Acute Effects of Wearing Different Surgical Face Masks during High-Intensity, Short-Rest Resistance Exercise on Cardiorespiratory and Pulmonary Function and Perceptual Responses in Weightlifters
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
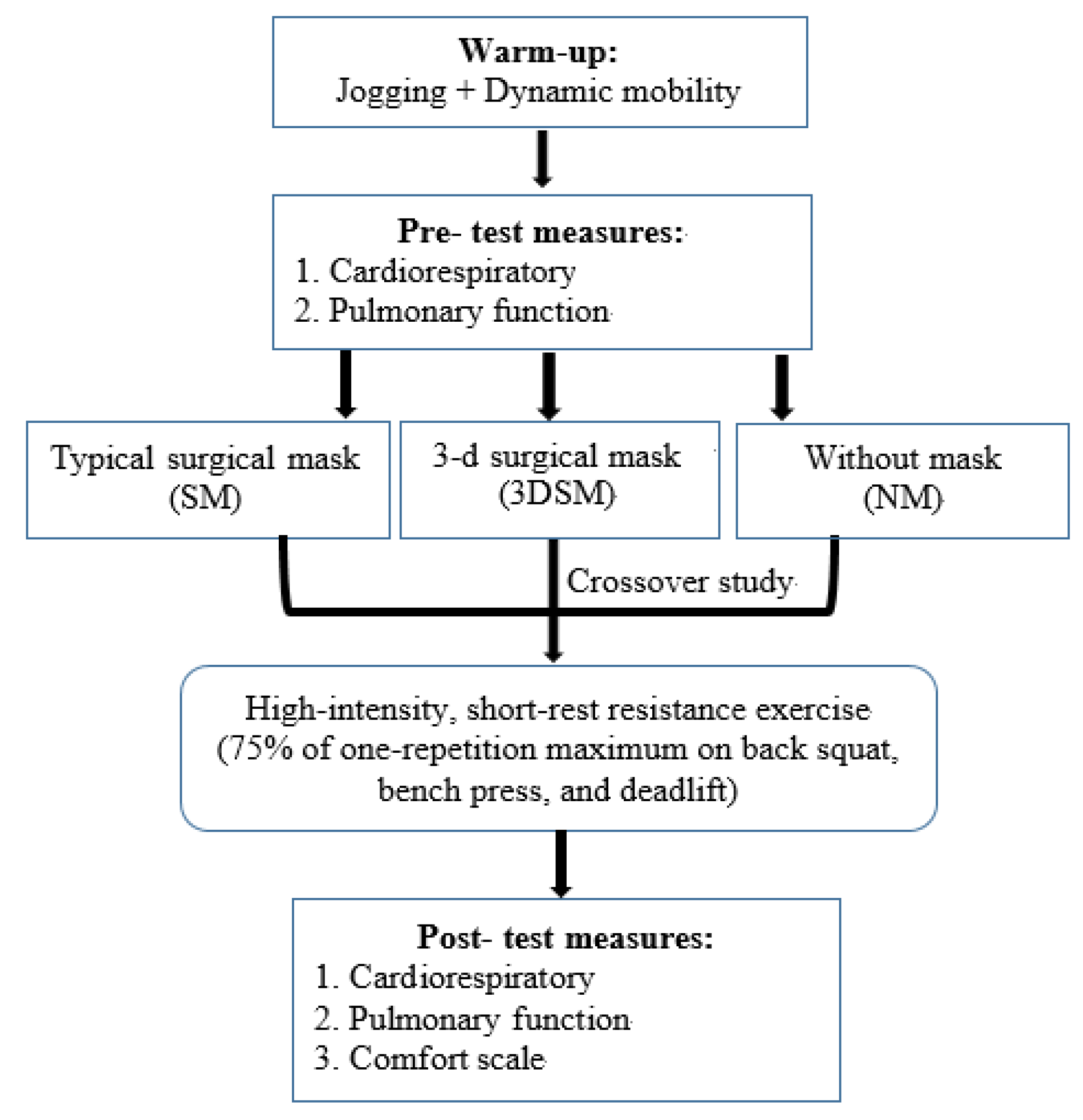
2.1. Experimental Design
2.2. Participants
2.3. Face Masks
2.4. Measurement of Dependent Variables
2.5. BP, baPWV, HR, and SpO2
2.6. Pulmonary Function Parameters
2.7. Comfort/Discomfort Scale
2.8. Whole-Body High-Intensity Short-Rest Resistance Exercise
2.9. Statistical Analysis
3. Results
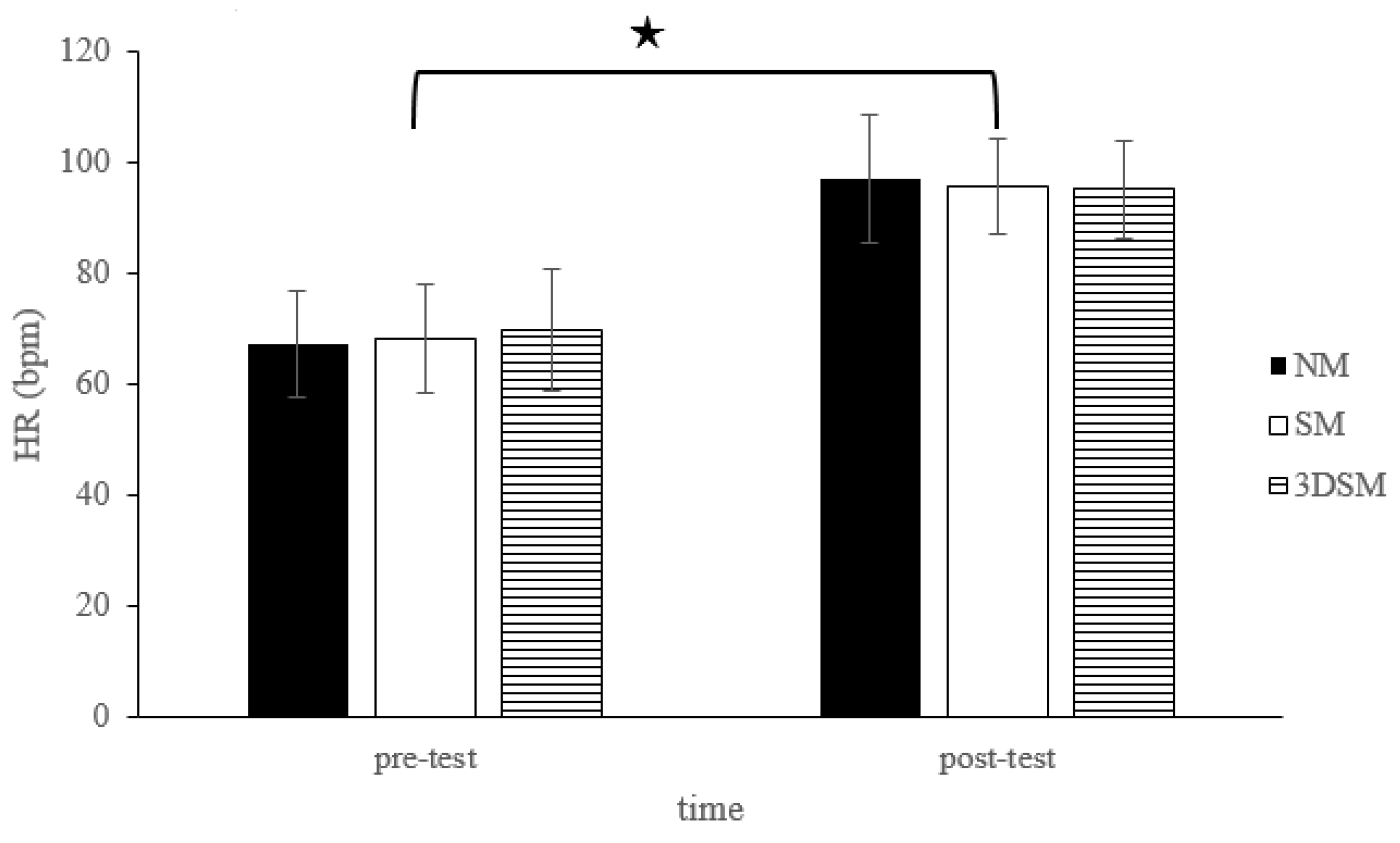
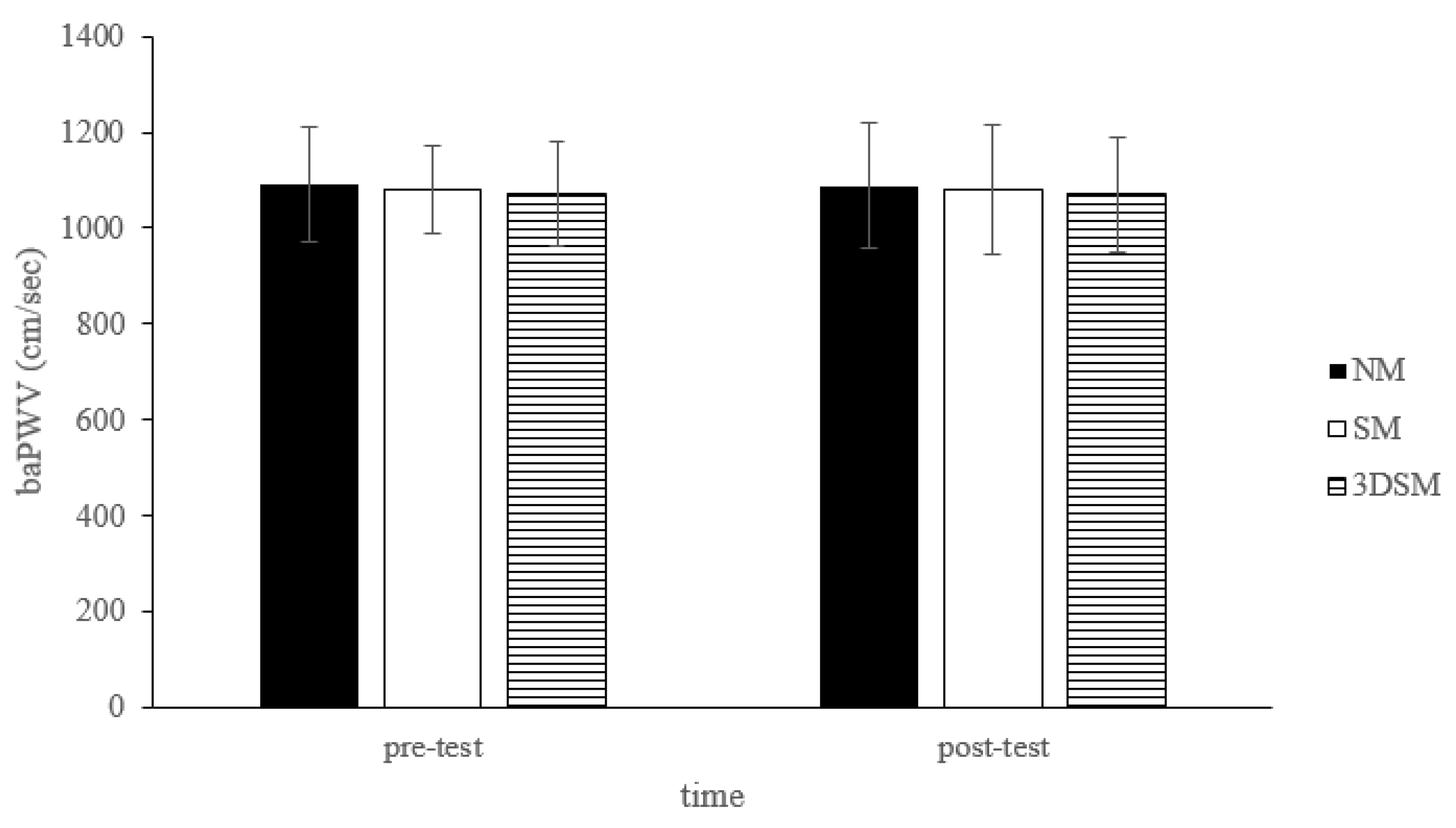
3.1. BP, baPWV, HR, and SpO2
3.2. Pulmonary Function Parameters
3.3. Perceived Exertion and Comfort/Discomfort Scale
4. Discussion
4.1. Cardiac Function
4.2. Pulmonary Function
4.3. Comfort/Discomfort Scale
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandrasekaran, B.; Fernandes, S. “Exercise with facemask; Are we handling a devil’s sword?”—A physiological hypothesis. Med. Hypotheses 2020, 144, 110002. [Google Scholar] [CrossRef] [PubMed]
- Sallis, R.; Young, D.R.; Tartof, S.Y.; Sallis, J.F.; Sall, J.; Li, Q.; Smith, G.N.; A Cohen, D. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: A study in 48 440 adult patients. Br. J. Sports Med. 2021, 55, 1099–1105. [Google Scholar] [CrossRef]
- Dixit, S. Can moderate intensity aerobic exercise be an effective and valuable therapy in preventing and controlling the pandemic of COVID-19? Med. Hypotheses 2020, 143, 109854. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Coronavirus Disease 2019 (COVID-19); WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters#exercising (accessed on 26 June 2022).
