Simple Summary
Besides showing the antibacterial activity of the Preyssler-type polyoxotungstate (POT) P5W30 with the ability to affect MRSA cells, we demonstrated that P5W30 also displays other proprieties, such as anti-quorum sensing and antibiofilm. These are biological activities that are reported for a POT for the first time. Quorum sensing and biofilm facilitate the bacterial colonization, antibiotic resistance and persistence in both the environment and host, and its impairment by POTs can greatly contribute to the control of bacterial infections, such as those caused by multiresistant bacteria. Moreover, antiviral activity was also observed using the enterovirus Qβ. NMR stability studies of P5W30 demonstrate that it remains intact, suggesting its responsibility in the described biological activities. Taken together, our results emphasize the potential biomedical use of POTs, particularly the Preyssler-type POT, to fight antibiotic-resistant MRSA strains and their ability to form biofilm, besides being a promising antiviral agent.
Abstract
The increase in bacterial resistance to antibiotics has led researchers to find new compounds or find combinations between different compounds with potential antibacterial action and with the ability to prevent the development of antibiotic resistance. Polyoxotungstates (POTs) are inorganic clusters that may fulfill that need, either individually or in combination with antibiotics. Herein, we report the ability of the polyoxotungstates (POTs) with Wells-Dawson P2W18, P2W17, P2W15, and Preyssler P5W30 type structures to differently affect Gram-negative and Gram-positive microorganisms, either susceptible or resistant to antibiotics. The compound P5W30 showed the highest activity against the majority of the tested bacterial strains in comparison with the other tested POTs (P2W15, P2W17 and P2W18) that did not show inhibition zones for the Gram-negative bacteria, A. baumanii I73775, E. coli DSM 1077, E. coli I73194, K. pneumoniae I7092374, and P. aeruginosa C46281). Generally, the results evidenced that Gram-positive bacteria are more susceptible to the POTs tested. The compound P5W30 was the one most active against S. aureus ATCC 6538 and MRSA16, reaching <0.83 mg·mL−1 (100 μM) and 4.96 mg·mL−1 (600 μM), respectively. Moreover, it was verified by NMR spectroscopy that the most promising POT, P5W30, remains intact under all the experimental conditions, after 24 h at 37 °C. This prompted us to further evaluate the anti-quorum sensing activity of P5W30 using the biosensor Chromobacterium violaceum CV026, as well as its antibiofilm activity both individually and in combination with the antibiotic cefoxitin against the methicillin-resistant Staphylococcus aureus 16 (MRSA16). P5W30 showed a synergistic antibacterial effect with the antibiotic cefoxitin and chloramphenicol against MRSA16. Moreover, the antibiofilm activity of P5W30 was more pronounced when used individually, in comparison with the combination with the antibiotic cefoxitin. Finally, the antiviral activity of P5W30 was tested using the coliphage Qβ, showing a dose-dependent response. The maximum inactivation was observed at 750 μM (6.23 mg·mL−1). In sum, P5W30 shows anti-quorum sensing and antibiofilm activities besides being a potent antibacterial agent against S. aureus and to exhibit antiviral activities against enteric viruses.
1. Introduction
Antibiotic resistance (AR) is an ancient natural process that is based on different mechanisms, mainly associated with (1) enzymatic inactivation or alteration of the antibacterial agent, (2) protection, modification, or replacement of the molecular target, (3) impediment of antibiotic permeation into the cell, and (4) active efflux pump from the cell [1,2]. The misuse of antibiotics in both human therapy and veterinary medicine allowed this natural process to evolve at an ever-increasing rate, thereby jeopardizing the control of severe bacterial infections [2]. AR, due to its prevalence in the environment and in food, is not only a problem in clinics [3]. In 2017, the most serious bacterial pathogens associated with health care infections (HCI) were classified by the World Health Organization (https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed, accessed on 17 December 2021) into three groups of priority, critical, high, and medium, for the development of new antibiotics to control the infections caused by these bacterial pathogens. In the group of critical and high-priority ones are the designated ESKAPE bacterial pathogens (Enterococcus faecium vancomycin-resistant [VRE], Staphylococcus aureus, methicillin-resistant, vancomycin-intermediate and resistant [MRSA/VRSA], Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa and Enterobacteriaceae carbapenem-resistant and third-generation cephalosporin-resistant). The ESKAPE pathogens are deadly, infectious bacteria that are brilliant at developing multiresistant patterns. Apart from the fact that they are genetically different, their mechanisms of resistance consist of the previously mentioned antibiotic target change, inactivation, decreased uptake, and drug efflux pumps [2]. The ability of pathogens to adhere to biotic or abiotic surfaces is a driving force in their antibiotic resistance and virulence [4,5]. The process of biofilm formation is established by a series of events, namely the initial adhesion, permanent adhesion, production of exopolysaccharides that confer a spatial structure, biofilm maturation, and disaggregation [6]. The protection of sessile cells (adherent) against antimicrobial agents, including phagocyte attack, is guaranteed by the exopolysaccharide matrix [7,8].
Biofilms are a source of contamination of medical devices, such as prosthetic joint, urinary catheters, central venous catheters, among others, and are of great concern in health care [9]. Biofilm formation, expression of virulence factors, toxin production, and antibiotic resistance are regulated by the chemical cell–cell communication system denominated by quorum sensing (QS). The QS system functions in coordination with cell density, permitting the adaptation of the microbial cells to environmental changes involving the production and detection of secreted signaling molecules designated by autoinducers [10]. The use of QS is common in both Gram-positive and Gram-negative bacteria. However, their signaling molecules are different. In Gram-negative bacteria, the autoinducer molecules are acyl-homoserine lactones (AHLs) or other molecules that are produced from the enzymatic cofactor S-adenosylmethionine (SAM), whereas the autoinducers in Gram-positive bacteria are peptides [10,11]. Autoinducers freely pass the bacterial membrane and the process continues with them attaching themselves to their specific membrane or cytoplasmic receptor, initiating a change in gene expression in a multitude (from dozen to hundreds) of genes that aid critical physiological processes [4,12].
Four main mechanisms of quorum quenching can be highlighted: (1) the inhibition of signal molecule synthesis, (2) inactivation or enzymatic degradation of signal molecules, (3) competition with signal molecules–receptor analogues, and (4) prevention of the signal transduction cascades [13]. The fact that quorum-quenching molecules do not affect the bacterial growth means that the risk of these molecules to induce resistance is minor. Due to the importance of QS in the bacterial pathogenesis process, it becomes an ideal target to control bacterial pathogens with an increased interest to identify QS inhibitors (quorum quenching) [12,13,14]. Several QS inhibitors have been identified, and among them, different phytochemicals [15,16], bacteriophages [17,18], and metal nanoparticles [19,20,21]. The efforts to find compounds able to break the development of antibiotic resistance led to the investigation of metal oxides as antibacterial agents [22,23,24,25,26,27,28]. These metal oxides can easily interact with the bacterial cell membrane due to their small size and active surface ligands, forcing the hyperpolarization of the bacterial cells, and at the same time, immobilizing Mg2+ transporters, disturbing the operation of ribosomes, which leads to the disruption of cellular viability without inducing antibiotic resistance [29]. Classic polyoxometalates (POMs) are a well-known group of anionic polynuclear metal oxides (containing VV, TaV, NbV, WVI and MoVI usually in their highest oxidation state) with distinct and chemically changeable cluster structures [30,31,32]. Besides, POMs can include non-metal ions (e. g. PV, AsV, SiIV) and one or more of the addenda metal oxo fragments may be absent and/or substituted by other 3d- (e.g., FeIII, CoII NiII) or 4f-metal ions (e.g., CeIII, NdIII, GdIII). Due to their structural diversity, POMs have shown specific physicochemical properties responsible for discrete chemical and biological applications, such as catalysis [33], protein crystallization [34], anticancer [35], antibacterial [36,37], and antidiabetic activities [38,39], among others. Phosphotungstates, and especially the doughnut-shaped Preyssler [Xn+P5W30O110](15−n)− (X = Na+, Ag+, Ca2+, etc.) anion, have shown great potential as antibacterial [24,29,40,41], antitumor [42,43,44], and antiviral agents [45]. Featured for their ultra-small sizes, favorable thermal and hydrolytic stability, and well-defined surface structures with high affinity to biomacromolecules, phosphotungstates of Dawson and Preyssler archetypes (Figure 1) are explored here for their effect on biological events that contribute to bacterial virulence, such as quorum sensing and biofilm formation. Once the polyoxotungstate (POT) showed powerful antibacterial, QS and antibiofilm activities, the antiviral activity (here P5W30) was further examined using the enterovirus Qβ.
