Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Study Area
2.2. Isolation and Identification of Mycoplasma Species
2.3. Antimicrobial Susceptibility Testing via Broth Microdilution Method
2.4. Molecular Identification of Enrofloxacin-Resistant Mycoplasma Species by Conventional PCR and DNA Sequencing Techniques
2.4.1. DNA Extraction
2.4.2. Conventional PCR Assays and Cycling Parameters
2.5. Statistical Analysis
3. Results
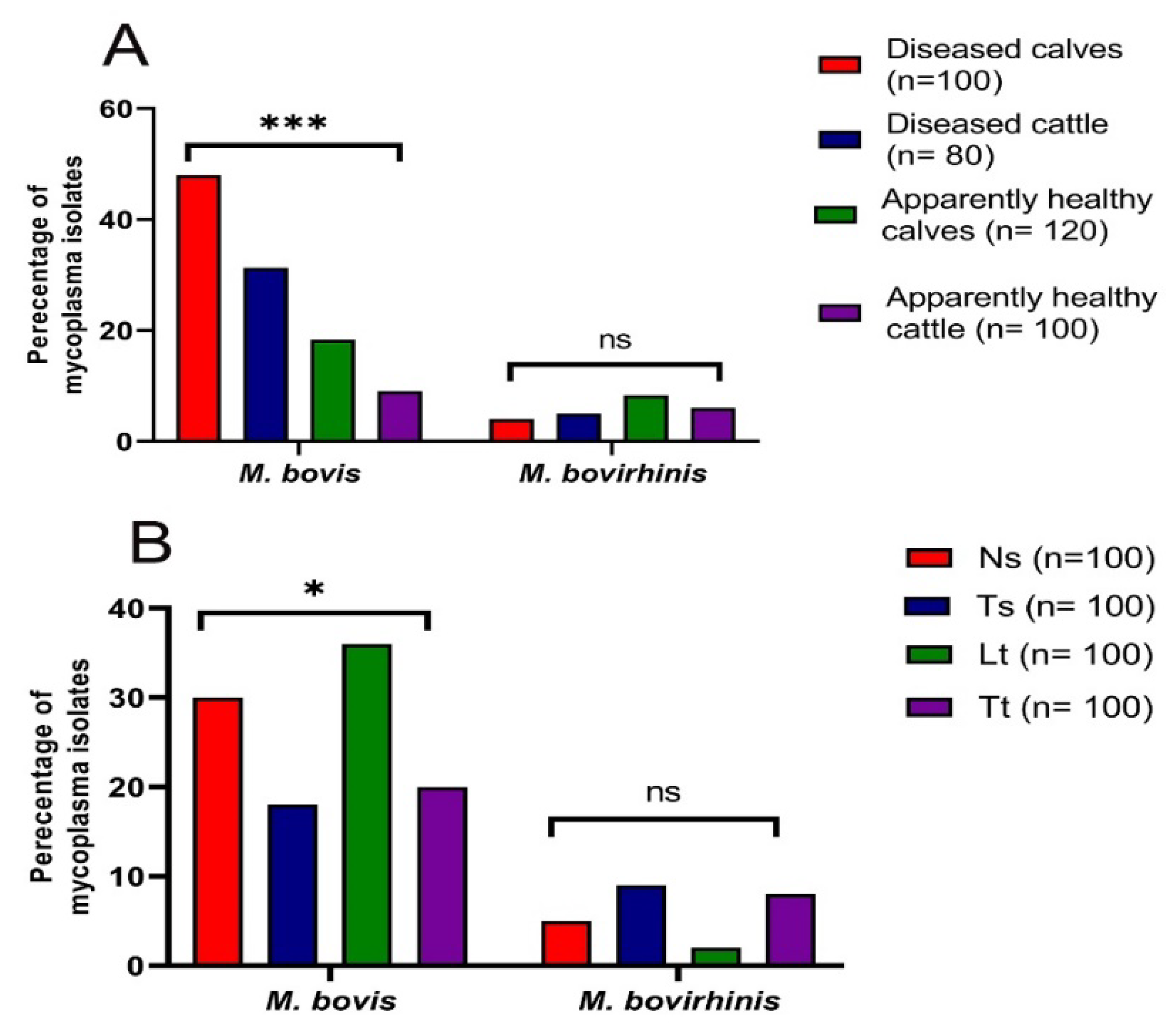
3.1. Prevalence of Mycoplasma Species in Cattle
3.2. Antimicrobial Susceptibility Testing of Mycoplasma Isolates
3.3. Molecular Identification of Enrofloxacin-Resistant Mycoplasma Isolates from Different Sources
4. Discussion
5. Conclusions
6. Supplementary Aspect of Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lysnyansky, I.; Ayling, R.D. Mycoplasma bovis: Mechanisms of resistance and trends in antimicrobial susceptibility. Front. Microbiol. 2016, 7, 595. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Sheehy, P.A.; Hazelton, M.S.; Bosward, K.L.; House, J.K. A review of mycoplasma diagnostics in Cattle. J. Vet. Intern. Med. 2018, 32, 1241–1252. [Google Scholar] [CrossRef]
- Calcutt, M.J.; Lysnyansky, I.; Sachse, K.; Fox, L.K.; Nicholas, R.A.J.; Ayling, R.D. Gap analysis of Mycoplasma bovis disease, diagnosis and control: An aid to identify future development requirements. Transbound. Emerg. Dis. 2018, 65, 91–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isa, F.M. Mycoplasma bovis: A Neglected Pathogen in Nigeria-A Mini Review. Dairy Vet. Sci. J. 2017, 4, 555633. [Google Scholar]
- Hale, H.H.; Helmboldt, C.F.; Plastridge, W.N.; Stula, E.F. Bovine mastitis caused by a Mycoplasma spp. Cornell Vet. 1962, 52, 582–591. [Google Scholar] [PubMed]
- Tardy, F.; Aspan, A.; Autio, T.; Ridley, A.; Tricot, A.; Colin, A.; Strube, M.L. Mycoplasma bovis in nordic european countries: Emergence and dominance of a new clone. J. Pathog. 2020, 9, 875. [Google Scholar] [CrossRef]
- Punyapornwithaya, V.; Fox, L.K.; Hancock, D.D.; Gay, J.M.; Alldredge, J.R. Association between an outbreak strain causing Mycoplasma bovis mastitis and its asymptomatic carriage in the herd: A case study from Idaho, USA. Prev. Vet. Med. 2010, 93, 66–70. [Google Scholar] [CrossRef]
- Hazelton, M.S.; Morton, J.M.; Parker, A.M.; Sheehy, P.A.; Bosward, K.L.; Malmo, J.; House, J.K. Whole dairy herd sampling to detect subclinical intramammary Mycoplasma bovis infection after clinical mastitis outbreaks. Vet. Microbiol. 2020, 244, 108662. [Google Scholar] [CrossRef]
- Oliveira, T.E.S.; Pelaquim, I.F.; Flores, E.F.; Massi, R.P.; Valdiviezo, M.J.J.; Pretto-Giordano, L.G.; Headley, S.A. Mycoplasma bovis and viral agents associated with the development of bovine respiratory disease in adult dairy cows. Trans. Emerg. Dis. 2020, 67, 82–93. [Google Scholar] [CrossRef]
- Bras, A.L.; Barkema, H.W.; Woodbury, M.R.; Ribble, C.S.; Perez-Casal, J. Windeyer, M. Clinical presentation, prevalence and risk factors associated with Mycoplasma bovis-associated disease in farmed bison (Bison bison) herds in western Canada. J. Am. Vet. Med. Assoc. 2017, 250, 1167–1175. [Google Scholar] [CrossRef]
- Ozdemir, U.; Turkyilmaz, M.; Nicholas, A. Detection and antibiotic susceptibility of Mycoplasma bovis and other respiratory disease pathogens from pneumonic lung samples in a calf rearing unit. Madr. J. Vet. Med. Res. 2019, 1, 8–12. [Google Scholar]