- Fikenzer, S.; Uhe, T.; Lavall, D.; Rudolph, U.; Falz, R.; Busse, M.; Hepp, P.; Laufs, U. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin. Res. Cardiol. 2020, 109, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Egger, F.; Blumenauer, D.; Fischer, P.; Venhorst, A.; Kulenthiran, S.; Bewarder, Y.; Zimmer, A.; Böhm, M.; Meyer, T.; Mahfoud, F. Effects of face masks on performance and cardiorespiratory response in well-trained athletes. Clin. Res. Cardiol. 2021, 111, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Driver, S.; Reynolds, M.; Brown, K.; Vingren, J.L.; Hill, D.W.; Bennett, M.; Gilliland, T.; McShan, E.; Callender, L.; Reynolds, E.; et al. Effects of wearing a cloth face mask on performance, physiological and perceptual responses during a graded treadmill running exercise test. Br. J. Sports Med. 2021, 56, 107–113. [Google Scholar] [CrossRef]
- Umutlu, G.; Acar, N.E.; Sinar, D.S.; Akarsu, G.; Güven, E.; Yildirim, I. COVID-19 and physical activity in sedentary individuals: Differences in metabolic, cardiovascular, and respiratory responses during aerobic exercise performed with and without a surgical face masks. J. Sports Med. Phys. Fit. 2022, 62, 851–858. [Google Scholar] [CrossRef]
- Epstein, D.; Korytny, A.; Isenberg, Y.; Marcusohn, E.; Zukermann, R.; Bishop, B.; Minha, S.; Raz, A.; Miller, A. Return to training in the COVID-19 era: The physiological effects of face masks during exercise. Scand. J. Med. Sci. Sports 2020, 31, 70–75. [Google Scholar] [CrossRef]
- Shaw, K.; Butcher, S.; Ko, J.; Zello, G.A.; Chilibeck, P.D. Wearing of Cloth or Disposable Surgical Face Masks has no Effect on Vigorous Exercise Performance in Healthy Individuals. Int. J. Environ. Res. Public Health 2020, 17, 8110. [Google Scholar] [CrossRef]