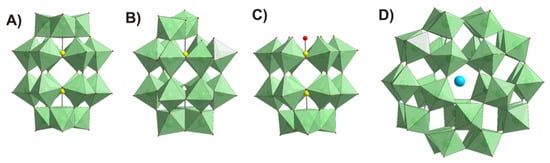
Figure 1.
Polyhedral representation of POT structures tested in this study: (A) intact Wells-Dawson anion [α-PV2WVI18O62]6− P2W18; (B) mono-lacunary Wells-Dawson anion [α2-PV2WVI17O61]10− P2W17; (C) tri-lacunary Wells-Dawson anion [PV2WVI15O56]12− P2W15; (D) Preyssler anion [NaPV5WVI30O110]14− P5W30. Color code: {WO6}, green; P, yellow; O, red; Na, cyan.
Thus, in addition to the antibacterial and antiviral activities previously described, as well as other biological activities, such as peroxidase immobilization, inhibition of aquaporin, amyloid-beta aggregation, and sarco(endo) reticulum calcium ATPases (SERCA)/plasma membrane calcium ATPases (PMCA) activities [24,29,40,41,42,43,44,45,46,47,48] (Table S1), we show that P5W30 is also capable to impair quorum sensing and biofilm formation, as illustrated in Table S1 and Figure 2. As can be seen, the first antiviral study was reported in 1990 [45], but the majority of biological investigations have only been reported in the last five years [24,29,40,41,42,43,44,46,49] (Figure 2).
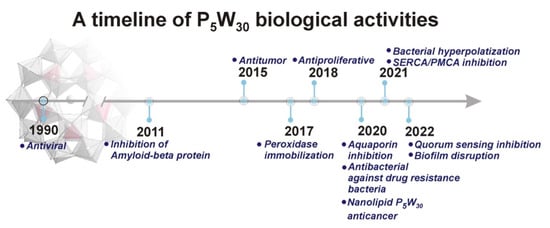
Figure 2.
Timeline for P5W30 biological activities: (1) 1990, Antiviral, (2) 2011, Amyloid beta inhibition; (3) 2015, Antitumor, (4) 2017, Peroxidase immobilization; (5) 2018, Antibacterial; (6) 2020, aquaporin inhibition; antimelanoma activity; (7) 2020, drug-resistance bacteria; (8) Nanolipid-loaded Preyssler for anticancer (9) 2021, bacterial hyperpolarization; (10) 2021, SERCA/PMCA inhibition; (11) 2022, antiquorum sensing; (12) biofilm disruption.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
The tested microorganisms are indicated in Table 1. All bacterial strains were maintained in Brain Heart Infusion (BHI) (Oxoid, Basingstock, UK), supplemented with glycerol (25% [v/v]) at −80 °C, except Chromobacterium violaceum CV026, which was maintained in Luria Broth Base (LB) (Sigma-Aldrich, St. Louis, MO, USA). The culture media Mueller–Hinton broth (MHB) and Gelose Mueller–Hinton (GMH) were purchased from Biokar Diagnostics (Beauvais). Prior to use, bacteria were transferred to fresh BHI agar plates and incubated at 37 °C. The recovery of Streptococcus pneumoniae D39 was performed in BHI supplemented with 5% sheep blood (Oxoid, Basingstock, UK) at 37 °C under microaerophilic conditions using an anaerobic jar.

Table 1.
Microorganisms used in the study.
2.2. Polyoxometalates
The POTs used in this study are the intact Wells-Dawson K6[α-PV2WVI18O62]·14H2O (abbreviated P2W18) mono-lacunary Wells-Dawson K10[α2-PV2WVI17O61]·20H2O (P2W17), tri-lacunary Wells-Dawson K12[α-PV2WVI15O56]·24H2O (P2W15), and Preyssler (NH4)14[NaPV5WVI30O110]·31H2O (P5W30) (Figure 1 and Table 2). The POTs were synthesized according to published procedures [50,51] and their purity was confirmed by infrared, 31P and 183W NMR spectroscopy (Figure 3, Figure 4, Figure 5 and Figures S1–S4; Table S2). Stock solutions of POTs were freshly prepared by dissolving the solid compound in water and keeping the solution at 4 °C. To determine the antibacterial activity of the POTs, 4 mM solutions of each compound was prepared and subsequently diluted in Mueller–Hinton culture medium to obtain the appropriate final concentrations (Table S3).

Table 2.
POTs used in this study.
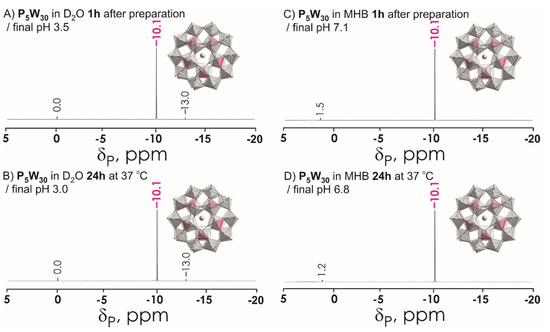
Figure 3.
31P NMR spectra of P5W30 10 mM solutions (A) in D2O recorded approximately 1 h after preparation; (B) in D2O recorded after incubation for 24 h at 37 °C; (C) in Mueller–Hinton broth (MHB) recorded approximately 1 h after preparation; (D) in Mueller–Hinton broth (MHB) recorded after incubation for 24 h at 37 °C. Signals at 0 ppm, 1.2, and 1.5 correspond to free phosphate HxPO4(3−x)− (x = 0–3). The signal at −10.1 ppm corresponds to 5 equivalent P ions in P5W30 shown in magenta in polyhedral presentation. Color code: {WO6}, light grey; {PO4}, magenta; O, red; Na, grey.
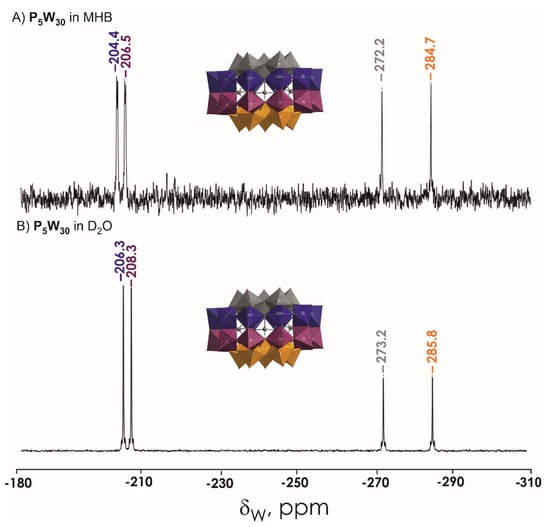
Figure 4.
183W NMR spectra of 20 mM (NH4)14[NaPV5WVI30O110] P5W30 solution in (A) MHB and (B) D2O. The signals correspond to four types of W ions in P5W30 shown in different colors. Color code: {WO6}, grey, blue, plum, orange; P, grey; O, red.
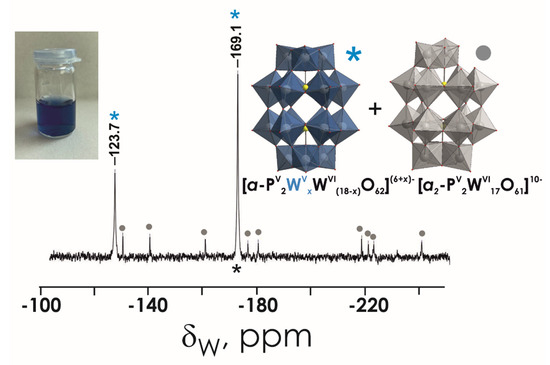
Figure 5.
183W NMR spectrum of P2W18 20 mM solutions in MHB. The signal at −123.7 and −169.1 ppm corresponding to two types of W ions in reduced P2W18 are depicted with a blue asterisk, and 9 signals of monolacunary P2W17 are marked with grey circle. Color code: {WO6}, grey or blue; P, yellow; O, red.
NMR Spectroscopy
183W NMR and 31P NMR spectra were recorded with a Bruker FT-NMR spectrometer Avance Neo 500 MHz (Bruker, Rheinstetten, Germany) at 25 °C. Chemical shifts were measured relative to 1 M Na2WO4 and 85% H3PO4. 183W NMR samples were prepared in 2.7 mL solvent with a POT concentration of 10 mM and measured in 10 mm tubes. The experimental time for 183W NMR was ca. 60 h, with a standard pulse program at 20.836 MHz and a 63° flip angle with 1 s relaxation delay. Subsequently, 31P NMR spectra were measured at 202.53 MHz in standard 5 mm tubes.