- Ammar, A.M.; Abd El-Hamid, M.I.; Hashem, Y.M.; El-Malt, R.M.S.; Mohamed, H.M. Mycoplasma bovis: Taxonomy, Characteristics, Pathogenesis and Antimicrobial Resistance. Zagazig Vet. J. 2021, 49, 444–461. [Google Scholar] [CrossRef]
- Khalil, D.; Becker, C.A.; Tardy, F. Alterations in the quinolone resistance-determining regions and fluoroquinolones resistance in clinical isolates and laboratory-derived mutants of Mycoplasma bovis not all genotypes may be equal. Appl. Environ. Microb. 2016, 82, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, A.M.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; Azab, D.S.; Albogami, S.; Bendary, M.M. Molecular detection of fluoroquinolone resistance among multidrug-, extensively drug-, and pan-drug-resistant Campylobacter species in Egypt. J. Antibiot. 2021, 10, 1342. [Google Scholar] [CrossRef]
- Sato, T.; Okubo, T.; Usui, M.; Higuchi, H.; Tamura, Y. Amino acid substitutions in gyrA and parC are associated with fluoroquinolones resistance in Mycoplasma bovis isolates from Japanese dairy calves. J. Vet. Med. Sci. 2013, 75, 1063–1065. [Google Scholar] [CrossRef] [Green Version]
- Redgrave, L.S.; Sutton, S.B.; Webber, M.A.; Piddock, L.J. Fluoroquinolones resistance: Mechanisms, impact on bacteria, and role in evolutionary success. Trend. Microbiol. 2014, 22, 438–445. [Google Scholar] [CrossRef]
- Megid, R.; Nicholas, R.A.J.; Miles, R.J. Biochemical characterization of Mycoplasma bovirhinis, Mycoplasma dispar and recent bovine isolates of Mycoplasma canis. Vet. Res. Commun. 2001, 25, 1–12. [Google Scholar] [CrossRef]
- Lysnyansky, I.; Mikula, I.; Gerchman, I.; Levisohn, S. Rapid detection of a point mutation in the parC gene associated with decreased susceptibility to fluoroquinolones in Mycoplasma bovis. Antimicrob. Agents Chemother. 2009, 53, 4911–4914. [Google Scholar] [CrossRef] [Green Version]
- Kairova, M.; Rakhimzhanova, D. Molecular genetic techniques and oligonucleotides for mycoplasma identification—A review. Acta Vet. Brno 2021, 89, 317–332. [Google Scholar] [CrossRef]
- Heaton, V.J.; Ambler, J.E.; Fisher, L.M. Potent antipneumococcal activity of gemifloxacin is associated with dual targeting of gyrase and topoisomerase IV, an in vivo target preference for gyrase and enhanced stabilization of cleavable complexes in vitro. Antimicrob. Agents Chemother. 2000, 44, 3112e7. [Google Scholar] [CrossRef] [Green Version]
- Sulyok, K.M.; Kreizinger, Z.; Wehmann, E.; Lysnyansky, I.; Bányai, K.; Marton, S.; Jerzsele, Á.; Rónai, Z.; Turcsányi, I.; Makrai, L.; et al. Mutations associated with decreased susceptibility to seven antimicrobial families in field and laboratory-derived Mycoplasma bovis strains. Antimicrob. Agents Chemother. 2017, 61, e01983-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, R.; Baker, S. Recovery of mycoplasmas from animals. Mycoplasma protocols. Methods Mol. Biol. 1998, 104, 37–43. [Google Scholar] [PubMed]
- Shahzad, W.; Munir, R.; Rana, M.Y.; Ahmad, R.; Khan, M.S.; Akbar, G.; Ijaz, M.; Mehmood, F. Prevalence, molecular diagnosis and treatment of Mycoplasma conjunctivae isolated from infectious keratoconjunctivitis affected Lohi sheep maintained at Livestock Experiment Station, Bahadurnagar, Okara, Pakistan. Trop. Anim Health Prod. 2013, 45, 737–742. [Google Scholar] [CrossRef]
- Freundt, E.A.; Andrews, B.E.; Erno, H.; Kunze, M.; Black, F.T. The sensitivity of Mycoplasmatales to sodium polyanethol sulfonate and digitonin. Zentralbl. Bakteriol. Parasitenkd. Infektioskr. Hyg. Abt. Orig. 1973, 225, 104–112. [Google Scholar]