- Rosa, B.V.; Rossi, F.E.; Moura, H.; Santos, A.; Véras-Silva, A.S.; Ribeiro, S.L.G.; Nakamura, F.Y.; dos Santos, M.A.P. Effects of FFP2/N95 face mask on low- and high-load resistance exercise performance in recreational weight lifters. Eur. J. Sport Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Ramos-Campo, D.; Pérez-Piñero, S.; Muñoz-Carrillo, J.; López-Román, F.; García-Sánchez, E.; Ávila-Gandía, V. Acute Effects of Surgical and FFP2 Face Masks on Physiological Responses and Strength Performance in Persons with Sarcopenia. Biology 2021, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.M.; Sommer, A.J.; Starkoff, B.E.; Devor, S.T. Crossfit-Based High-Intensity Power Training Improves Maximal Aerobic Fitness and Body Composition. J. Strength Cond. Res. 2013, 27, 3159–3172. [Google Scholar] [CrossRef] [Green Version]
- Schlegel, P. CrossFit(R) Training Strategies from the Perspective of Concurrent Training: A Systematic Review. J. Sports Sci. Med. 2020, 19, 670–680. [Google Scholar]
- Heavens, K.R.; Szivak, T.K.; Hooper, D.R.; Dunn-Lewis, C.; Comstock, B.A.; Flanagan, S.D.; Looney, D.P.; Kupchak, B.R.; Maresh, C.M.; Volek, J.S.; et al. The Effects of High Intensity Short Rest Resistance Exercise on Muscle Damage Markers in Men and Women. J. Strength Cond. Res. 2014, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Domingues, W.J.R.; Soares, A.; Cavalcante, B.R.; Da Silva, R.R.M.; Nunhes, P.M.; Da Silva, G.M.G.; Bezerra, E.; Oliveira, N.L.; Oliveira, J. Influence of the order of aerobic and resistance exercise on hemodynamic responses and arterial stiffness in young normotensive individuals. J. Bodyw. Mov. Ther. 2019, 24, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Dantas, T.S.P.; Aidar, F.J.; de Souza, R.F.; de Matos Gama, D.; Ferreira, A.R.P.; de Almeida Barros, N. Evaluation of a CrossFit[R] Session on Post-Exercise Blood Pressure. J. Exerc. Physiol. Online 2018, 21, 44. [Google Scholar]