2.3. Screening of Antibacterial Activity by Agar Diffusion
Each bacterial strain was previously grown in GMH at 37 °C for 24 h. From this culture a loop was transferred to 10 mL of MHB, and the incubation was done at 37 °C overnight. Afterwards, 8 mL of the previous culture (OD600nm = 0.8–1.0) was transferred into 40 mL of liquefied MH agar medium. The mixture was poured into a sterile Petri dish, which was kept at room temperature, in a laminar flow cabinet, until solidification of the inoculated culture medium. Then, using an inverted sterile Pasteur pipette, 6 mm diameter wells were made into which 40 µL of each compound at the concentrations to be tested were dispensed: for P5W30, 0.83 mg·mL-1 (100 µM), 1.65 mg·mL-1 (200 µM), 2.48 mg·mL-1 (300 µM), 3.31 mg·mL-1 (400 µM), and 4.13 mg·mL-1 (500 µM). Incubation was conducted at 37 ± 1 °C for 24 h. The assay with the S. pneumoniae D39 bacteria was carried out in Columbia agar (Oxoid, Basingstock, UK) supplemented with 5% sheep blood (Oxoid, Basingstock, UK) and under microaerophilic conditions. Three biological replicates and two technical replicates were performed. After 24 h incubation, the inhibition zone was determined (Table S4).
Determination of the Minimum Inhibitory Concentration
The minimum inhibitory concentration (MIC) was determined by microdilution using 96-well flat bottom microplates (Sarstedt Inc, Nümbrecht, Germany). Previously, each bacterial strain was grown in GMH at 37 °C for 24 h. From this culture a loop was transferred to 10 mL of MHB, and the incubation was done at 37 °C overnight. An inoculum density of 5 × 105 CFU·mL−1 was used. The total volume in each well was 200 μL and the concentrations tested ranged between 0.44 and 9.82 mg·mL−1. The tested concentrations for all POMs were from 100 to 1000 μM. For example, for the compound P5W30, the tested concentrations were 0.83 mg·mL−1 (100 μM), 1.65 mg·mL−1 (200 μM), 3.31 mg·mL−1 (400 μM), 4.96 mg·mL−1 (600 μM), 6.61 mg·mL−1 (800 μM), and 8.26 mg·mL−1 (1000 μM). The antibiotic chloramphenicol (30 μg·mL−1) was used as control. A set of wells containing only the culture medium was included as the negative control. Three biological and three technical replicates for each strain were used. The incubation of the microplate was performed at 37 °C for 24 h. The bacterial growth was followed by spectrophotometry (OD600 nm) in a microplate reader (Tecan Infinite M200, Tecan, Austria). The MIC value was considered the lowest concentration of the compound that caused the inhibition of the bacterial growth (95–100%). The lowest concentration that did not allow the recovery of cells in GMH plates was considered the minimum bactericidal concentration (MBC). The MIC values for the MRSA16 strain for the antibiotics erythromycin, vancomycin, cefoxitin, and chloramphenicol were determined and the resistant profile was established according to the conventional breakpoints [55].
To determine further compound interactions with antibiotics to combat MRSA16 strain, the combinations of P5W30 with the antibiotics chloramphenicol, cefoxitin, and vancomycin were evaluated using POT concentrations from 100 to 1000 μM. For example, for P5W30, values were 0.83 mg·mL−1 (100 μM), 1.65 mg·mL−1 (200 μM), 3.31 mg·mL−1 (400 μM), 4.96 mg·mL−1 (600 μM), 6.61 mg·mL−1 (800 μM), and 8.26 mg·mL−1 (1000 μM). The tested concentrations of vancomycin were 0.5 μg·mL−1 and 2 μg·mL−1 and the concentration of cefoxitin was 2 μg·mL−1. The Fractional Inhibitory Concentration (FIC) index (ΣFIC) was calculated for the combination of compound P5W30 with the antibiotic against MRSA16 using Equations (1) and (2):
- Equation (1):
- Equation (2):The ƩFIC was calculated according to the equation ƩFIC = FIC(P5W30) + FIC(antibiotic)
The interpretation of ƩFIC was done according to EUCAST [56].
2.4. Inhibition of Quorum Sensing
The anti-quorum-sensing assay was performed in a 96-well flat-bottom microplate (Sarsted Inc, Nümbrecht, Germany) using the biosensor Chromobacterium violaceum CV026 [57]. The biosensor was cultivated on LB agar plates at 30 °C for 24 h. A loop from this culture was transferred into 10 mL of LB broth following incubation in a water-bath at 30 °C overnight. A volume of 100 μL of the overnight culture with an optical density (OD600nm) of 1.2 was transferred to 100 μL of LB broth, followed by the addition of N-hexanoyl-homoserine lactone (C6-HSL) (Sigma-Aldrich, St. Louis, MO, USA) to a final concentration of 0.2 μg·mL−1 in the control (induction of violacein formation) and P5W30 samples (inhibition of violacein formation). Control wells with no C6-HSL or P5W30 were included. The tested concentrations of P5W30 were 100 μM (0.83 mg·mL−1), 200 μM (1.65 mg·mL−1), 300 μM (2.48 mg·mL−1), and 400 μM (3.31 mg·mL−1). The incubation was performed at 30 °C over 24 h. After this time interval the inhibition of violacein was evaluated. The optical density of the wells was measured at 585 nm in a microplate reader (Tecan Infinite M200, Tecan, Austria). The assays were performed in triplicate.
For growth control a similar microplate without the addition of C6-HSL was prepared. The antibiotic chloramphenicol (30 μg·mL−1) was used as control. The bacterial growth was followed by spectrophotometry (OD600nm) in a microplate reader (Tecan Infinite M200, Tecan, Austria). The assays were also performed in triplicate.
2.5. Inhibition of Biofilm Formation
The antibiofilm activity of the compound P5W30 was determined according to the method described by Walker et al. [58] applying slight modifications. Briefly, Staphylococcus aureus methicillin-resistant 16 (MRSA16) was grown on BHI agar plates at 37 °C for 24 h. From this culture one isolated colony was transferred into 10 mL of BHI broth and incubated at 37 °C in a water-bath overnight with agitation (120 rpm). Plastic coverslips (unbreakable; 22 × 22 mm; Fisherbrand) were distributed in 6-well flat-bottom plates (Greiner Bio-One GmbH, Kremsmünster, Austria) and sterilized in a flow cabinet for 2 h under ultraviolet light. From the overnight bacterial culture 300 μL was transferred into 2700 μL of BHI broth and this suspension was transferred into each well covering the coverslip. The formation of biofilm was allowed to be produced for 24 h at 37 °C. After biofilm formation, the bacterial culture was eliminated and each well was washed 4 times with phosphate-buffered saline (PBS) to remove non-adherent cells. The biofilm slides were treated with P5W30 (2× MIC, 10 mg·mL−1) and with P5W30 plus cefoxitin (0.7 mg·mL−1 + 2 μg·mL−1) for 6 h and 24 h. Non-treated coverslips were used as control. The quantification of the sessile cells (adherent) was conducted by washing each coverslip 4 times with PBS. Each coverslip was transferred into 10 mL of BHI supplemented with 0.05% Tween 80. The tube was sonicated for 7 min at 4 °C. After sonication the coverslip was immediately removed and serial dilutions were prepared by transferring 100 μL of the sonicated culture into 900 μL of PBS. The viable counts were determined by the drop method [59] in BHI agar plates that were incubated at 37 °C for 24 h. Three biological and two technical replicates were used.
2.6. Antiviral Activity
The antiviral activity of the compound P5W30 was evaluated using a microplate dilution method [60] followed by the double-layer agar [61]. The Qβ phage was exposed to P5W30 at concentrations of 250 μM (2.08 mg·mL−1), 500 μM (4.15 mg·mL−1), and 750 μM (6.23 mg·mL−1) over 24 h. Afterwards, the treated phage suspensions were serially diluted using PBS. For the microplate technique 180 µL of the host bacterial culture in exponential phase (OD600nm = 0.25–0.3) was distributed through a 96-well flat-bottom microplate, and the wells were inoculated with 20 µL of each phage dilution. The bacterial culture in BHI without the compound and the bacterial culture supplemented with the compound at the tested concentrations were used as control. The microplate was incubated at 37 °C and bacterial lysis was monitored every 6 h using a microplate reader (OD600nm) (Infinite M200, Tecan, Austria). The lowest two dilutions that show bacterial lysis were selected for quantification of the number of phage particles by the double-layer agar. For this, 90 μL of the phage dilution was transferred into 2 mL of the host bacterium culture (OD600nm = 0.25–0.3) and the contact between the phage and the bacterial host was allowed for 30 min at 37 °C with agitation (120 rpm) in a water-bath. After 30 min of contact, the bacterial and phage suspensions were transferred into 5 mL of semi-solid agar (0.75% (w/v)), which was then poured onto plates previously prepared with a first layer of solid medium. The inoculated plates were incubated at 37 °C for 24 h. Three biological and three technical replicates were performed.