- Poveda, J.B.; Nicholas, R. Serological identification of mycoplasmas by growth and metabolism inhibition tests. Mycoplasma Protoc. Methods Mol. Biol. 1998, 104, 105–111. [Google Scholar]
- Clyde, W.A. Mycoplasma species identification based upon growth inhibition by specific antisera. J. Immunol. 1964, 92, 958–965. [Google Scholar] [PubMed]
- Lauerman, L.H. Mycoplasmas of the bovine respiratory tract. In Mycoplasmosis in Animals: Laboratory Diagnosis; Whitford, H.W., Rosenbusch, R.F., Lauerman, L.H., Eds.; Iowa State University Press: Ames, IA, USA, 1994; pp. 50–56. [Google Scholar]
- Hannan, P.C. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary Mycoplasma species. Vet. Res. 2000, 31, 373–395. [Google Scholar] [CrossRef] [Green Version]
- Jelinski, M.; Kinnear, A.; Gesy, K.; Andrés-Lasheras, S.; Zaheer, R.; Weese, S.; McAllister, T.A. Antimicrobial sensitivity testing of Mycoplasma bovis isolates derived from Western Canadian feedlot cattle. Microorganisms 2020, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals; Approved Standard—Second Edition; NCCLS Document M31-A2; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002; Volume 22, p. 57. [Google Scholar]
- Godinho, K.S. Susceptibility testing of tulathromycin: Interpretative breakpoints and susceptibility of field isolates. Vet. Microbiol. 2008, 129, 426–432. [Google Scholar] [CrossRef] [Green Version]
- Gerchman, I.; Levisohn, S.; Mikula, I. In vitro antimicrobial susceptibility of Mycoplasma bovis isolated in Israel from local and imported cattle. Vet. Microbiol. 2009, 137, 268–275. [Google Scholar] [CrossRef]
- Catania, S.; Bottinelli, M.; Fincato, A.; Gastaldelli, M.; Barberio, A.; Gobbo, F.; Vicenzoni, G. Evaluation of Minimum Inhibitory Concentrations for 154 Mycoplasma synoviae isolates from Italy collected during 2012–2017. PLoS ONE 2019, 14, e0224903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, Y.R.; Ros Bascuñana, C.; Bölske, G.; Mattsson, J.G.; Fernandez Molina, C.; Johansson, K.E. In vitro amplification of the 16S rRNA genes from Mycoplasma bovis and Mycoplasma agalactiae by PCR. Vet. Microbiol. 1995, 47, 183–190. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1989; Volume 1. [Google Scholar]
- Alberto, A.; Addis, M.F.; Chessa, B.; Cubaddu, T.; Profiti, M.; Rosati, S.; Ruiu, A.; Pittau, M. Molecular and antigenic characterization of a Mycoplasma bovis strain causing an outbreak of infectious keratoconjunctivitis. J. Vet. Diagn. Investig. 2006, 18, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salama, S.E.M.; Kholy, M.M.; Talkhan, O.F.A.; Mosallam, S.A.; Atwa, E.I. Bacterial agent of respiratory manifestation in cattle and associated biochemical alterations in Menoufiea Governorate. Nat. Sci. 2009, 7, 26-30.–30. [Google Scholar]
- Kobayashi, H.; Hirose, K.; Worarach, A.; Paugtes, P.; Ito, N.; Morozumi, T.; Yamamoto, K. In vitro amplification of the 16S rRNA genes from Mycoplasma bovirhinis, Mycoplasma alkalescens and Mycoplasma bovigenitalium by PCR. J. Vet. Med. Sci. 1998, 60, 1299–1303. [Google Scholar] [CrossRef] [Green Version]
- Al-Farha, A.A.B.; Hemmatzadeh, F.; Khazandi, M.; Hoare, A.; Petrovski, K. Evaluation of effects of Mycoplasma mastitis on milk composition in dairy cattle from South Australia. BMC Vet. Res. 2017, 13, 351. [Google Scholar] [CrossRef] [Green Version]
- Hata, E.; Harada, T.; Itoh, M. Relationship between antimicrobial susceptibility and multilocus sequence type of Mycoplasma bovis isolates and development of a method for rapid detection of point mutations involved in decreased susceptibility to macrolides, lincosamides, tetracyclines, and spectinomycin. Appl. Environ. Microbiol. 2019, 85, e00575-19. [Google Scholar]