- Kingsley, J.D.; Tai, Y.L.; Mayo, X.; Glasgow, A.; Marshall, E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur. J. Sport Sci. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Tai, Y.L.; Gerhart, H.; Mayo, X.; Kingsley, J.D. Acute resistance exercise using free weights on aortic wave reflection characteristics. Clin. Physiol. Funct. Imaging 2016, 38, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Shaw, S.B.; Dullabh, M.; Forbes, G.; Brandkamp, J.-L.; Shaw, I. Analysis of physiological determinants during a single bout of Crossfit. Int. J. Perform. Anal. Sport 2015, 15, 809–815. [Google Scholar] [CrossRef]
- Chao, H.-H.; Liao, Y.-H.; Chou, C.-C. Influences of Recreational Tennis-Playing Exercise Time on Cardiometabolic Health Parameters in Healthy Elderly: The ExAMIN AGE Study. Int. J. Environ. Res. Public Health 2021, 18, 1255. [Google Scholar] [CrossRef]
- Meaney, E.; Alva, F.; Moguel, R.; Meaney, A.; Alva, J.; Webel, R. Formula and nomogram for the sphygmomanometric calculation of the mean arterial pressure. Heart 2000, 84, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, M.; Javadi, H.; Pourbehi, M.R.; Mogharrabi, M.; Rayzan, M.; Semnani, S.; Jallalat, S.; Amini, A.; Abbaszadeh, M.; Barekat, M.; et al. The association of rate pressure product (RPP) and myocardial perfusion imaging (MPI) findings: A preliminary study. Perfusion 2012, 27, 207–213. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.-P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetière, P. Heart-Rate Profile during Exercise as a Predictor of Sudden Death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; Van Der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tokura, H.; Guo, Y.; Wong, A.; Wong, T.; Chung, J.; Newton, E. Effects of wearing N95 and surgical facemasks on heart rate, thermal stress and subjective sensations. Int. Arch. Occup. Environ. Health 2005, 78, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Lässing, J.; Falz, R.; Pökel, C.; Fikenzer, S.; Laufs, U.; Schulze, A.; Hölldobler, N.; Rüdrich, P.; Busse, M. Effects of surgical face masks on cardiopulmonary parameters during steady state exercise. Sci. Rep. 2020, 10, 22363. [Google Scholar] [CrossRef]