2.7. Transmission Electron Microscopy
The effect of P5W30 on the MRSA16 cells was analyzed by TEM as previously described by El-Guendouz [62]. For this, 1 mL of bacterial cultures exposed to P5W30 at concentration of 1211 μM (10 mg·mL−1) and P5W30 plus cefoxitin 85 μM + 400 μM (0. 7 mg·mL−1 + 2 μg·mL−1) was centrifuged for 10 min at 12,000× g at 4 °C. Afterwards, the bacterial cells were resuspended in a mixture of fixatives composed of 2.5% (v/v) glutaraldehyde and 4% formaldehyde (w/v) in PBS, maintained at room temperature and protected from light for 2 h. The fixatives were eliminated by centrifugation at 12,000× g for 20 min and the bacterial cells were resuspended in 1% (w/v) paraformaldehyde and maintained at 4 °C until further processing. The fixative was removed by washing with PBS. Bacterial pellets were resuspended in 10% w/v gelatin (Sigma, St. Louis, MO, USA) in PBS and kept on ice until the solidification of gelatin and small cubes could be cut. The samples were post-fixed by incubation on ice for 2 h in the dark with 1% osmic acid anhydride (OsO4) (EMS, Hatfield, PA, USA) in PBS, washed twice with PBS and twice with water before dehydration. Dehydration was accomplished by incubation for 10 min at room temperature 50%, 70%, 96%, and 100% ethanol (Merck, Darmstadt, Germany). The sample was momentarily washed with Epon 812 (EMS) and incubated overnight in the same resin at room temperature. Samples were mounted in molds (EMS) and the Epon 812 was allowed to polymerize in an incubator at 65 °C. Ultra-thin sections were obtained using an ultra-microtome (Leica ultracut R, Leica, Wetzlar, Germany) contrasted with saturated uranyl acetate (Merck, Darmstadt, Germany) in water for 15 min followed by Reynolds lead citrate (Merck, Darmstadt, Germany) for 3 min. Bacterial morphology examined by TEM was performed with a Hitachi H8100 (Hitachi High-Technologies Corporation, Tokyo, Japan). Digital images were acquired using a bottom-mounted CCD Keen-View camera (Olympus Soft Imaging Solutions GmbH, Munich, Germany).
2.8. Statistical Analysis
The data were analyzed for statistical significance by one ANOVA using GraphPad Prism (version 9.0) (GraphPad Software, San Diego, CA, USA, www.graphpad.com, accessed on 17 December 2021). Statistical significance was considered at p < 0.05; when the analysis was statistically significant, Tukey’s post-hoc test was performed.
3. Results and Discussion
3.1. Stability Studies
The stability of POTs was tested in water and in MHB by 31P and 183W NMR spectroscopy. The aqueous solutions of P5W30, P2W18, and P2W17 contain the initial anions [NaPV5WVI30O110]14−, [α-PV2WVI18O62]6−, and [α2-PV2WVI17O61]10−, respectively, even after 24 h incubation at 37 °C (Figure 3A,B and Figures S2–S4). Regarding P2W15 (Figure S4), after dissolution in water, it immediately rearranged to P2W17, which is very stable under these conditions [63]. In MHB medium, which was used for antibacterial studies, P2W18 partially (around 30% based on the integration of 31P signals) hydrolyses to the monolacunary anion [α2-PV2WVI17O61]10- (Figure 4 and Figure S2C,D). Conversely, Preyssler POT is still the only species present in MHB (Figure 2C,D and Figure 3A,B), which is consistent with previous reports [29,41], indicating the stability of this anion under physiological conditions. In MHB solutions of P2W17 and P2W15, before and after incubation, the predominant (100% for P2W17 and 97% for P2W15) POT is [α2-PV2WVI17O61]10−. It is worth noting that solutions of P2W18 (Figure 5) and P5W30 turned blue after dissolution in MHB, indicating a reduction in WVI ions.
3.2. Antibacterial Studies
In order to assess the potential antibacterial activity of POTs, we first screened the antibacterial activity of the four POTs, the intact Wells-Dawson K6[α-PV2WVI18O62]·14H2O (P2W18), mono-lacunary Wells-Dawson K10[α2-PV2WVI17O61]·20H2O (P2W17), tri-lacunary Wells-Dawson K12[α-PV2WVI15O56]·24H2O (P2W15), and Preyssler-type (NH4)14[NaPV5WVI30O110]·31H2O (P5W30), on different Gram-negative and Gram-positive strains, either susceptible or resistant to antibiotics (Table S4). The antibacterial activity was examined using the agar diffusion technique and the MIC values of all polyoxotungstates were determined by microdilution (Table 3). The observed inhibition zones produced by the tested compounds using the agar diffusion technique are summarized in Table S4. The compound P5W30 showed the highest activity against the majority of the tested bacterial strains in comparison with the other tested POTs (P2W15, P2W17 and P2W18) that did not show inhibition zones for the Gram-negative bacteria, A. baumanii I73775, E. coli DSM 1077, E. coli I73194, K. pneumoniae I7092374, and P. aeruginosa C46281). S. aureus ATCC 6538 was the most susceptible, followed by S. pneumoniae D39 to the four polyoxotungstates tested. No inhibition zone was observed against E. coli DSM 1077 by the action of P5W30, in contrast to the multiresistant strain E. coli I73194, which showed a slight susceptibility that increased with the concentration (Table S4). The MIC values of the polyoxotungstates for S. aureus ATCC 6538 and two MRSA strains are summarized in Table 3. The compound P5W30 was more active against S. aureus ATCC 6538 and MRSA16, reaching <0.83 and 4.96 mg·mL−1, respectively (Table 3). This last strain showed the highest MIC values of 7.01 mg·mL−1, 7.87 mg·mL−1 and 7.76 mg·mL−1 for other three POTs P2W15, P2W17, and P2W18, respectively (Table 3). The MIC values against all three organisms (Table 3) for thee Wells-Dawson POTs P2W15, P2W17, and P2W18 are very close. The similar values for P2W15 and P2W17 correlate with speciation data, showing that the same monolacunary anion [α2-PV2WVI17O61]10− is exclusively present in P2W15 and P2W17 solutions (Figures S3 and S4). Although in the MHB solution of P2W18, both intact [α-PV2WVI18O62]6− and monolacunary [α2-PV2WVI17O61]10− anions co-exist (Figure S2), the presence of [α-PV2WVI18O62]6− does not affect the antibacterial efficacy compared to P2W15 and P2W17.

Table 3.
MIC values for the tested polyoxotungstates (mg·mL−1).
Our findings, together with those obtained by others [22,24,25,64], highlight the potential use of POMs, including POTs, to combat bacterial infections, even those caused by multiresistant strains. Nevertheless, it is important to emphasize the usefulness of using a significant panel of bacterial strains, as evidenced by the results observed in the current study of MRSA strains, where MRSA16 showed a higher MIC value to the tested P2W15 (>2×, P2W17 (>2.5×), and P2W18 (>2.5×), in comparison with MRSA15, and the lowest MIC value difference was observed for P5W30 (>1.5×). It is important to highlight that the strain MRSA16 showed resistance to erythromycin that, according to EUCAST [55], also evidences resistance to the antibiotics azithromycin, clarithromycin, and roxithromycin, antibiotics in the macrolide group. This group of antibiotics inhibits the translation process, therefore, inhibiting protein synthesis [64]. The resistance of S. aureus to these antibiotics is associated with the modification of the target location of macrolide antibiotics, which is mediated by adenyl-N-methyltransferase erythromycin-resistance methylase (Erm) enzymes, codified by the erm genes [64]. Another mechanism of resistance to macrolide antibiotics is linked with msr genes that codify for ATP binding cassette (ABC) transporters [65]. MRSA16 also showed resistance to chloramphenicol. This antibiotic acts by binding to the 50S ribosomal subunit, thus, blocking the bacterial protein synthesis, and the most common mechanism of resistance identified in S. aureus is through the enzymatic inactivation that is carried out by the enzyme chloramphenicol acetyltransferase [66]. Other mechanisms of resistance to chloramphenicol are the extrusion of the antibiotic through an efflux mechanism driven by the chloramphenicol/florfenicol exporter, the 23S rRNA methyl transferase that is also involved in the resistance to linezolid [66,67]. We are aware of the broad mechanisms of resistance to antibiotics and the difficulty to combat infections caused by such multiresistant strains.