- Abd El-Hamid, M.I.; Bendary, M.M. Comparative phenotypic and genotypic discrimination of methicillin resistant and susceptible Staphylococcus aureus in Egypt. Cell. Mol. Biol. 2015, 61, 101–112. [Google Scholar]
- Ammar, A.M.; Abd El-Hamid, M.I.; Eid, S.E.A.; El Oksh, A.S. Insights into antimicrobial resistance and virulence genes of emergent multidrug resistant avian pathogenic Escherichia coli in Egypt: How closely related are they? Revue Méd. Vét. 2015, 166, 304–314. [Google Scholar]
- Ammar, A.M.; Attia, A.M.; Abd El-Aziz, N.K.; Abd El Hamid, M.I.; El-Demerdash, A.S. Class 1 integron and associated gene cassettes mediating multiple-drug resistance in some foodborne pathogens. Int. Food Res. J. 2016, 23, 332–339. [Google Scholar]
- Ammar, A.M.; Attia, A.M.; Marwa, I.; Abd El-Hamid, M.I.; El-Shorbagy, I.M.; Shaimaa, A.; Abd El-Kader, S.A. Genetic basis of resistance waves among methicillin resistant Staphylococcus aureus isolates recovered from milk and meat products in Egypt. Cell. Mol. Biol. 2016, 62, 7–15. [Google Scholar] [PubMed]
- Bendary, M.M.; Solyman, S.M.; Azab, M.M.; Mahmoud, N.F.; Hanora, A.M. Genetic diversity of multidrug resistant Staphylococcus aureus isolated from clinical and non clinical samples in Egypt. Cell. Mol. Biol. 2016, 62, 55–61. [Google Scholar] [PubMed]
- Abd El-Aziz, N.K.; Abd El-Hamid, M.I.; Bendary, M.M.; El-Azazy, A.A.; Ammar, A.M. Existence of vancomycin resistance among methicillin resistant S. aureus recovered from animal and human sources in Egypt. Slov. Vet. Res. 2018, 55, 221–230. [Google Scholar]
- Abd El-Hamid, M.I.; Awad, N.F.S.; Abo-Shama, U.H.; Yousreya, M.H.; Abdel-Rahman, M.A. In vitro evaluation of various antimicrobials against field avian Mycoplasma gallisepticum and Mycoplasma synoviae isolates in Egypt. Poult. Sci. J. 2019, 98, 6281–6288. [Google Scholar] [CrossRef]
- Abd El-Hamid, M.I.; Bendary, M.M.; Merwad, A.M.A.; Elsohaby, I.; Ghaith, D.M.; Alshareef, W.A. What is behind phylogenetic analysis of hospital-, community and livestock-associated methicillin-resistant Staphylococcus aureus? Transbound. Emerg. Dis. 2019, 66, 1506–1517. [Google Scholar] [PubMed]
- Awad, N.F.S.; Abd El-Hamid, M.I.; Hashem, Y.M.; Erfan, A.M.; Abdelrahman, B.A.; Mahmoud, H.I. Impact of single and mixed infections with Escherichia coli and Mycoplasma gallisepticum on Newcastle disease virus vaccine performance in broiler chickens: An in vivo perspective. J. Appl. Microbiol. 2019, 127, 396–405. [Google Scholar] [CrossRef]
- Ammar, A.M.; El-Naenaeey, E.S.Y.; El-Malt, R.M.S.; El-Gedawy, A.A.; Khalifa, E.; Elnahriry, S.S.; Abd El-Hamid, M.I. Prevalence, antimicrobial susceptibility, virulence and genotyping of Campylobacter jejuni with a special reference to the anti-virulence potential of Eugenol and beta-resorcylic acid on some multi-drug resistant isolates in Egypt. J. Anim. Sci. 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.M.; El-Naenaeey, E.S.Y.; El-Hamid, M.I.A.; El-Gedawy, A.A.; El-Malt, R.M.S. Campylobacter as a Major Foodborne Pathogen: A Review of Its Characteristics, Pathogenesis, Antimicrobial Resistance and Control. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 609–619. [Google Scholar] [CrossRef]