- Polito, M.D.; Farinatti, P. The Effects of Muscle Mass and Number of Sets During Resistance Exercise on Postexercise Hypotension. J. Strength Cond. Res. 2009, 23, 2351–2357. [Google Scholar] [CrossRef]
- Simão, R.; Fleck, S.J.; Polito, M.; Monteiro, W.; Farinatti, P. Effects of Resistance Training Intensity, Volume, and Session Format on the Postexercise Hypotensive Response. J. Strength Cond. Res. 2005, 19, 853–858. [Google Scholar] [CrossRef]
- Brito, A.D.F.; De Oliveira, C.V.C.; Santos, M.D.S.B.; Santos, A.D.C. High-intensity exercise promotes postexercise hypotension greater than moderate intensity in elderly hypertensive individuals. Clin. Physiol. Funct. Imaging 2013, 34, 126–132. [Google Scholar] [CrossRef]
- Brito, A.D.F.; Brasileiro-Santos, M.D.S.; de Oliveira, C.V.C.; da Nóbrega, T.K.S.; Forjaz, C.L.D.M.; Santos, A.D.C. High-Intensity Resistance Exercise Promotes Postexercise Hypotension Greater than Moderate Intensity and Affects Cardiac Autonomic Responses in Women Who Are Hypertensive. J. Strength Cond. Res. 2015, 29, 3486–3493. [Google Scholar] [CrossRef]
- Cavalcante, P.A.; Rica, R.; Evangelista, A.; Serra, A.; Junior, F.P.; Kilgore, L.; Baker, J.; Bocalini, D.; Junior, A.F. Effects of exercise intensity on postexercise hypotension after resistance training session in overweight hypertensive patients. Clin. Interv. Aging 2015, 10, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho-Júnior, H.J.; Irigoyen, M.-C.; Aguiar, S.D.S.; Gonçalves, I.D.O.; Câmara, N.O.S.; Cenedeze, M.A.; Asano, R.Y.; Rodrigues, B.; Uchida, M.C. Acute effects of power and resistance exercises on hemodynamic measurements of older women. Clin. Interv. Aging 2017, 12, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Bopp, M.; Botta, F.; Nussbaumer, M.; Schäfer, J.; Roth, R.; Schmidt-Trucksäss, A.; Hanssen, H. Lower Body vs. Upper Body Resistance Training and Arterial Stiffness in Young Men. Endoscopy 2015, 36, 960–967. [Google Scholar] [CrossRef]