In the study of Gumerova et al. [24], P5W30 showed a MIC value of 1 μg·mL−1 for the respiratory bacterial pathogen Moraxella catarrhalis ATCC 2346, whereas against the Gram-positive bacteria S. aureus, ATCC 29213 and Enterococcus faecalis ATCC 29212 MIC values were increased to 16 μg·mL−1 and 8 μg·mL−1, respectively. The difference in susceptibility to P5W30 between the tested S. aureus strain in the current study and the S. aureus ATCC 29213 tested in [24] is caused by the lower susceptibility of S. aureus ATCC 6538. Regarding the susceptibility of E. coli strains, the one tested in [24] (E. coli ECM 1556) showed no susceptibility to this compound, as observed in the current study for E. coli DSM 1077. In contrast, as mentioned, the multiresistant strain E. coli I73194 exhibited a very weak susceptibility in the agar diffusion technique.
The Preyssler-type POT P5W30 (Figure 1D), which shows the most potent antibacterial activity, was further combined with antibiotics and investigated as an antibiofilm, anti-quorum, and antiviral agent. According to EUCAST recommendations [56], the effect of the combination of P5W30 with the antibiotic chloramphenicol was additive (ΣFIC = 0.79), whereas for vancomycin, it was indifferent (ΣFIC = 2.0), and for cefoxitin, the observed effect was synergistic (ΣFIC = 0.20). Planktonic cells (in suspension) are known to be more susceptible in comparison with sessile cells (adherent) that are very resistant to the impact of several stress conditions, including antibiotics and other antibacterial agents [15,68]. TEM observations were performed in order to evaluate the impact of the treatment of planktonic MRSA16 cells either with P5W30 at 2× MIC value (10 mg·mL−1), or with P5W30 (0.7 mg·mL−1) combined with cefoxitin (2 μg·mL−1). The effect of these treatments on MRSA16 is shown in Figure 6. The MRSA16 cells were severely damaged by P5W30 at 2× MIC value, showing pronounced shape deformation and loss of cell integrity, resembling protoplasts, in contrast with the bacterial cells treated with the combination of P5W30 and cefoxitin, which showed mainly a disturbed cell wall (Figure 6). The interaction of P2W18 with MRSA cell walls has been reported [22], and this interaction occurs with the penetration of the cell wall by the compound leading to its reduction inside the cells (MRSA cells remain blue for about 12 h). The proposed mechanism is associated with the participation of P2W18 in the electron transfer system for respiration enrolling the NADH/ubiquinone/cytochrome-c, which displays a negative redox potential, able to reduce P2W18 [22]. Another reported effect of exposure of MRSA strains to P2W18 is changes at the transcriptome level; namely, the transcripts of mecA and pbp genes are impaired [22]. We can assume that P5W30 could act similarly to P2W18 against MRSA16. Recently, it was described that P5W30 modulates the cell growth rate via the hyperpolarization of bacterial cells and the so-resulted blocking of the magnesium ion flux into bacterial cells [29]. Moreover, it was described that for both B. subtilis and MRSA, the MIC values of P5W30 in combination with spectinomycin decreased by approximately 10-fold, from about 6–7 mg·mL−1 to 0.7 mg·mL−1 [29]. These values are in good agreement with the ones obtained in the current study for P5W30 against MRSA. Taken together, all of these results point to the possibility of realizing the maximum potential of POMs as antibiotics, while mitigating the resistance of pathogenic bacteria.
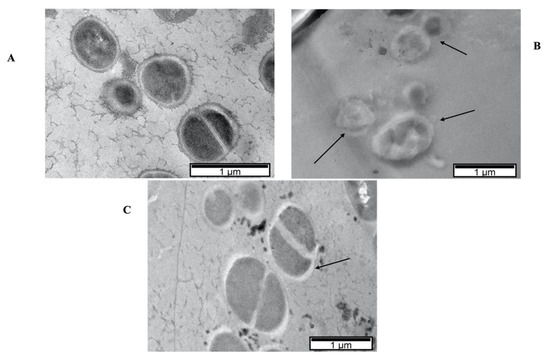
Figure 6.
TEM micrographs of MRSA16 cells treated with P5W30 at 2 × MIC value (1211 μM [10 mg·mL−1]) (B) and with the combination of P5W30 with cefoxitin (85 μM + 400 μM [0.7 mg·mL−1 + 2 μg·mL−1]) (C) and control cells (no agent) (A). The arrows in (B) indicate the severe damage to the bacterial cells caused by the exposure to the compound with loss of cell integrity and in (C) the damaged cell wall that will be mainly caused by the combined action of cefoxitin with P5W30.
3.3. Anti-Quorum-Sensing Activity
The anti-quorum-sensing activity of P5W30 was evaluated using the biosensor C. violaceum CV026. The production of violacein by the biosensor strain, the production of which is regulated by the quorum-sensing system [69], was inhibited (p < 0.0001) at all concentrations tested: 100 μM [0.83 mg·mL−1], 200 μM [1.65 mg·mL−1], 300 μM [2.48 mg·mL−1], and 400 μM [3.31 mg·mL−1] (Figure 7A). No inhibition of the biosensor growth was observed at any tested concentration in the control microplate (no addition of C6-HSL) in comparison with the antibiotic chloramphenicol, for which a 3 h lag phase was observed (Figure 7B). To the best of our knowledge, the current study is the first to report the anti-quorum-sensing activity of a POT compound. As previously mentioned, there are different quorum-quenching mechanisms [13], and future investigations will be of interest to determine the quorum-sensing inhibition mechanisms used by P5W30 and their impact on reductions in virulence.
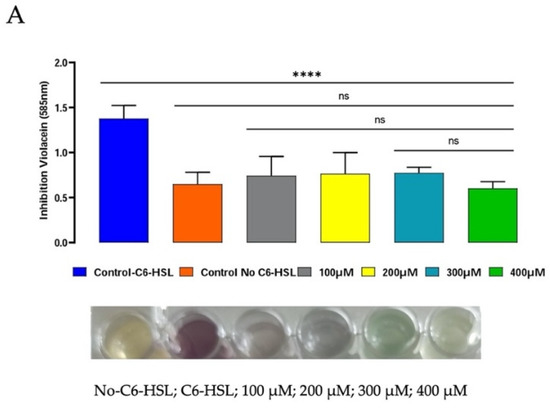
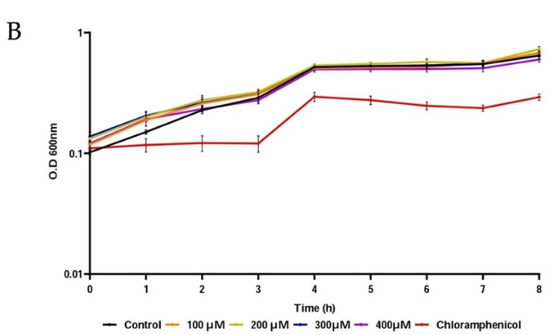
Figure 7.
Anti-quorum-sensing activity of P5W30 at concentrations 100 μM (0.83 mg·mL−1), 200 μM (1.65 mg·mL−1), 300 μM (2.48 mg·mL−1), and 400 μM (3.31 mg·mL−1). (A) Effect of P5W30 on violacein production (OD585nm). (B) Effect of P5W30 on C. violaceum CV026 growth. Error bars represent standard deviation (n = 3). **** p < 0.0001, ns—not significant.
3.4. Antibiofilm Activity
For the evaluation of the antibiofilm properties, the MRSA16 cells were allowed to form biofilms in coverslips over 24 h, and afterwards, were treated for 6 h and 24 h with the compound P5W30 at a concentration of 1211 μM (10 mg·mL−1, 2× MIC) and with the combination of P5W30 with the antibiotic cefoxitin at concentrations of 85 μM + 470 μM (0.7 mg·mL−1 + 2 μg·mL−1). The 2× MIC value of P5W30 and the combination of P5W30 with the antibiotic was selected, taking into account the higher resistance of sessile cells that is not affected either by P5W30 at the MIC value of or by the antibiotic cefoxitin at the MIC value. The results are represented in Figure 8. The disruption of the biofilm produced by MRSA16 was significantly different (p < 0.0001) both from the treatment with P5W30 individually or in combination with the antibiotic cefoxitin after 6 h of treatment. However, the exposure of sessile cells to P5W30 alone was more detrimental (p < 0.05) in comparison with the combination (Figure 8A). The exposure of sessile cells over 24 h to P5W30 individually or in combination with cefoxitin significantly affected the MRSA16 biofilm (p < 0.0001) in comparison with the control (Figure 8B). However, as observed for the 6 h treatment, the exposure of sessile cells for 24 h to P5W30 alone showed to be more efficient (p < 0.001) in the disruption of sessile cells in comparison with the combination (Figure 8B). As expected, the exposure of MRSA16 sessile cells to the concentration of 2× MIC was not sufficient to eliminate these cells; instead, a significant reduction was observed. This result can be explained by the difficulty of P5W30 to penetrate the matrix of exopolysaccharides that impregnates the aggregated cells in the biofilm. It is possible that the combination of P5W30 with the antibiotic cefoxitin at concentrations of 85 μM + 470 μM (0.7 mg·mL−1 + 2 μg·mL−1), although in a more limited way, can disrupt the aggregated cells by interfering with the exopolysaccharide layer. It is important to stress that the antibiotic cefoxitin acts by inhibiting the biosynthesis of the bacterial cell wall by binding to transpeptidases (penicillin-binding proteins, PBPs), and it is resistant to the action of extended-spectrum β-lactamases [70]. Therefore, it is very likely that the binding of the cefoxitin to PBPs will be strongly affected by the exopolysaccharide matrix that protects sessile cells.