- Bendary, M.M.; Ibrahim, D.; Mosbah, R.A.; Mosallam, F.; Hegazy, W.A.H.; Awad, N.F.S.; Alshareef, W.A.; Alomar, S.Y.; Zaitone, S.A.; Abd El-Hamid, M.I. Thymol Nanoemulsion: A new therapeutic option for extensively drug resistant foodborne pathogens. J. Antibiot. 2021, 10, 25. [Google Scholar] [CrossRef]
- Ibrahim, D.; Abdelfattah-Hassan, A.; Badawi, M.; Ismail, T.A.; Bendary, M.M.; Abdelaziz, A.M.; Mosbah, R.A.; Mohamed, D.; Arisha, A.H.; El-Hamid, M.I.A. Thymol nanoemulsion promoted broiler chicken’s growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021, 11, 7742. [Google Scholar] [CrossRef]
- Gabinaitiene, A.; Siugzdaite, J.; Zilinskas, H.; Siugzda, R.; Petkevicius, S. Mycoplasma bovis and bacterial pathogens in the bovine respiratory tract. J. Vet. Med. 2011, 56, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Müştak, H.K. Diagnosis of Mycoplasma bovis infection in cattle by ELISA and PCR. Kafkas Üniversitesi Vet. Fakültesi Derg. 2014, 20, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Francis, M.I.; Ankeli, P.I.; Kwanashie, C.N.; Adamu, J.; Allam, L.; Raji, M.A.; Scacchia, M. Detection of Mycoplasma bovis from cattle presented for slaughter in Adamawa and Taraba States, Northeastern Nigeria. Vet. Sci. 2020, 6, 109–115. [Google Scholar] [CrossRef]
- Boonyayatra, S.; Fox, L.K.; Gay, J.M.; Sawant, A.; Besser, T.E. Discrimination between Mycoplasma and Acholeplasma species of bovine origin using digitonin disc diffusion assay, nisin disc diffusion assay, and conventional polymerase chain reaction. J. Vet. Diagn. Investig. 2012, 24, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Tawab, A.; Awad, A.; Elhofy, F.; Hassan, N.I.; Ramdan, M. Identification and genetic characterization of Mycoplasma spp. affecting respiratory system in Egyptian cattle. Benha Vet. Med. J. 2021, 40, 21–26. [Google Scholar] [CrossRef]
- García-Galán, A.; De la Fe, C.; Gomis, J.; Bataller, E.; Sánchez, A.; Quereda, J.J.; Gómez-Martín, A. The addition of Lactobacillus spp. negatively affects Mycoplasma bovis viability in bovine cervical mucus. BMC Vet. Res. 2020, 16, 251. [Google Scholar] [CrossRef]
- El-Shafey, D.Y. Advanced Studies on Mycoplasma bovis in Cattle. Ph.D. Thesis, Cairo University, Giza, Egypt, 2005. [Google Scholar]
- Soehnlen, M.K.; Kariyawasam, S.; Lumadue, J.A.; Pierre, T.A.; Wolfgang, D.R.; Jayarao, B.M. Molecular epidemiological analysis of Mycoplasma bovis isolates from the Pennsylvania Animal Diagnostic Laboratory showing genetic diversity. J. Dairy Sci. 2011, 94, 1893–1899. [Google Scholar] [CrossRef] [Green Version]
- Fawkia, I.A.E.R. In vitro antibiotic sensitivity of different strains of Mycoplasma and Acholeplasma isolated from feeder calves. Proceeding Third Sci. Congr. JESCD 1995, 1, 173–177. [Google Scholar]
- El-Shabiny, L.M.; Abou El-Makarem, M.M.; Nada, H.S. Mycoplasma isolated from cattle lungs and their Pathogenicity study. Egypt. J. Agric. Res. 1999, 77, 421–431. [Google Scholar]
- Gagea, M.I.; Bateman, K.G.; Shanahan, R.A.; van Dreumel, T.; McEwen, B.J.; Carman, S.; Archambault, M.; Caswell, J.L. Natural occurring Mycoplasma bovis associated pneumonia and polyarthritis in feedlot beef calves. J. Vet. Diagn. Investig. 2006, 18, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Kusiluka, L.J.; Kokotovic, B.; Ojeniyi, B.; Friis, N.F.; Ahrens, P. Genetic variations among Mycoplasma bovis strains isolated from Danish cattle. FEMS Microbiol. Lett. 2000, 192, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Dudek, K.; Nicholas, R.A.; Szacawa, E.; Bednarek, D. Mycoplasma bovis infections—Occurrence, diagnosis and control. J. Pathog. 2020, 9, 640. [Google Scholar] [CrossRef] [PubMed]
