| Amoxicillin/clavulanic acid | AMC | 20/10 | ≤13 | ≥18 | ≥32/16 | ≤8/4 |
| Aztreonam | ATM | 30 | ≤17 | ≥21 | ≥16 | ≤4 |
| Cefoxitin | FOX | 30 | ≤14 | ≥18 | ≥32 | ≤8 |
| Cefoperazone | CPZ | 75 | ≤15 | ≥21 | ≥64 | ≤16 |
| Cefepime | CPM | 30 | ≤18 | ≥25 | ≥16 | ≤2 |
| Piperacillin/tazobactam | TZP | 100/10 | ≤17 | ≥21 | ≥128/4 | ≤16/4 |
| Chloramphenicol | C | 30 | ≤12 | ≥18 | ≥32 | ≤8 |
| Imipenem | IPM | 10 | ≤19 | ≥23 | ≥4 | ≤1 |
| Erythromycin | E | 15 | ||||
| Gentamycin | CN | 10 | ≤12 | ≥15 | ≥16 | ≤4 |
| Ciprofloxacin | CIP | 5 | ≤15 | ≥21 | ≥4 | ≤1 |
| Sulfamethoxazole/trimethoprim | SXT | 23.75/1.25 | ≤10 | ≥16 | ≥4/76 | ≤2/38 |
| Tetracycline | TE | 30 | ≤11 | ≥15 | ≥16 | ≤4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendary, M.M.; Abd El-Hamid, M.I.; Alhomrani, M.; Alamri, A.S.; Elshimy, R.; Mosbah, R.A.; Bahnass, M.M.; Omar, N.N.; Al-Sanea, M.M.; Elmanakhly, A.R.; et al. What Is behind the Correlation Analysis of Diarrheagenic E. coli Pathotypes? Biology 2022, 11, 1004. https://doi.org/10.3390/biology11071004
Bendary MM, Abd El-Hamid MI, Alhomrani M, Alamri AS, Elshimy R, Mosbah RA, Bahnass MM, Omar NN, Al-Sanea MM, Elmanakhly AR, et al. What Is behind the Correlation Analysis of Diarrheagenic E. coli Pathotypes? Biology. 2022; 11(7):1004. https://doi.org/10.3390/biology11071004
Chicago/Turabian StyleBendary, Mahmoud M., Marwa I. Abd El-Hamid, Majid Alhomrani, Abdulhakeem S. Alamri, Rana Elshimy, Rasha A. Mosbah, Mosa M. Bahnass, Nasreen N. Omar, Mohammad M. Al-Sanea, Arwa R. Elmanakhly, and et al. 2022. "What Is behind the Correlation Analysis of Diarrheagenic E. coli Pathotypes?" Biology 11, no. 7: 1004. https://doi.org/10.3390/biology11071004
APA StyleBendary, M. M., Abd El-Hamid, M. I., Alhomrani, M., Alamri, A. S., Elshimy, R., Mosbah, R. A., Bahnass, M. M., Omar, N. N., Al-Sanea, M. M., Elmanakhly, A. R., Safwat, N. A., & Alshareef, W. A. (2022). What Is behind the Correlation Analysis of Diarrheagenic E. coli Pathotypes? Biology, 11(7), 1004. https://doi.org/10.3390/biology11071004