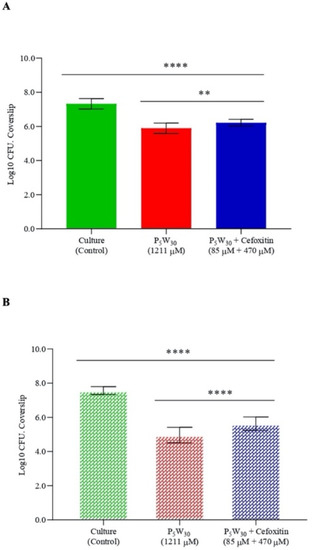
Figure 8.
Antibiofilm activity of the compound P5W30 individually (2× MIC, 1211 μM) and in combination with the antibiotic cefoxitin (85 μM + 470 μM [0.7 mg·mL−1 + 2 μg·mL−1]) against MRSA16 after 6 h of exposure (A) and after 24 h of exposure (B). Data are expressed as the mean of three biological and two technical replicates. The error bars represent the standard deviation. **** p < 0.0001, ** p < 0.05.
As for the anti-quorum-sensing activity, to the best of our knowledge, the current study is the first that describes the antibiofilm action of a POT compound.
3.5. Antiviral Activity
Finally, the antiviral activity of the compound P5W30 was evaluated against the enterovirus Qβ at concentrations of 250 μM (2.08 mg·mL−1), 500 μM (4.15 mg·mL−1), and 750 μM (6.23 mg·mL−1). The impact of the compound on the phage infectivity is represented in Figure 9. The reduction in viral particles increased with the concentration of P5W30 tested. The lowest reduction value (p < 0.0001) was observed at a concentration of 250 μM, achieving the maximum reduction at the highest concentration tested of 750 μM (reduction of 8.11± 0.14 Log10 PFU·mL−1) (Figure 9). The coliphage Qβ of the Leviviridae family is a single-stranded, positive-sense RNA phage that is commonly used as a surrogate to mimic the behavior of pathogenic enteric viruses [71]. The ability of POTs to inactivate RNA virus has been reported [47] and the proposed mechanisms of Flu V (influenza virus) inactivation by POTs can occur by inhibiting the virus fusion with the membrane or by inhibiting the binding of the virus to the cell [47]. It is possible that the mechanism of the inactivation of the coliphage Qβ by P5W30 will be via inhibiting attachment of the phage to the host cells. However, such a hypothesis requires validation.
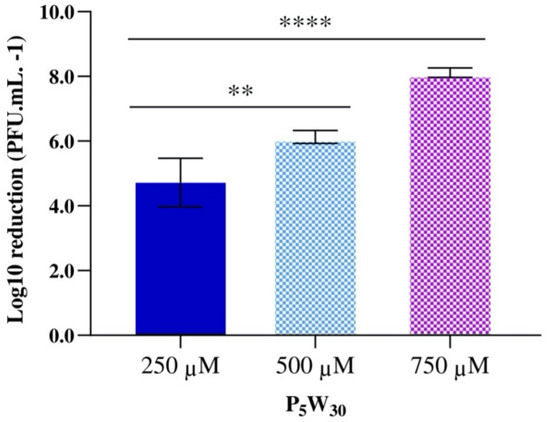
Figure 9.
Antiviral activity of the compound P5W30 against the enterovirus Qβ. Data are expressed as the mean of three biological and three technical replicates. The error bars represent the standard deviation. **** p < 0.0001, ** p < 0.05.
In sum, in addition to the antibacterial and antiviral activities, we showed that P5W30 was also able to impair quorum sensing and biofilm formation, as illustrated in Table S1 and Figure 2. Regarding the mode of P5W30 action and in general for POMs, several potential mechanisms have been evoked and recently summarized [34,35,36,37,39,72], pointing towards promising biological applications in the near future.
4. Conclusions
In addition to showing the antibacterial activity of P5W30 with the ability to affect MRSA cells, we demonstrated that P5W30 also displays other properties, such as anti-quorum sensing and antibiofilm properties. These are biological activities that are reported for a polyoxotungstate for the first time. Quorum sensing and biofilms facilitate bacterial colonization, antibiotic resistance, and persistence in both the environment and host, and its impairment by POT can greatly contribute to the control of bacterial infections, such as those caused by multiresistant bacteria. Moreover, antiviral activity was also observed using the enterovirus Qβ. NMR stability studies of P5W30 demonstrate that it remains intact, suggesting that it is an active species in the described biological activities. Taken together, our results emphasize the potential biomedical use of POTs, particularly Preyssler-type, to combat antibiotic-resistant MRSA strains and their ability to form biofilms, in addition to being a promising antiviral agent. The molecular mechanisms of action of the various biological activities described still need to be elucidated, which will lead to a new understanding on a molecular basis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11070994/s1, Table S1: Biological activities of Preyssler-type POMs, Figure S1: IR spectra of the Wells-Dawson (WD) and Preyssler POTs used in this study, Table S2: Comparison of experimental and reported wavenumbers for P–O vibrations, which are the most important to destinguish between phosphotungstates within one family. Table S3: Experimental conditions and shifts obtained from 31P and 183W NMR spectroscopic studies performed on the tested POTs, Figure S2: 31P NMR spectra of 10 mM K6[α-PV2WVI18O62]P2W18 solutions (A) in D2O recorded approximately 1 h after preparation; (B) in D2O recorded after incubation for 24 h at 37 °C; (C) in Mueller-Hinton broth (MHB) recorded approximately 1 h after preparation; (D) in Mueller-Hinton broth (MHB) recorded after incubation for 24 h at 37 °C, Figure S3: 31P NMR spectra of 10 mM K10[α2-PV2WVI17O61] P2W17 solutions (A) in D2O recorded approximately 1 h after preparation; (B) in D2O recorded after incubation for 24 h at 37 °C; (C) in Mueller-Hinton broth (MHB) recorded approximately 1 h after preparation; D) in Mueller-Hinton broth (MHB) recorded after incubation for 24 h at 37 °C, Figure S4: 31P NMR spectra of 10 mM K12[PV2WVI15O56] P2W15 solutions (A) in D2O recorded approximately 1 h after preparation; (B) in D2O recorded after incubation for 24 h at 37 °C; (C) in Mueller-Hinton broth (MHB) recorded approximately 1 h after preparation; (D) in Mueller-Hinton broth (MHB) recorded after incubation for 24 h at 37 °C, Table S4: Inhibition zones (mm) produced by the tested polyoxotungstates; K12[PV2WVI15O56] 24H2O (P2W15), K10[α2-PV2WVI17O61] 20H2O (P2W17), K6[α-PV2WVI18O62] 14H2O (P2W18) and (NH4)14[NaPV5WVI30O110]·31H2O (P5W30) ranging from 100 to 500 µM.
Author Contributions
L.F. was the principal researcher responsible for the study. L.F. and M.A. planned the design of the study and made a substantial contribution to the drafting of the manuscript. A.M. and J.M. collaborated in the experiments. L.J. and I.N. contributed with the TEM analysis. N.I.G. and A.R. collaborated in the synthesis, characterization, and stability studies of all POMs and contributed greatly with the drafting of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FCT—Foundation for Science and Technology—through project UIDB/04326/2020 (M.A.) and LA/P/0101/2020; the Austrian Science Fund (FWF): P33927 (N.I.G.); P33089 (A.R.); and the University of Vienna (N.I.G. and A.R.). The authors are grateful to Algarve Biomedical Center (AD-ABC) for Open Access Funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the current study are available within the article and Supplementary Material.