- Margineda, C.A.; Zielinski, G.O.; Jurado, S.B.; Ferrella, A.; Mozgovoj, M.; Alcaraz, A.C.; Lopez, A. Mycoplasma bovis pneumonia in feedlot cattle and dairy calves in Argentina. Brazil. J. Vet. Pathol. 2017, 10, 79–86. [Google Scholar] [CrossRef]
- Li, C.; Zaheer, R.; Kinnear, A.; Jelinski, M.; McAllister, T.A. Comparative Microbiomes of the respiratory tract and joints of feedlot cattle mortalities. Microorganisms 2022, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Rifatbegovic, M.; Assunção, P.; Poveda, J.B.; Pasic, S. Isolation of Mycoplasma bovis from the respiratory tract of cattle in Bosnia and Herzegovina. Vet. Rec. 2007, 160, 484. [Google Scholar] [CrossRef]
- Hashem, Y.M.; Mousa, W.S.; Abdeen, E.E.; Abdelkhalek, H.M.; Nooruzzaman, M.; El-Askary, A.; Wareth, G. Prevalence and molecular characterization of Mycoplasma species, Pasteurella multocida, and Staphylococcus aureus isolated from calves with respiratory manifestations. J. Anim. 2022, 12, 312. [Google Scholar] [CrossRef]
- Maunsell, F.P.; Donovan, G.A. Mycoplasma bovis Infections in Young Calves. Food Anim. Pract. 2009, 25, 139–177. [Google Scholar] [CrossRef]
- Nicholas, R.A.J.; Fox, L.K.; Lysnyansky, I. Mycoplasma mastitis in cattle: To cull or not to cull. Vet. J. 2016, 216, 142–147. [Google Scholar] [CrossRef]
- Sulyok, K.M.; Kreizinger, Z.; Fekete, L.; Hrivnák, V.; Magyar, T.; Jánosi, S.; Gyuranecz, M. Antibiotic susceptibility profiles of Mycoplasma bovis strains isolated from cattle in Hungary, Central Europe. BMC Vet. Res. 2014, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Klein, U.; de Jong, A.; Moyaert, H.; El Garch, F.; Leon, R.; Richard-Mazet, A.; Ayling, R.D. Antimicrobial susceptibility monitoring of Mycoplasma hyopneumoniae and Mycoplasma bovis isolated in Europe. Vet. Microbiol. 2017, 204, 188–193. [Google Scholar] [CrossRef]
- Khalil, D.; Becker, C.A.; Tardy, F. Monitoring the decrease in susceptibility to ribosomal RNAs targeting antimicrobials and its molecular basis in clinical Mycoplasma bovis isolates over time. Microb. Drug Resist. 2017, 23, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Sayed, M.E.; Ali Aisha, R.; Abdallah, H.M.; Marwa, I.A.; El-mowalid, G.A. Marjoram extract down-regulates the expression of Pasteurella multocida adhesion, colonization and toxin genes: A potential mechanism for its antimicrobial activity. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Elmowalid, G.A.; Marwa, I.; Abd El-Wahab, A.M.; Atta, M.; Abd El-Naser, G.; Attia, A.M. Garlic and ginger extracts modulated broiler chicks innate immune responses and enhanced multidrug resistant Escherichia coli O78 clearance. Comp. Immunol. Microbiol. Infec Dis. 2019, 66, 101334. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, M.I.; El-Naenaeey, E.Y.; Kandeel, T.M.; Hegazy, W.A.H.; Mosbah, R.A.; Nassar, M.S.; Bakhrebah, M.A.; Abdulaal, W.H.; Alhakamy, N.A.; Bendary, M.M. Promising Antibiofilm Agents: Recent Breakthrough against Biofilm Producing Methicillin-Resistant Staphylococcus aureus. Antibiotics 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Aljazzar, A.; Abd El-Hamid, M.I.; El-Malt, R.M.S.; El-Gharreb, W.R.; Abdel-Raheem, S.M.; Ibrahim, A.M.; Abdelaziz, A.M.; Ibrahim, D. Prevalence and Antimicrobial Susceptibility of Campylobacter Species with Particular Focus on the Growth Promoting, Immunostimulant and Anti-Campylobacter jejuni Activities of Eugenol and Trans-Cinnamaldehyde Mixture in Broiler Chickens. Animals 2022, 12, 905. [Google Scholar] [CrossRef]