- Shein, S.L.; Whitticar, S.; Mascho, K.K.; Pace, E.; Speicher, R.; Deakins, K. The effects of wearing facemasks on oxygenation and ventilation at rest and during physical activity. PLoS ONE 2021, 16, e0247414. [Google Scholar] [CrossRef]
- Albiol, L.A.; Paya, N.S.; Garnacho-Castaño, M.A.; Cano, L.G.; Cobo, E.P.; Maté-Muñoz, J.L.; Garnacho-Castaño, M.V. Ventilatory efficiency during constant-load test at lactate threshold intensity: Endurance versus resistance exercises. PLoS ONE 2019, 14, e0216824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohya, T.; Yamanaka, R.; Hagiwara, M.; Oriishi, M.; Suzuki, Y. The 400- and 800-m Track Running Induces Inspiratory Muscle Fatigue in Trained Female Middle-Distance Runners. J. Strength Cond. Res. 2016, 30, 1433–1437. [Google Scholar] [CrossRef]
- Chevrolet, J.C.; Tschopp, J.M.; Blanc, Y.; Rochat, T.; Junod, A.F. Alterations in inspiratory and leg muscle force and recovery pattern after a marathon. Med. Sci. Sports Exerc. 1993, 25, 501–507. [Google Scholar] [CrossRef]
- Ozkaplan, A.; Rhodes, E.C.; Sheel, A.W.; Taunton, J.E. A comparison of inspiratory muscle fatigue following maximal exercise in moderately trained males and females. Eur. J. Appl. Physiol. 2005, 95, 52–56. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, J.U.; Williams, J.S. Effects of acute exercise on inspiratory muscle strength and endurance in untrained women and men. J. Sports Med. Phys. Fit. 2010, 50, 268–273. [Google Scholar]
- Poon, E.T.-C.; Zheng, C.; Wong, S.H.-S. Effect of Wearing Surgical Face Masks During Exercise: Does Intensity Matter? Front. Physiol. 2021, 12, 775750. [Google Scholar] [CrossRef]