Acknowledgments
The authors gratefully acknowledge Mathea Sophia Galanski, Ricarda Ofenschüssl, and Susanne Felsinger for 31P and 183W NMR measurements, NMR Core Facility, Faculty of Chemistry, University of Vienna. The authors acknowledge Município de Loulé for the support provided during the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davies, J. Origins and evolution of antibiotic resistance. Microbiologia 1996, 12, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Gudda, F.O.; Waigi, M.G.; Odinga, E.S.; Yang, B.; Carter, L.; Gao, Y. Antibiotic-contaminated wastewater irrigated vegetables pose resistance selection risks to the gut microbiome. Environ. Pollut. 2020, 264, 114752. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Hernandez, A.L.; DePas, W.H.; Park, J.H.; Teschler, J.K.; Hartmann, R.; Jeckel, H.; Drescher, K.; Beyhane, S.; Newman, D.K.; Yildiz, F.H. Upregulation of virulence genes promotes Vibrio cholerae biofilm hyperinfectivity. Proc. Natl. Acad. Sci. USA 2020, 117, 11010–11017. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm activity as a health issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Thurlow, L.R.; Hanke, M.L.; Fritz, T.; Angle, A.; Aldrich, A.; Williams, S.H.; Engebretsen, I.L.; Bayles, K.W.; Horswill, A.R.; Kielian, T. Staphylococcus aureus Biofilms Prevent Macrophage Phagocytosis and Attenuate Inflammation In Vivo. J. Immunol. 2011, 186, 6585–6596. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-Associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [Green Version]
- Papenfort, K.; Bassler, B.L. Quorum sensing signal-response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016, 14, 576–588. [Google Scholar] [CrossRef] [Green Version]
- Aframian, N.; Eldar, A. A Bacterial Tower of Babel: Quorum-Sensing Signaling Diversity and Its Evolution. Annu. Rev. Microbiol. 2020, 74, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Ampomah-Wireko, M.; Luo, C.; Cao, Y.; Wang, H.; Nininahazwe, L.; Wu, C. Chemical probe of AHL modulators on quorum sensing in Gram-Negative Bacteria and as antiproliferative agents: A review. Eur. J. Med. Chem. 2021, 226, 113864. [Google Scholar] [CrossRef] [PubMed]
- Paluch, E.; Rewak-Soroczyńska, J.; Jędrusik, I.; Mazurkiewicz, E.; Jermakow, K. Prevention of biofilm formation by quorum quenching. Appl. Microbiol. Biotechnol. 2020, 104, 1871–1881. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.; Ding, Y.; Cao, J.; Sun, Y.; Wang, F.; Ju, S.; Yu, J. Non-antibiotic methods against Pseudomonas aeruginosa include QS inhibitors: A narrative review. Ann. Palliat. Med. 2021, 10, 6926–6935. [Google Scholar] [CrossRef]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing phytochemicals as anti-virulent agents to attenuate quorum sensing-regulated virulence factors and biofilm formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2021, 15, 1695–1718. [Google Scholar] [CrossRef] [PubMed]
- Anju, V.T.; Busi, S.; Ranganathan, S.; Ampasala, D.R.; Kumar, S.; Suchiang, K.; Kumavath, R.; Dyavaiah, M. Sesamin and sesamolin rescues Caenorhabditis elegans from Pseudomonas aeruginosa infection through the attenuation of quorum sensing regulated virulence factors. Microb. Pathog. 2021, 155, 104912. [Google Scholar] [CrossRef]
- Horváth, M.; Kovács, T.; Koderivalappil, S.; Ábrahám, H.; Rákhely, G.; Schneider, G. Identification of a newly isolated lytic bacteriophage against K24 capsular type, carbapenem resistant Klebsiella pneumoniae isolates. Sci. Rep. 2020, 10, 5891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, J.; Melo, L.D.R.; Poeta, P.; Igrejas, G.; Ferraz, M.P.; Azeredo, J.; Monteiro, F.J. Lytic bacteriophages against multidrug-resistant Staphylococcus aureus, Enterococcus faecalis and Escherichia coli isolates from orthopaedic implant-associated infections. Int. J. Antimicrob. Agents 2019, 54, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Garza-Cervantes, J.A.; Mendiola-Garza, G.; de Melo, E.M.; Dugmore, T.I.J.; Matharu, A.S.; Morones-Ramirez, J.R. Antimicrobial activity of a silver-microfibrillated cellulose biocomposite against susceptible and resistant bacteria. Sci. Rep. 2020, 10, 7281. [Google Scholar] [CrossRef]
- García-Lara, B.; Saucedo-Mora, M.A.; Roldán-Sánchez, J.A.; Pérez-Eretza, B.; Ramasamy, M.; Lee, J.; Coria-Jimenez, R.; Tapia, M.; Varela-Guerrero, V.; García-Contreras, R. Inhibition of quorum-sensing-dependent virulence factors and biofilm formation of clinical and environmental Pseudomonas aeruginosa strains by ZnO nanoparticles. Lett. Appl. Microbiol. 2015, 61, 299–305. [Google Scholar] [CrossRef]
- Rao, H.; Choo, S.; Mahalingam, S.R.R.; Adisuri, D.S.; Madhavan, P.; Akim, A.M.; Chong, P.P. Approaches for mitigating microbial biofilm-related drug resistance: A focus on micro- and nanotechnologies. Molecules 2021, 26, 1870. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Suzuki, T.; Fujita, Y.; Oda, M.; Matsumoto, N.; Yamase, T. Enhancement of antibacterial activity of β-lactam antibiotics by [P2W18O62]6−, [SiMo12O40]4−, and [PTi2W10O40]7− against methicillin-resistant and vancomycin-resistant Staphylococcus aureus. J. Inorg. Biochem. 2006, 100, 1225–1233. [Google Scholar] [CrossRef]
- Yu, X.; Chen, C.; Peng, J.; Shi, Z.; Shen, Y.; Mei, J.; Ren, Z. Antibacterial-active multilayer films composed of polyoxometalate and Methyl Violet: Fabrication, characterization and properties. Thin Solid Films 2014, 571, 69–74. [Google Scholar] [CrossRef]
- Gumerova, N.; Al-Sayed, E.; Krivosudský, L.; Čipčić-Paljetak, H.; Verbanac, D.; Rompel, A. Antibacterial Activity of Polyoxometalates Against Moraxella catarrhalis. Front. Chem. 2018, 6, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Farzana, R.; Iqra, P.; Hunaiza, T. Antioxidant and antimicrobial effects of polyoxometalates. Microbiol. Curr. Res. 2018, 2, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Leite, A.; Bessa, L.J.; Silva, A.M.G.; Gameiro, P.; de Castro, B.; Rangel, M. Antibacterial activity of naphthyl derived bis-(3-hydroxy-4-pyridinonate) copper(II) complexes against multidrug-resistant bacteria. J. Inorg. Biochem. 2019, 197, 110704. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, G.; Saoncella, O.; Kaner, P.; Altinkaya, S.A.; Figoli, A.; Bonchio, M.; Carraro, M. Chitosan-polyoxometalate nanocomposites: Synthesis, characterization and application as antimicrobial agents. J. Clust. Sci. 2014, 25, 839–854. [Google Scholar] [CrossRef] [Green Version]
- Daima, H.K.; Selvakannan, P.R.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic influence of polyoxometalate surface corona towards enhancing the antibacterial performance of tyrosine-capped Ag nanoparticles. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Yu, Q.; Liu, Y.; Yin, P. Bacterial hyperpolarization modulated by polyoxometalates for solutions of antibiotic resistance. J. Inorg. Biochem. 2021, 220, 111463. [Google Scholar] [CrossRef]
- Pope, M. Heteropoly and Isopoly Oxometalates, Inorganic; Springer: Berlin/Heidelberg, Germany, 1983; ISBN 978-3-662-12006-4. [Google Scholar]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in solution: Speciation under spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Stuckart, M.; Monakhov, K.Y. Polyoxometalates as components of supramolecular assemblies. Chem. Sci. 2019, 10, 4364–4376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rompuy, L.S.; Parac-Vogt, T.N. Interactions between polyoxometalates and biological systems: From drug design to artificial enzymes. Curr. Opin. Biotechnol. 2019, 58, 92–99. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [Green Version]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasenknopf, B. Polyoxometalates: Introduction to a class of inorganic compounds and their biomedical applications. Front. Biosci. 2005, 10, 275–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Lara, E.; Treviño, S.; Sánchez-Gaytán, B.L.; Sánchez-Mora, E.; Castro, M.E.; Meléndez-Bustamante, F.J.; Méndez-Rojas, M.A.; González-Vergara, E. Decavanadate salts of cytosine and metformin: A combined experimental-theoretical study of potential metallodrugs against diabetes and cancer. Front. Chem. 2018, 6, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