- Mosallam, F.M.; Helmy, E.A.; Bendary, M.M.; El-Batal, I.A. Potency of a novel synthesized Ag- eugenol nanoemulsion for treating some bacterial and fungal pathogens. J. Mater. Res. 2021, 36, 1524–1537. [Google Scholar] [CrossRef]
- Ghaly, M.; Shaheen, A.; Bouhy, A.; Bendary, M. Alternative therapy to manage otitis media caused by multidrug-resistant fungi. Arch. Microbiol. 2020, 1, 10. [Google Scholar] [CrossRef]
- Bell, C.J.; Blackburn, P.; Elliott, M.; Patterson, T.I.; Ellison, S.; Lahuerta-Marin, A.; Ball, H.J. Investigation of polymerase chain reaction assays to improve detection of bacterial involvement in bovine respiratory disease. J. Vet. Diagn. Investig. 2014, 26, 631–634. [Google Scholar] [CrossRef] [Green Version]
- Ayling, R.D.; Bashiruddin, S.E.; Nicholas, R.A. Mycoplasma species and related organisms isolated from ruminants in Britain between 1990 and 2000. Vet. Rec. 2004, 155, 413–416. [Google Scholar] [CrossRef]
- Piddock, L.J. Mechanisms of fluoroquinolone resistance: An update 1994–1998. Drugs 1999, 58, 11–18. [Google Scholar] [CrossRef]
- Chernova, O.A.; Medvedeva, E.S.; Mouzykantov, A.A.; Baranova, N.B.; Chernov, V.M. Mycoplasmas and their antibiotic resistance: The problems and prospects in controlling infections. Acta Nat. 2016, 8, 24–34. [Google Scholar] [CrossRef] [Green Version]



| Sample Type (Symbol) | Animal Health Condition | Total | |||
|---|---|---|---|---|---|
| Apparently Healthy (n = 55) | Diseased (n = 45) | ||||
| Calve (n = 30) | Adult Cattle (n = 25) | Calve (n = 25) | Adult Cattle (n = 20) | ||
| Nasal swab (Ns) | 30 | 25 | 25 | 20 | 100 |
| Tracheal swab (Ts) | 30 | 25 | 25 | 20 | 100 |
| Lung tissue (Lt) | 30 | 25 | 25 | 20 | 100 |
| Tracheal tissue (Tt) | 30 | 25 | 25 | 20 | 100 |
| Total | 120 | 100 | 100 | 80 | 400 |
| Antimicrobial Class | Antimicrobial Agent (Symbol) | MIC Breakpoint (µg/mL) | Reference | ||
|---|---|---|---|---|---|
| Sensitive | Intermediate Sensitive | Resistant | |||
| Fluroquinolones | Enrofloxacin (ENR) | ≤0.25 | 0.5−1 | ≥2 | [32] |
| Macrolides | Tulathromycin (TUL) | ≤16 | 32 | ≥64 | [31] |
| Tilmicosin (TIL) | ≤8 | 16 | ≥32 | [32] | |
| Tylosin (TYL) | ≤8 | - | ≥16 | [28] | |
| Spiramycin (SP) | ≤2−8 | - | >8 | ||
| Tetracyclines | Doxycycline (DO) | ≤1 | 2−4 | ≥8 | |
| Aminoglycosides | Spectinomycin (SPT) | ≤32 | 64 | ≥128 | [30] |
| Phenicols | Florfenicol (FF) | ≤2 | 4 | ≥8 | |
| Specificity (Target Gene) | Primer Sequence (5′-3′) | PCR Amplified Product (bp) | Reference |
|---|---|---|---|
| Genus Mycoplasma (16S rRNA) | F: AGACTCCTACGGGAGGCAGCA R: ACTAGCGAT TCCGACTTCATG | 1000 | [36] |
| M. bovis (16S rRNA) | F: CCTTTTAGATTGGGATAGCGGATG R: CCGTCAAGGTAGCATCATTTCCTAT | 360 | [34,37] |
| M. bovirhinis (16S rRNA) | F: GCTGATAGAGAGGTCTATCG R: ATTACTCGGGCAGTCTCC | 316 | [38,39] |
| QRDRs (gyrA) | F: GACGAATCATCTAGCGAG R: GCCTTCTAGCATCAAAGTAGC | 531 | [18,40] |
| QRDRs (parC) | F: GAGCAACAGTTAAACGATTTG R: GGCATAACAACTGGCTCTT | 488 | [18,40] |
| Sample Type (Symbol, No.) | ** Total No. of Mycoplasma Isolates from Various Samples of Investigated Animals (%) | Total * | |||
|---|---|---|---|---|---|
| Diseased Calves (n = 100) | Diseased Cattle (n = 80) | Apparently Healthy Calves (n = 120) | Apparently Healthy Cattle (n = 100) | ||
| Nasal swab (Ns, 100) | 12 (48) | 8 (40) | 9 (30) | 6 (24) | 35 (35) |
| Tracheal swab (Ts, 100) | 10 (40) | 5 (25) | 8 (26.7) | 4 (16) | 27 (27) |
| Lung tissue (Lt, 100) | 19 (76) | 12 (60) | 7 (23.3) | 0 (0) | 38 (38) |