| NM | SM | 3DSM | p | ||||
|---|---|---|---|---|---|---|---|
| Pre Test | Post Test | Pre Test | Post Test | Pre Test | Post Test | ||
| SBP (mmHg) | 121.05 ± 10.19 | 113.55 ± 10.33 | 119.00 ± 9.99 | 114.40 ± 8.91 | 118.35 ± 9.23 | 113.80 ± 11.73 | 0.17 |
| DBP (mmHg) | 67.45 ± 6.21 | 63.20 ± 6.60 | 64.85 ± 7.56 | 61.60 ± 6.83 | 64.90 ± 6.27 | 61.80 ± 7.61 | 0.59 |
| HR (bpm) | 67.30 ± 9.50 | 97.05 ± 11.70 | 68.35 ± 9.84 | 95.85 ± 8.65 | 69.70 ± 11.06 | 95.20 ± 8.85 | 0.15 |
| RPP | 8172.00 ± 1509.74 | 11,030.60 ± 1779.71 | 8146.85 ± 1430.19 | 10,966.35 ± 1320.17 | 8281.90 ± 1665.14 | 10,865.95 ± 1763.83 | 0.62 |
| MAP (mmHg) | 85.32 ± 6.93 | 79.99 ± 7.49 | 82.90 ± 7.96 | 79.20 ± 7.30 | 82.72 ± 6.81 | 79.13 ± 8.69 | 0.29 |
| SpO2 (%) | 97.30 ± 0.93 | 96.80 ± 1.36 | 97.25 ± 0.85 | 97.15 ± 0.88 | 97.20 ± 1.00 | 96.95 ± 0.89 | 0.33 |
| baPWV (cm/sec) | 1091.25 ± 119.63 | 1087.20 ± 130.90 | 1078.85 ± 91.91 | 1080.30 ± 135.99 | 1071.55 ± 107.46 | 1070. 10 ± 121.00 | 0.97 |
| ABI index | 1.05 ± 0.06 | 0.99 ± 0.09 | 1.08 ± 0.08 | 1.02 ± 0.01 | 1.08 ± 0.08 | 0.98 ± 0.09 | 0.33 |
| NM | SM | 3DSM | p | ||||
|---|---|---|---|---|---|---|---|
| Pre Test | Post Test | Pre Test | Post Test | Pre Test | Post Test | ||
| FVC (l) | 3.87 ± 0.69 | 3.90 ± 0.74 | 3.99 ± 0.64 | 3.90 ± 0.58 | 3.86 ± 0.72 | 3.90 ± 0.65 | 0.07 |
| FEV1 (l) | 3.60 ± 0.61 | 3.69 ± 0.67 | 3.72 ± 0.59 | 3.69 ± 0.57 | 3.62 ± 0.66 | 3.67 ± 0.59 | 0.08 |
| TV (l) | 0.88 ± 0.35 | 0.97 ± 0.38 | 1.10 ± 0.43 | 1.19 ± 0.52 | 1.04 ± 0.37 | 1.11 ± 0.49 | 0.95 |
| FEV/FVC (%) | 92.56 ± 6.83 | 94.44 ± 5.96 | 93.26 ± 7.62 | 94.44 ± 7.14 | 93.57 ± 6.35 | 93.95 ± 6.44 | 0.15 |
| MVV (L/min) | 134.19 ± 30.66 | 135.37 ± 30.55 | 144.65 ± 33.46 | 145.01 ± 30.23 | 137.34 ± 38.75 | 141.56 ± 33.50 | 0.60 |
| NM | SM | 3DSM | F | p | |
|---|---|---|---|---|---|