- Haider, A.; Zarschler, K.; Joshi, S.A.; Smith, R.M.; Lin, Z.; Mougharbel, A.S.; Herzog, U.; Müller, C.E.; Stephan, H.; Kortz, U. Preyssler-Pope-Jeannin polyanions [NaP5W30O110]14– and [AgP5W30O110]14–: Microwave-assisted synthesis, structure, and biological activity. Z. Anorg. Allg. Chem. 2018, 644, 752–758. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, K.; Li, M.; Hu, C.; Yin, P. Sustained release of Ag+ confined inside polyoxometalates for long-lasting bacterial resistance. Chem. Commun. 2020, 56, 5287–5290. [Google Scholar] [CrossRef]
- Fu, L.; Gao, H.; Yan, M.; Li, S.; Li, X.; Dai, Z.; Liu, S. Polyoxometalate-based organic-inorganic hybrids as antitumor drugs. Small 2015, 11, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The aquaporin-3-inhibiting potential of polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [Green Version]
- Razavi, S.F.; Bamoharram, F.F.; Hashemi, T.; Shahrokhabadi, K.; Davoodnia, A. Nanolipid-loaded Preyssler polyoxometalate: Synthesis, characterization and invitro inhibitory effects on HepG2 tumor cells. Toxicol. Vitr. 2020, 68, 104917. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.L.; Schinazi, R.F.; Weeks, M.S. Anti-HIV-1 activity, toxicity, and stability studies of representative structural families of polyoxometalates. J. Med. Chem. 1990, 33, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M.; Fraqueza, G.; Gumerova, N.I.; Rompel, A.; Cordoba-Granados, J.J.; Berrocal, M.; Mata, A.M. Comparison of SERCA and PMCA Inhibition Potential of Polyoxotungstates; Antunes, C., Costa, A., Palma, P., Galveias, A., Gastalho, C., Marques, M., Cancela, L., Aureliano, M., Eds.; Universidade de Évora: Évora, Portugal, 2021; ISBN 978-972-778-215-4. [Google Scholar]
- Shigeta, S.; Mori, S.; Yamase, T.; Yamamoto, N.; Yamamoto, N. Anti-RNA virus activity of polyoxometalates. Biomed. Pharmacother. 2006, 60, 211–219. [Google Scholar] [CrossRef]
- Müller, A.; Fedin, V.P.; Kuhlmann, C.; Bögge, H.; Hauptfleisch, B.; Fenske, H.-D.; Baum, G. ‘Adding’ stable functional complementary, nucleophilic and electrophilic clusters: A synthetic route to [{(SiW11O39)Mo3S4(H2O)3(μ-OH)}2]10− and [{(P2W17O61)Mo3S4(H2O)3(μ-OH)}2]14− as examples. Chem. Commun. 1999, 1189–1190. [Google Scholar] [CrossRef]
- Du, J.; Cao, M.D.; Feng, S.L.; Su, F.; Sang, X.J.; Zhang, L.C.; You, W.S.; Yang, M.; Zhu, Z.M. Two New Preyssler-Type Polyoxometalate-Based Coordination Polymers and Their Application in Horseradish Peroxidase Immobilization. Chem. A Eur. J. 2017, 23, 14614–14622. [Google Scholar] [CrossRef]
- Contant, R.; Klemperer, W.; Yaghi, O. Potassium octadecatungstodiphosphates (V) and related lacunary compounds. In Inorganic Syntheses; Ginsberg, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 104–111. [Google Scholar]
- Jeannin, Y.; Martin-Frere, J.; Choi, D.; Pope, M. The sodium pentaphosphato (V)-triacontatungstate anion isolated as the ammonium salt. In Inorganic Syntheses; Ginsberg, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; pp. 115–118. [Google Scholar]
- Dawson, B. The structure of the 9(18)-heteropoly anion in potassium 9(18)-tungstophosphate, K6(P2W18O62)·14H2O. Acta Crystallogr. 1953, 6, 113–126. [Google Scholar] [CrossRef] [Green Version]
- Finke, R.G.; Lyon, D.K.; Nomiya, K.; Weakley, T.J.R. Structure of nonasodium—triniobatopentadecawolframatodiphosphate-acetonitrile-water (1/2/23), Na9[P2W15Nb3O62]·2CH3CN·23H2O. Acta Cryst 1990, C46, 1592–1596. [Google Scholar] [CrossRef]
- Alizadeh, M.H.; Harmalker, S.P.; Jeannin, Y.; Martin-Frer, J.; Pope, M.T. A Heteropolyanion with fivefold molecular symmetry that contains a nonlabile encapsulated sodium ion. The structure and chemistry of [NaP5W30O110]14−. J. Am. Chem. Soc. 1985, 107, 2662–2669. [Google Scholar] [CrossRef]
- EUCAST, European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2019. Available online: http://www.eucast.org (accessed on 2 December 2019).
- EUCAST, European Committee on Antimicrobial Susceptibility Testing. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinelli, D.; Grossmann, G.; Séquin, U.; Brandl, H.; Bachofen, R. Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum. BMC Microbiol. 2004, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.N.; Horswill, A.R. A coverslip-based technique for evaluating Staphylococcus aureus biofilm formation on human plasma. Front. Cell. Infect. Microbiol. 2012, 2, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Hyg. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, M.R. Simple colorimetric microplate test of phage lysis in Salmonella enterica. J. Microbiol. Methods 2007, 69, 394–398. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, L.; Antunes, M.D.; Aazza, S.; Duarte, J.; Silvério, A.R. Antimicrobial, antiviral and antioxidant activities of “água-mel” from Portugal. Food Chem. Toxicol. 2013, 56, 136–144. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Lourenço, J.P.; Rosa da Costa, A.M.; Miguel, M.G.; Barrocas Dias, C.; Manhita, A.; Jordao, L.; Nogueira, I.; Faleiro, M.L. Magnetite nanoparticles functionalized with propolis against methicillin resistant strains of Staphylococcus aureus. J. Taiwan Inst. Chem. Eng. 2019, 102, 25–33. [Google Scholar] [CrossRef]
- Lampl, R.; Breibeck, J.; Gumerova, N.I.; Galanski, M.S.; Rompel, A. Wells–Dawson phosphotungstates as mushroom tyrosinase inhibitors: A speciation study. Sci. Rep. 2021, 11, 19354. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M. Mechanisms of resistance to macrolide antibiotics among Staphylococcus aureus. Antibiotics 2021, 10, 1406. [Google Scholar] [CrossRef]
- Ojo, K.K.; Striplin, M.J.; Ulep, C.C.; Close, N.S.; Zittle, J.; Luis, H.; Bernardo, M.; Leitao, J.; Roberts, M.C. Staphylococcus efflux msr(A) gene characterized in Streptococcus, Enterococcus, Corynebacterium, and Pseudomonas isolates. Antimicrob. Agents Chemother. 2006, 50, 1089–1091. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, S.; Kehrenberg, C.; Doublet, B.; Cloeckaert, A. Molecular basis of bacterial resistance to chloramphenicol and florfenicol. FEMS Microbiol. Rev. 2004, 28, 519–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udo, E.E.; Boswihi, S.S.; Mathew, B.; Noronha, B.; Verghese, T. Resurgence of chloramphenicol resistance in methicillin-resistant Staphylococcus aureus due to the acquisition of a variant florfenicol exporter (Fexav)-mediated chloramphenicol resistance in Kuwait hospitals. Antibiotics 2021, 10, 1250. [Google Scholar] [CrossRef] [PubMed]
- Lamret, F.; Varin-Simon, J.; Velard, F.; Terryn, C.; Mongaret, C.; Colin, M.; Gangloff, S.C.; Reffuveille, F. Staphylococcus aureus Strain-Dependent Biofilm Formation in Bone-Like Environment. Front. Microbiol. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuhashi, M.; Tamaki, S. Enzymatic studies on the mechanism of action of cefoxitin. J. Antibiot. 1982, 2, 888–901. [Google Scholar]
- Bastin, G.; Loison, P.; Vernex-Loset, L.; Dupire, F.; Challant, J.; Majou, D.; Boudaud, N.; Krier, G.; Gantzer, C. Structural Organizations of Qβ and MS2 Phages Affect Capsid Protein Modifications by Oxidants Hypochlorous Acid and Peroxynitrite. Front. Microbiol. 2020, 11, 1157. [Google Scholar] [CrossRef] [PubMed]
- Aureliano, M. The future is bright for polyoxometalates. BioChem 2022, 2, 8–26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).