| Tracheal tissue (Tt, 100) | 11 (44) | 4 (20) | 8 (26.7) | 5 (20) | 28 (28) |
| * Total (400) | 52 (52) | 29 (36.3) | 32 (26.7) | 15 (15) | 128 (32) |
| Antimicrobial Class | Antimicrobial Agent (Symbol) | No. of Mycoplasma Isolates Showing MIC Values of the Tested Antimicrobials (μg/mL) * | MIC50 | MIC90 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.0078 | 0.0156 | 0.0312 | 0.0625 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | ||||
| Fluoroquinolones | Enrofloxacin (ENR) | - | - | - | - | - | 5 | 10 | 15 | 41 | 4 | 1 | 2 | 2 |
| Macrolides | Tulathromycin (TUL) | - | 7 | 6 | 4 | 5 | 14 | 16 | 24 | - | - | - | 0.5 | 1 |
| Tilmicosin (TIL) | 13 | 29 | 27 | 7 | - | - | - | - | - | - | - | 0.0156 | 0.0312 | |
| Tylosin (TYL) | - | - | - | - | 17 | 18 | 22 | 16 | 3 | - | 0.5 | 1 | ||
| Spiramycin (SP) | - | - | 37 | 3 | 36 | - | - | - | - | - | - | 0.0625 | 0.125 | |
| Tetracyclines | Doxycycline (DO) | - | - | - | - | - | - | - | 5 | 3 | 35 | 33 | 4 | 8 |
| Aminoglycosides | Spectinomycin (SPT) | - | 6 | 6 | - | - | - | 10 | 12 | 28 | 14 | - | 2 | 4 |
| Phenicols | Florfenicol (FF) | - | - | - | - | - | - | 6 | 26 | 17 | 27 | - | 2 | 4 |
| No. of Mycoplasma Species and Total Mycoplasma Isolates (%) Showing Susceptibility Patterns Against the Examined Antibiotic | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial Class | Antimicrobial Agent (Symbol) | M. bovis (n = 61) | M. bovirhinis (n = 15) | Total Mycoplasma Isolates (n = 76) | ||||||
| R | I | S | R | I | S | R | I | S | ||
| Fluoroquinolones | Enrofloxacin (ENR) | 41 (67.2) | 20 (32.8) | - | 5 (33.3) | 10 (66.7) | - | 46 (60.5) | 30 (39.5) | - |
| Macrolides | Tulathromycin (TUL) | - | - | 61 (100) | - | - | 15 (100) | - | - | 76 (100) |
| Tilmicosin (TIL) | - | - | 61 (100) | - | - | 15 (100) | - | - | 76 (100) | |
| Tylosin (TYL) | - | - | 61 (100) | - | - | 15 (100) | - | - | 76 (100) | |
| Spiramycin (SP) | - | - | 61 (100) | - | - | 15 (100) | - | - | 76 (100) | |
| Tetracyclines | Doxycycline (DO) | 33 (54.1) | 28 (45.9) | - | - | 10 (66.7) | 5 (33.3) | 33 (43.4) | 38 (50) | 5 (6.6) |
| Aminoglycosides | Spectinomycin (SPT) | - | - | 61 (100) | - | - | 15 (100) | - | - | 76 (100) |
| Phenicols | Florfenicol (FF) | - | 21 (34.4) | 40 (65.6) | - | 10 (66.7) | 5 (33.3) | - | 31 (40.8) | 45 (59.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ammar, A.M.; Abd El-Hamid, M.I.; Mohamed, Y.H.; Mohamed, H.M.; Al-khalifah, D.H.M.; Hozzein, W.N.; Selim, S.; El-Neshwy, W.M.; El-Malt, R.M.S. Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt. Biology 2022, 11, 1083. https://doi.org/10.3390/biology11071083
Ammar AM, Abd El-Hamid MI, Mohamed YH, Mohamed HM, Al-khalifah DHM, Hozzein WN, Selim S, El-Neshwy WM, El-Malt RMS. Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt. Biology. 2022; 11(7):1083. https://doi.org/10.3390/biology11071083
Chicago/Turabian StyleAmmar, Ahmed M., Marwa I. Abd El-Hamid, Yousreya H. Mohamed, Heba M. Mohamed, Dalal H. M. Al-khalifah, Wael N. Hozzein, Samy Selim, Wafaa M. El-Neshwy, and Rania M. S. El-Malt. 2022. "Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt" Biology 11, no. 7: 1083. https://doi.org/10.3390/biology11071083
APA StyleAmmar, A. M., Abd El-Hamid, M. I., Mohamed, Y. H., Mohamed, H. M., Al-khalifah, D. H. M., Hozzein, W. N., Selim, S., El-Neshwy, W. M., & El-Malt, R. M. S. (2022). Prevalence and Antimicrobial Susceptibility of Bovine Mycoplasma Species in Egypt. Biology, 11(7), 1083. https://doi.org/10.3390/biology11071083