| Humidity | 5.95 ± 2.65 | 5.48 ± 2.18 | 5.22 ± 2.77 | 0.42 | 0.67 |
| Heat | 5.18 ± 2.77 | 5.13 ± 2.38 | 5.05 ± 2.74 | 0.01 | 0.99 |
| Breathing resistance | 3.55 ± 1.90 * | 5.25 ± 1.78 | 3.50 ± 1.88 * | 4.37 | 0.005 |
| Itchiness | 1.27 ± 1.92 | 1.13 ± 1.41 | 1.60 ± 1.89 | 0.38 | 0.69 |
| Tightness | 2.52 ±1.42 * | 4.13 ± 2.57 | 1.60 ± 1.53 * | 8.88 | <0.001 |
| Saltiness | 1.48 ± 1.99 | 1.20 ± 1.50 | 1.55 ± 2.33 | 0.17 | 0.84 |
| Feeling unfit | 4.15 ± 2.08 | 4.53 ± 1.87 | 3.32 ± 2.57 | 1.56 | 0.22 |
| Odor | 1.23 ± 1.52 | 2.08 ± 1.88 | 2.23 ± 2.19 | 1.63 | 0.20 |
| Fatigue | 4.50 ± 2.09 | 4.68 ± 2.35 | 3.88 ± 2.69 | 0.62 | 0.54 |
| Overall discomfort | 4.78 ± 1.63 | 4.98 ± 1.83 | 4.35 ± 2.12 | 0.58 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-Y.; Chiu, C.-H.; Hsu, C.-H.; Chou, C.-C.; Hsu, S.-M.; Shapu, L.-B.; Chao, T.-C.; Chen, C.-H. Acute Effects of Wearing Different Surgical Face Masks during High-Intensity, Short-Rest Resistance Exercise on Cardiorespiratory and Pulmonary Function and Perceptual Responses in Weightlifters. Biology 2022, 11, 992. https://doi.org/10.3390/biology11070992
Wang S-Y, Chiu C-H, Hsu C-H, Chou C-C, Hsu S-M, Shapu L-B, Chao T-C, Chen C-H. Acute Effects of Wearing Different Surgical Face Masks during High-Intensity, Short-Rest Resistance Exercise on Cardiorespiratory and Pulmonary Function and Perceptual Responses in Weightlifters. Biology. 2022; 11(7):992. https://doi.org/10.3390/biology11070992
Chicago/Turabian StyleWang, Shin-Yuan, Chih-Hui Chiu, Chin-Hsien Hsu, Chun-Chung Chou, Shuo-Min Hsu, Lu-Bi Shapu, Tai-Chen Chao, and Che-Hsiu Chen. 2022. "Acute Effects of Wearing Different Surgical Face Masks during High-Intensity, Short-Rest Resistance Exercise on Cardiorespiratory and Pulmonary Function and Perceptual Responses in Weightlifters" Biology 11, no. 7: 992. https://doi.org/10.3390/biology11070992







